Abstract
Free essential oil methanolic extracts from three different geographical populations of Lippia graveolens in México were screened for antioxidant and antimutagenic properties by the DPPH and Kado microsuspension assay, respectively. Total phenolic and flavonoid contents as well as HPLC identification and quantification of naringenin and rosmarinic acid were also carried out. In addition, a taxonomical phenetic analysis was performed. The L. graveolens extracts showed varying content of phenols and flavonoids. Significant concentration of rosmarinic acid was found for the first time in the species. All the extracts were capable of scavenging DPPH radicals in a concentration dependent fashion; the IC50 values correlate with the phenolic content. None of the extracts was toxic to TA100 and TA98 strains at the concentrations tested; moreover, the extracts at a concentration equivalent to 200 μg of gallic acid inhibited a 39 and 30% the mutagenicity induced by 4-nitro-o-phenylenediamine and sodium azide, respectively. The results suggest that the Mexican oregano is a source of polar bioactive ingredients for the food industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oregano is a common name for some flavor filled culinary and medicinal herbs. Most of them belong to the genus Origanum (Lamiaceae) and Lippia (Verbenaceae). One of the most widely used species is Lippia graveolens Kunth (L. berlandieri Schauer) known as Mexican oregano, an aromatic plant native of Southern North America, México, Guatemala, Nicaragua and Honduras. In the Mexican traditional medicine, the aerial parts of the plant are used as an antiseptic, antipyretic, analgesic, abortive, antispasmodic, anti-inflammatory agent and for the treatment of menstrual disorders and diabetes [1]. The chemical composition of the essential oil of L. graveolens has been determined by GC-MS. A high content of hydrocarbonated and oxigenated monoterpenes was found, being carvacrol, thymol and p-cymene the major constituents. In addition, the essential oil demonstrated significant antimicrobial and antioxidant activity [2, 3]. As far as other components, naringenin, pinocembrin, lapachenole and ten iridoids have been isolated [4, 5].
Most of the studies of L. graveolens have focused on the chemical composition and biological activities of the essential oil and few scientific reports regarding chemical characterization of the polar extracts as well as their biological activities are found [6]. Therefore, we report here a preliminary chemical analysis and the antioxidant and the antimutagenic properties of the polar extracts from different Mexican geographical populations of L. graveolens with the aim of proposed natural antioxidant agents to be used as phytoadditives in food, pharmaceutical and cosmetic industries.
Materials and Methods
Plant Material and Populations Analysis
The aerial parts of the L. graveolens plants were collected at the flowering time, from Peñamiller, State of Querétaro (Qro.); Victoria, State of Guanajuato (Gto.); and San Juan Raya, State of Puebla (Pue.) in August 2002. A voucher specimen of each plant was deposited at the Ethnobotanical Collection of the Herbarium of Querétaro “Dr. Jerzy Rzedowski” (QMEX) located at the School of Natural Sciences, University of Querétaro. To find a possible correlation among the morphological similarities of the three “oregano” populations, a taxonomical phenetic analysis was done with the Jump Statistics Made Visual (JMP) program. 20 morphometric characters out of 15 OTUs were used, representing 15 localities of the three Mexican states, including the three populations studied. The additional material for this analysis was obtained from herbarium vouchers from the IEB, MEXU, and QMEX herbaria. Air-dried, ground plant material (10 g) was extracted with hexane–acetone 1:1, followed by methanol in a Soxhlet apparatus. The extracts were evaporated to dryness in vacuum, and stored at 4°C for later used.
Determination of Total Phenolic and Flavonoid Contents
The total phenolic content of the extracts was determined according to the Folin–Ciocalteu colorimetric method [7]. Results are expressed as milligrams of gallic acid equivalents per gram of extract. The total flavonoid content was determined by the method described by Liu et al. [8]; the results are expressed as milligrams of (+)-catechin equivalents per gram of extract. All data are reported as the average of three measurements.
HPLC Analysis
Plant extracts were filtered through a 0.2 mm filter membrane and 20 μl were injected in triplicate into a reversed phase column (C18 Prep-Nova Pack HR, 60 Å, 6 μm, 3.6–300 mm), using a Waters HPLC system (Waters Corporation, Milford, MA, USA) which consisted of a quaternary pump (model 600), a photodiode array detector (model 996), an in-line vacuum degasser (MetaChem Technologies Inc.), and a Rheodyne injector (4793). Control of the equipment, data acquisition, processing, and management of chromatographic information were performed by the Millennium32 software program (Waters). Reported chromatographic conditions were used [9].
