Abstract
To evaluate the potential benefits and risks associated with tea consumption it is important to identify the constituents of this beverage. Levels of some minerals, caffeine and catechins in green tea samples commercialized in Portugal were evaluated. Potassium is the metal present in larger amount (92–151 mg/l). The content of sodium, calcium, fluoride, aluminium, manganese and iron were 35–69, 1.9–3.5, 0.80–2.0, 1.0–2.2, 0.52–1.9, 0.020–0.128 mg/l, respectively. Chromium and selenium were not detected. The resulting data showed considerable variability in catechins content. The levels of epigallocatechin gallate (EGCG) ranged from 117 to 442 mg/l, epicatechin 3-gallate (EGC) from 203 to 471 mg/l, epigallocatechin (ECG) from 16.9 to 150 mg/l, epicatechin (EC) from 25 to 81 mg/l and catechin (C) from 9.03 to 115 mg/l. Caffeine contents in the green tea infusions studied were between 141–338 mg/l. Green tea infusions provide significant amounts of catechins and could be an important source of some minerals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tea is an infusion of Camellia sinensis leaves. Native from China it is today the most widely consumed beverage in the world, aside water.
During the last decades, the beneficial health effects of green tea have been demonstrated in a number of studies. Tea drinking is associated with reduction of serum cholesterol, prevention of low density lipoprotein (LDL) oxidation and a decreased risk of cardiovascular disease and cancer [1, 2]. Due to these facts the popularity of this beverage has grown all over the world.
The chemistry of tea is complex: polyphenols, alkaloids, amino acids, glucides, proteins, volatile compounds, minerals and trace elements [3]. Polyphenols are the most biologically active group of tea components which have antioxidative, antimutagenic and anticarcinogenic effects [4, 5]. But there are other compounds in green tea with interest for human health like fluoride, caffeine, minerals and trace elements like, chromium and manganese [6–8]. The regular consumption of tea can contribute to the daily dietary requirements of some of these elements [9]. Due to the importance of minerals in tea several studies have been carried out in order to determine their levels in tea leaves and infusions [10–14].
To evaluate the nutritional contribution and the intoxication risk related with green tea consumption it is important to quantify micronutrients and toxic elements levels in this beverage. Therefore aluminium, chromium, selenium, manganese, iron, sodium, potassium, calcium, fluoride, caffeine and catechins were determined in green tea infusions available in Portugal.
Materials and Methods
Tea Samples
In Portugal the consumption of green tea is new. The market is growing and there are only a few brands of green tea. For this study were selected the most representative green tea brands accordingly to the information of the specialty shops. These tea samples were purchased from several markets and herbalists in Lisbon region. Six of them were sold in tea bags and the other three as loose-leaf tea. The samples were identified by the acronyms G, S, T, TN, TV, F, B, M and T. Each tea was analysed in triplicate using separate infusions.
The infusion time and ratio sample/water volume were selected based on the label instructions of the analyzed samples (infusion of a tea bag in a cup of boiling water (≈250 ml) and a brewing time from 5–10 min) in order to simulate the conditions used by the consumers at home.
A mass of 1.5 g of tea leaf (or tea leaf from a tea bag) was weighed in a beaker and 250 ml of boiling ultra-pure water were added. After 10 min, tea infusions were filtered into a standard flask of 250 ml and diluted to the mark with ultra-pure water. All the infusions were analyzed in the day of the preparation.
Methods
The fluoride concentration in green tea infusion was determined using a digital pH meter, with a fluoride selective electrode (Mettler Toledo, Switzerland) and a Ag/AgCl as reference electrode (Mettler Toledo, Switzerland) [15]. For calcium determination the EDTA titrimetric method was performed accordingly with the described by Clesceri et al. [15].
Potassium and sodium were analyzed by flame photometric method using a flame photometer 400 flame photometer Corning 450 (Corning Limited, Essex, England) [15].