1,1-Diphenyl-2-picrylhydrazyl (DPPH) Scavenging Activity
Radical scavenging activity (RSA) was determined using the stable radical DPPH, according to the method reported by Fukumoto and Mazza [10]. All reactions were conducted in 96 well microplates. Absorbances were recorded in a Versa Max Tunable Microplate Reader (Molecular Devices Co., Sunnyvale, USA). 20 μl of methanolic extract solution (50, 100, 200, 400, 500, 600, 800, and 1,000 μg/ml) was tested. All experiments were carried out in triplicate and repeated three times. The radical scavenging activities were expressed as IC50 values and Trolox equivalent antioxidant capacity (TEAC), the IC50 was calculated from the log–dose inhibition curve obtained by a nonlinear regression algorithm (Prism, 4.0, GraphPad) and the TEAC value was calculated employing a Trolox calibration curve.
Mutagenicity and Antimutagenicity Testing
The Kado microsuspension assay was used to evaluate the mutagenic and antimutagenic effect of the extracts [11, 12]. TA100 and TA98 Salmonella typhimurium strains and the Sodium azide (NaN3) and 4-Nitro-o-phenylenediamine (4-N-O-P) mutagens were included. Tester strains TA98 and TA100 were purchased from Molecular Toxicology Inc., of Bone NC (Annapolis, MD, USA). 0.01 ml of oregano extracts solutions (amount equivalent to 200 μg gallic acid/ml) were tested. Samples were tested in triplicate for each independent experiment performed.
Statistical Analysis
Experimental values are given as means ± standard deviation (SD). Statistical significance was determined by one-way variance analysis (ANOVA). Differences at P < 0.05 were considered to be significant. A Tukey test for comparison of multiple means was used.
Results and Discussion
Taxonomical phenetic analysis
The geographical localization of the collected samples of L. graveolens is shown in Fig. 1. For the population similarity analysis, a phenogram with two general groups of populations was obtained (Fig. 2). The first group formed two subgroups out of five populations. The second group also presented two subgroups out of ten populations. The populations studied for the chemical and biological analysis are in different groups; the ones from Victoria and Peñamiller are in the first one, and the one from San Juan Raya in the second. The morphological similarities seem geographically feasible with the first group, where Victoria and Peñamiller are included with populations from the semiarid region of Guanajuato and Querétaro. Nonetheless, when the whole localities are considered, few geographical correlation is found with plant morphology, since in the second group, populations from the same Guanajuato and Querétaro semiarid region are intermixed with the ones from the state of Puebla.
Phenogram indicating the “oregano” (Lippia graveolens) populations similarity, including the three localities sampled (stars). The localities are from the following states: Guanajuato: Atar G Atarjea, Vic G Victoria, Xich G Xichú; Puebla: Izuc. P Izúcar de Matamoros, Jolal P Jolal (San Juan Raya); Querétaro: Arroy Q Arroyo Seco, CaÊonCaQ Cañón de Cadereyta, JalpQ Jalpan, LandaQ Landa de Matamoros, MoraCaQ Mora (Cadereyta), PeÊQ Peñamiller, SjoaQ San Joaquín, Teq Q Tequisquiapan, Tierra CPQ Tierra Caliente (Peñamiller), VillaQ El Batán (Villa Corregidora)
Total phenols and flavonoids in L. graveolens extracts
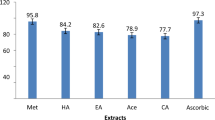
The extraction with hexane and acetone was performed to remove the essential oil components and thin layer chromatography of the methanolic extracts indicated the absence of carvacrol, thymol and cymene, the major constituents of the essential oil. Since phenolic substances have been shown to be responsible for the biological activities of plant extracts [13, 14], the total phenol and flavonoid content of the methanolic extracts were investigated and the results are shown in Table 1. The total phenol content, ranged around 211 mg and 270 mg gallic acid equivs./g of dried extract. The extracts from Puebla (MEP) and Querétaro (MEQ) have the major phenolic content. The total flavonoid content ranged from 136 and 200 mg (+)-catechin equivs./g with the order of increasing flavonoid content being MEG < MEP < MEQ. Rosmarinic acid and naringenin were identified and quantified by HPLC, by comparing the retention time with standards, and calculating the concentration from the respective calibration curves (Table 1). The extracts from Puebla and Querétaro showed similar rosmarinic acid content as the reported values for the European oregano extracts (Origanum vulgare; 25.63 mg/g extract [15] and 12.7 mg/g extract [16]), while the extract from Guanajuato (MEG) showed the lowest value. As far as we know, there are no references in the literature concerning the identification of rosmarinic acid in L. graveolens Kunth, therefore this is the first report. Naringenin has been isolated from the aerial parts of L. graveolens [4]; however, it was not detected in the MEQ extract.
DPPH Radical Scavenging
Free radicals play an important role in chronic diseases related to oxidative stress, such as diabetes, cancer and cardiovascular pathologies [13, 17]. Therefore the free radical scavenging properties of the extracts were determined by the DPPH assay, where the DPPH radical is reduced by the antioxidant compound to its hydrazine derivative. All the extracts were capable of scavenging DPPH radicals in a concentration dependent fashion. At a concentration equivalent to 1,000 μM gallic acid, the ARA and TEAC values of the extracts were similar to the values of gallic acid (1,000 μM). The estimated median inhibitory concentration (IC50) values for the extracts are shown in Table 2. It can be observed that the order of increasing IC50 for the extracts is MEQ < MEP < MEG. These results are in accordance with the phenolic content. As expected the extract with major phenolic and flavonoid content exhibited the better antioxidant capacity. However, compared with the controls BHT (IC50 = 56.15 ± 1 μg/ml) and Trolox (IC50 = 115 ± 7 μg/ml), the oregano extracts exhibited a less powerful antioxidant activity.
Mutagenicity and antimutagenicity testing
In order to choose the NaN3 and 4-N-O-P concentration for antimutagenicity assays in TA100 and TA98 a dose–response curve was done (NaN3 μg/ml: 50, 100, 200, 400, 800, and for 4-N-O-P μg/ml: 16, 32, 64, 125, 250, 500). None of the concentrations tested was toxic to the bacteria; 500 μg/ml of NaN3 was chosen for TA100 tester strain and 400 μg/ml of 4-N-O-P for TA98 strain. None of the extracts was toxic to the bacteria at the concentration tested since the numbers of revertants were similar to the spontaneous mutation (114 ± 12 revertants/plate for TA100 and 35 ± 5 revertants/plate for TA98). The free essential oil methanolic extract concentrations were adjusted to 200 μg equivalents of gallic acid to test the inhibitory effects on 4-N-O-P and NaN3 mutagenicity in TA98 and TA100 tester strains, respectively (Table 3). For TA98 tester strain, all the extracts showed an average of 39 percentage inhibition against 4-N-O-P mutagenicity. Similar results were showed for TA100 tester strain. MEQ showed more inhibition on NaN3 mutagenicity, but not statistical different to MEP. Kanazawa et al. [18] reported that methanolic extracts from fresh and dry European oregano (O. vulgare) at 10, 50 and 500 μg inhibited the mutagenicity against 3-amino-1-methyl-5H-pyrido[4,3-b] indole (Trp-P-2) in a dose-response manner (32, 86 and 96% for fresh oregano and 38, 90 and 90% for dry oregano), using incorporation plate assay with TA98 tester strain. The differences with our results can be explained due to the fact that we are testing an extract of L. graveolens that do not contained non-polar components.
In summary, the L. graveolens extracts showed a high content of phenolic compounds being the flavonoids the major constituents. Rosmarinic acid is present in concentrations compared to the reported values of the European Oregano (O. vulgare). All the extracts were capable of scavenging DPPH radicals in a concentration dependent fashion. The antimutagenic evaluation demonstrated that all extracts were not toxic to TA100 and TA98 strains at the concentrations tested, and showed a 39 and 30% average of antimutagenic effect on 4-nitro-o-phenylenediamine and sodium azide mutagenicity respectively. Mexican oregano can be proposed as a source of antioxidant and antimutagenic polar compounds, a bio-guided phytochemical study is currently in progress.