Aluminium, chromium, selenium, manganese and iron were determined by electrothermal atomic absorption spectrometry (EAAS) with Perkin Elmer Zeeman Atomic Spectrometer 4110ZL and Autosampler AS-72 from Perkin Elmer (Norwalk, Connecticut). The appropriate conditions for each metal were used [15]. The material used to prepare the infusions was washed with nitric acid and rinsed with ultra-pure water. The samples were analyzed after a pre-treatment with concentrate HNO3. 250 ml of green tea infusion were transferred to a beaker with boiling chips. In a hood, 5 ml of concentrate HNO3 were added. The beaker was covered with a ribbed watch glass to minimise contamination. After a slow boil, the solution was evaporated, on a hot plate (DataPlate®Digital, Biomolecular Inc. from Cole-Parmer International, Vernon Hills, U.S.A.), to about 150 ml. Another 5 ml of concentrate HNO3 were added until digestion was completed, as showed by a light yellow clear solution. The solution was brought to the lowest volume possible before precipitation (about 70–90 ml). The digested solution was transferred to a 100 ml volumetric flask and diluted to the mark with ultra-pure water. A reagent blank was prepared with the same acid and subjected to the same digestion procedure as the sample to correct impurities present in acid and reagent water.
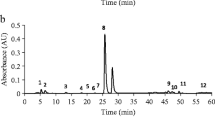
The method proposed by Fernandez et al. [16] was used for the simultaneous determination of catechins and caffeine in the tea infusions. A high-performance liquid chromatograph system equipped with a binary LC-6A pump controlled by a SCL-6B controller, a ultra violet detector model SPD-M10A DAD and a LC workstation (class LC-10 versão1) integrator all from Shimadzu (Kyoto, Japan) and a Lichrosper 100 RP-18 (5 μm), 250 mm × 4 mm column (Ecopack, Merck) were used. The tea infusions were filtered through a 0.45 μm filter and then 20 μL were injected for HPLC analysis.
Methods Validation
Accuracy was validated by recovery assays. Recoveries were calculated as the difference between the amount of analyte present in spiked and non-spiked samples divided by the amount of added analyte [17].
The linear range of each method was assessed by analyzing several solutions containing standards in different concentration levels.
Ten solutions containing the target compounds at the lowest concentration of linearity range were analyzed and the standard deviations (SD) were determined. The values of limit of detection (LOD) and limit of quantification (LOQ) were calculated using the formula (3 × SD) and (10 × SD) respectively [18].
The precision of each method was evaluated by carrying out ten replicate analyses of a standard solution, with a concentration equivalent to the lower limit of the linearity range. The relative standard deviation (RSD) should be lower than 10% accordingly with the requirements of Portuguese law for water for human consumption (DL 243/01, 5th September).
Results and Discussion
The linearity range, precision, recovery and limits of detection (LOD) and quantification (LOQ) obtained are shown in Table 1.
The results showed, for the majority of methods, a good precision under the analytical conditions used since the relative standard deviations are lower than 10%. Good recoveries between 80–115% were obtained.
The contents of the analyzed elements were determined in the tea samples. The results are summarized in Table 2.
Potassium was the element with higher content in all samples analyzed ranging from 92–151 mg/l. These concentrations were lower than those reported by Powell et al. [7] (221 mg/l), Matsuura et al. [11] (246 mg/l) and Fernández et al. [14] (94–259 mg/l). The levels of sodium in the nine infusions were much higher (36–69 mg/l) than those described in the literature [7, 11, 14]. Different concentrations of this element should be expected in the infusions since sodium is a highly extractable element and its content in green tea leaves can range from 33.9 mg/kg to 1760 mg/kg. [12]. The conditions under which the tea infusions were prepared are other possible explanation for the observed differences. Recent research suggests that the Na/K ratio is important to control blood pressure. A ratio of less than one is recommended. Since the tea samples analyzed have at least two or three times as much potassium as sodium, their consumption may be useful for lowering blood pressure.
Calcium is the most common mineral in the body. The obtained calcium levels in tea leaves ranged from 1.9–3.5 mg/l. These values are consistent with data reported previously [11].