Abbreviations
- DPPH:
-
1,1-Diphenyl-2-picrylhydrazyl
- MEG:
-
Guanajuato free oil methanolic extract
- MEP:
-
Puebla free oil methanolic extract
- MEQ:
-
Querétaro free oil methanolic extract
References
Pascual ME, Slowing K, Carretero E, Sánchez Mata D, Villar A (2001) Lippia: traditional uses, chemistry and pharmacology: a review. J Ethnopharmacol 76:201–214
Salgueiro LR, Cavaleiro C, Gonçalves MJ, Proença de Cunha A (2003) Antimicrobial activity and chemical composition of the essential oil of Lippia graveolens from Guatemala. Planta Med 69:80–83
Rocha GN, Gallegos IJA, González LR, Ramos GM, Rodríguez ME, Reynoso CR, Rocha UA, Roque RM (2007) Antioxidant effect of oregano (Lippia beralndieri v. Shauer) essential oil and mother liquors. Food Chem 102:330–335
Domínquez XA, Sánchez H, Suárez M, Baldas JH, González MR (1989) Chemical constituents of Lippia graveolens. Planta Med 55:208–209
Rastrelli L, Caceres A, Morales C, De Simona F, Aquino R (1998) Iridoids from Lippia graveolens. Phytochemistry 49:1829–1832
Arcila CL, Loarca GP, Lecona SU, González EM (2004) El orégano: propiedades, composición y actividad biológica de sus componentes. Arch Latinoam Nutr 54:100–111
Dewanto V, Wu X, Adom K, Lui R (2002) Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem 50:3010–3014
Liu M, Li XQ, Weber C, Lee CY, Brown J, Liu RH (2002) Antioxidant and antiproliferative activities of raspberries. J Agric Food Chem 50:2926–2930
Del Baño MJ, Lorente J, Castillo J, Benavente-García O, Del Río JA, Ortuño A, Quirin KW, Gerard D (2003) Phenolic diterpenes, flavones, and rosmarinic acid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis. Antioxidant activity. J Agric Food Chem 51:4247–4253
Fukumoto LR, Mazza G (2000) Assessing antioxidant and prooxidant activities of phenolic compounds. J Agric Food Chem 48:3597–3604
Kado NY, Langley D, Eisenstadt E (1983) A simple modification of the Salmonella liquid incubation assay. Increased sensitivity for detecting mutagens in human urine. Mutat Res 121:25–32
Cardador-Martínez A, Albores A, Bah M, Calderón-Salinas V, Castaño-Tostado E, Guevara-González R, Shimada-Miyasaka A, Loarca-Piña G (2006) Relationship among antimutagenic, antioxidant and enzymatic activities of methanolic extract from common beans (Phaseolus vulgaris L). Plant Foods Hum Nutr 61:161–168
Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T (2005) Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutat Res 579:200–213
Fresco P, Borges F, Diniz C, Marques MPM (2006) New insights on the anticancer properties of dietary polyphenols. Med Res Rev 26:747–766
Shan B, Cai YZ, Sun M, Corke H (2005) Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agric Food Chem 53:7749–7759
Exarchou V, Nenadis N, Tsimidou M, Gerothanassis IP, Troganis A, Boskou D (2002) Antioxidant activities and phenolic composition of extracts from Greek oregano, Greek sage, and summer savory. J Agric Food Chem 50:5294–5299
Willcox JK, Ash SL, Catignani GL (2004) Antioxidants and prevention of chronic disease. Crit Rev Food Sci 44:275–295
Kanazawa K, Kazuyasu HK, Samejima K, Ashida H, Danno G (1995) Specific desmutagens (antimutagens) in oregano against a dietary carcinogen, Trp-P-2, are galangin and quercetin. J Agric Food Chem 43:404–409
Acknowledgement
The authors are grateful to the oregano producers. This research has been supported by the Mexican Consejo Nacional de Ciencia y Tecnología, through grant CONACYT 50596-Q.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martínez-Rocha, A., Puga, R., Hernández-Sandoval, L. et al. Antioxidant and Antimutagenic Activities of Mexican Oregano (Lippia graveolens Kunth). Plant Foods Hum Nutr 63, 1–5 (2008). https://doi.org/10.1007/s11130-007-0061-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-007-0061-9