The anticariogenic effect of fluoride is well known. The fluoride content of most foods is low (less than 0.05 mg/100 g). Camellia sinensis plant takes fluoride from soil and accumulates it in the leaves [10]. The fluoride levels of the analyzed samples were between 0.80 and 2.0 mg/l, in agreement with previous findings [10, 13]. Simpson et al. [19] showed that 34% of tea fluoride was retained in the oral cavity, suggesting that this drink is an effective vehicle for delivering fluoride in the oral cavity helping to prevent dental decay. Some concern has been raised over excessive intakes of fluoride causing fluorosis (mottling and decolouration of the teeth). In areas with fluorinated water supply the consumption of several cups of green tea per day could lead to an excessive ingestion of fluoride.
Analysis of variance of the results of Na, K, Ca and F showed statistically significant differences in content of these elements among the different teas (p < 0.05).
The analysis of Al, Mn, Cr, Fe by EAAS showed that these trace elements are present in green tea infusions over a wide range of concentrations (Table 2). Among them, selenium is the element present with a lower concentration (<1.2 μg/l). In most tea leaves the level of this element is 0.1 μg/g but Xie et al. [9] detected higher concentrations (7.5 μg/g) in some tea samples produced in a region with selenium rich soils.
Tea contains only trace amounts of iron (0.043 and 0.03–0.17 mg/l) [11, 14]. In the present study iron levels were between 0.020–0.128 mg/l. The bioavailability of this element is influenced by polyphenols found in tea that can markedly inhibit the absorption of nonheme iron [20].
Tea is an important source of manganese and chromium which are essential nutrients required for normal metabolism [6–9]. Mean chromium intakes from food and water range from 52 to 943 μg/day. Chromium was not detected in the green tea samples analyzed and for this reason the contribution of these infusions for the daily intake of this element is insignificant.
Manganese is present in small amounts in foods [9]. The levels of manganese detected (0.52–1.9 mg/l) were lower than those described in other studies [11, 14]. According to Powell et al. [7] only 40% of the manganese is in a potentially bioavailable form, so a cup of green tea (225 ml) may contribute with 10% of the average daily dietary intake of this metal.
Tea plants take up a substantial amount of aluminium however this beverage is not among the dietary sources of aluminium associated with an increased risk of Alzheimer’s disease [1]. The content of aluminium detected (1.0–2.1 mg/l) was lower than that obtained by Fernández et al. [14]. This element, like iron, has poor biovailability due to the actions of polyphenols, which avidly bind to trivalent metal and prevent their intestinal absorption.
A considerable variability in metal and trace elements content was observed in the green tea samples. Although present in the infusions not all minerals are available in our organisms after the consumption of a cup of tea since absorption of most minerals is very variable and incomplete [21].
The main classes of polyphenols present in green tea are flavanols and flavonols. These compounds constitute 16–30% of the dry weight of fresh leaf [22]. Catechins (flavan-3-ols) are the predominant form found. Fresh green tea leaves contain five major catechins: catechin (C), (−)-epicatechin (EC), (−)-epicatechin 3-gallate (ECG), (−)-epigallocatechin (EGC) and (−)-epigallocatechin gallate (EGCG). These compounds are water-soluble and contribute to bitterness and astringency of green tea. Flavonols such as quercetin, kaempferol, myricitin and their glycosides can also be found in green tea [23, 24].
The concentrations of catechins and caffeine are presented in Table 3.
The obtained results indicated that in the majority of the samples the EGCG was the catechin present at the highest concentration, while catechin showed the lowest level. The detected values of catechins in the samples analyzed are similar to those described by Wang et al. [25], except for catechin (C) and ECG but different from those reported by Del Rio et al. [24] and Lin et al. [26]. The differences observed can be related with sample preparation methodologies since different brewing times and ratio leaves/water were used. The comparison of the results is sometimes difficult due to the lack of uniformity in the conditions used to prepare the infusions. The amount of leaf to be used per amount of water is of critical importance. However there are other factors to consider like the temperature and the time of infusion. An enhancement of caffeine and catechins extraction is observed as the temperature increases. The catechin content of the tea infusion increases 30–40% when extracted for a period of 10 min. The leaf age is also a very important factor because old leaves contain less caffeine but more EGCG and total catechins than young ones [27].
Because individual catechins have different antioxidative and health properties is important to know the concentration of each one of them in the infusions. Toshi et al. [28] had observed that the antioxidant activity of the green tea is higher in the teas that contain higher levels of EGCG and EGC.
The consumption of a cup of tea may contribute to the intake of 90–230 mg of catechins, so a moderate consumption may provide significant amounts of these potential beneficial compounds. The data available until now do not allow us to predict the amount of polyphenols that have physiological activity in humans. In the last years several works had been conducted in order to study the biological effects, bioavailability and metabolism of tea polyphenols in humans. These studies are sometimes discordant and variability among individuals is also observed. Most of the ingested catechins are rapidly and extensively metabolized [(−)-5-(3′,4′, 5′-trihydroxyphenyl)-gamma-valerolactone (M4) and (−)-5-(3′, 4′-dihydroxyphenyl)-gamma-valerolactone (M6)]. Substantial amounts of these metabolites are present in plasma and urine after tea consumption so, these compounds had been used as biomarkers. However the pharmokinetics of M4 and M6 requires further clarification in order to define appropriate dose levels [1].
The levels of caffeine in infusions were between 141–338 mg/l. The detected values are in agreement with previous findings [25, 26, 27]. For the healthy adult population moderate daily caffeine intake at a dose level up to 400 mg/kg (6 mg/kg of body weight) is not associated with adverse effects [29].
Tea has been studied extensively for its biological action but there are few data on the catechin content of green tea infusions. Most of the studies have determined the catechin contents of fresh leaf after extraction with organic solvents.
This work is a contribution for the chemical characterization of green tea infusions. The obtained results suggested that there are significative differences in the chemical composition of green tea brands commercialized in Portugal. However, further studies are needed in order to obtain a more representative data.
In conclusion, green tea consumption may contribute to daily intake of some minerals, such as manganese.
Abbreviations
- C:
-
(+) catechin
- EC:
-
(−) epicatechin
- ECG:
-
(−)−epicatechin gallate
- EGC:
-
(−)−epigallocatechin
- EGCG:
-
(−)−epigallocatechin gallate
- HPLC:
-
High-performance liquid chromatography
- M4:
-
(−)-5-(3′,4′, 5′-trihydroxyphenyl)-gamma-valerolactone
- M6:
-
(−)-5-(3′, 4′-dihydroxyphenyl)-gamma-valerolactone
References
MacKay DL, Blumberg JB (2000) The role of tea in human health: an update. J Am Coll Nutr 21:1–13
Chung FL, Schwartz J, Herzog CR, Yang YM (2003) Tea and cancer prevention: studies in animals and humans. J Nutr 133:3268S–3274S
Stagg GV, Millin DJ (1975) The nutritional and therapeutic value of tea—a review. J Sci Food Agric 26:1439–1459
Higdon JV, Frei B (2003) Tea catechins and polyphenols: health effects, metabolism, and antioxidant function. Crit Rev Food Sci Nutr 43:89–143
Yao LH, Jiang YM, Shi J, Tomás-Barberán FA, Datta N, Singanusong R, Chen SS (2004) Flavonoids in food and their health benefits. Plant Foods Hum Nutr 59(3):113–122
Cabrera C, Gimenez R, Lopez MC (2003) Determination of tea components with antioxidant activity. J Agric Food Chem 51(15):4427–4435
Powell JJ, Burden TJ, Thompson RPH (1998) In vitro mineral availability from digested tea: a rich dietary source of manganese. Analyst 123:1721–1724
Hope S-J, Daniel K, Gleason KL, Comber S, Nelson M, Powell J (2006) Influence of tea drinking on manganese intake, manganese status and leucocyte expression of MnSOD and cytosolic aminopeptidase. Eur J Clin Nutr 60:1–8
Xie MY, von Bohlen A, Klockenkämper R, Jian XH, Günther K (1998) Multielement analysis of Chinese tea (Camellia sinensis) by total-reflection X-ray fluorescence. Z Lebensm Unters Forsch A 207:31–38
Fung KF, Zhang ZQ, Wong JWC, Wong MH (1999) Fluoride contents in tea and soil from tea plantations and the release of fluoride into tea liquor during infusion. Environ Pollut 104:197–205
Matsuura H, Hokura A, Katsuki F, Itoh A, Haraguchi H (2001) Multielement determination and speciation of major-to-trace elements in black tea leaves by ICP-AES and ICP-MS with the aid of size exclusion chromatography. Anal Sci 17:391–398
Fernandez-Caceres PL, Martin MJ, Pablos F, Gonzalez AG (2001) Differentiation of tea (Camellia sinensis) varieties and their geographical origin according to their metal content. J Agric Food Chem 49:4775–4779
Behrendt A, Oberste V, Wetzel WE (2002) Fluoride concentration and pH of iced tea products. Caries Res 36:405–410
Fernández PL, Pablos F, Martín MJ, González AG (2002) Multi-element analysis of tea beverages by inductively coupled plasma atomic emission spectrometry. Food Chem 76:483–489
Cleresci LS, Greenberg AE, Eaton AD (2005) Part 3030 E, 3113, 3114 C, 3500-Na, 3500-K and 4500-F. In: APHA-AWWA-WEF (eds) Standard Methods for the Examination of Water and Wastewater. 21st edn, Washington, DC
Fernandez PL, Martin MJ, González AG, Pablos F (2000) HPLC determination of catechins in tea. Differentiation of green, black and instant tea. Analyst 125:421–425
Burns DT, Danzer K, Townsend A (2002) Use of the term “recovery” and “apparent recovery” in analytical procedures (IUPAC Recommendations 2002). Pure Appl Chem 74(11):2201–2205
The United States Pharmacopeia and National Formulary (USP 30-NF 25) (2007) Validation of Compendial Procedures <1225>
Simpson A, Shaw L, Smith AJ (2001) The bio-availability of fluoride from black tea. J Dentistry 29(1):15–21
Samman S, Sandstrom B, Toft MB, Bukhave K, Jensen M, Sorensen SS, Hansen M (2001) Green tea or rosemary extract added to foods reduces nonheme-iron absorption. Am J Clin Nutr 73(3):607–612
Fairweather-Tait SJ (1999) The importance of trace element speciation in nutritional sciences. Fresenius J Anal Chem 363(5–6):536–540
Graham HN (1991) Green tea composition, consumption, and polyphenol chemistry. Prev Med 21:334–350
Balentine DA, Wiseman AS, Bouwens LCM (1997) The chemistry of tea flavonoids. Crit Rev Food Sci Nutr 37(8):693–704
Del Rio D, Stewart AJ, Mullen W, Burns J, Lean ME, Brighenti F, Crozier A (2004) HPLC-MSn analysis of phenolic compounds and purine alkaloids in green and black tea. J Agric Food Chem 52(10):2807–2815
Wang H, Helliwell K, You X (2000) Isocratic elution for the determination of catechins, caffeine and gallic acid in green tea using HPLC. Food Chem 68:115–121
Lin JK, Lin C-L, Liang Y-C, Lin-Shiau S-Y, Juan I-M (1998) Survey of catechins, gallic acid and methylxanthines in green, oolong, pu-erh and black teas. J Agric Food Chem 46:3635–3642
Lin Y-S, Wu S-S, Lin J-K (2003) Determination of tea polyphenols and caffeine in tea flowers (Camellia sinensis) and their hydroxil radical scavenging and nitric oxide suppressing effects. J Agric Food Chem 51:975–980
Toschi TG, Bordoni A, Hrelia S, Bendini A, Lercker G, Biagi PL (2000) The protective role of different green tea extracts after oxidative damage is related to their catechin composition. J Agric Food Chem 48(9):3973–3978
Nawrot P, Jordan S, Eastwood J, Rotstein J, Hugenholtz A, Feeley M (2003) Effects of caffeine on human health. Food Addit Contam 20(1):1–30
Acknowledgment
The authors thank all collaborators of the following laboratories: Laboratório de Hidrologia e Análises Hidrológicas of FFUL (Faculdade de Farmácia da Universidade de Lisboa) and LALA (Laboratório de Águas do Litoral Alentejano).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reto, M., Figueira, M.E., Filipe, H.M. et al. Chemical Composition of Green Tea (Camellia sinensis) Infusions Commercialized in Portugal. Plant Foods Hum Nutr 62, 139–144 (2007). https://doi.org/10.1007/s11130-007-0054-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-007-0054-8