Abstract
Pathways of intramolecular conversion and intermolecular electronic excitation energy transfer (EET) in the photosynthetic apparatus of purple bacteria remain subject to debate. Here we experimentally tested the possibility of EET from the bacteriochlorophyll (BChl) Soret band to the singlet S2 level of carotenoids using femtosecond pump–probe measurements and steady-state fluorescence excitation and absorption measurements in the near-ultraviolet and visible spectral ranges. The efficiency of EET from the Soret band of BChl to S2 of the carotenoids in light-harvesting complex LH2 from the purple bacterium Ectothiorhodospira haloalkaliphila appeared not to exceed a few percent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary processes of photosynthesis in purple bacteria (excitation energy migration via the light-harvesting antenna system to the reaction centers (RCs) and energy conversion in the RCs) are based on two types of pigment molecules. The main pigment is bacteriochlorophyll a (BChl), and auxiliary pigments are carotenoids (Cars). BChl has four bands (Qy, Qx, Bx, and By) in the visible and near-infrared regions of the absorption spectrum. The overlapping bands Bx and By in the near ultraviolet are often referred to as a single band (Soret band, Scheer 2006). Carotenoid molecules show an absorption band in the 400–550 nm region corresponding to the transition S0 → S2 (11A g − → 11B u −). The lowest singlet excited state, S1 (21A g −), is optically forbidden because it has the same symmetry as the ground state (Polívka and Sundström 2004).
Absorption of light quanta by antenna pigment molecules leads to processes of intramolecular conversion and intermolecular excitation energy transfer (EET). These processes continue until the excitation appears on the longest-wavelength absorption band of the RC.
The photosynthetic apparatus absorbs light from ultraviolet to infrared. Since light quanta can be absorbed by different bands of two pigment types, internal conversion and EET occur via several pathways. There are many publications on EET from Cars to BChl (see reviews Polívka and Sundström 2004; Polívka and Frank 2010; Frank and Polivka 2008; Sundström 2008; Slouf et al. 2013). Transfer of energy from BChl to Cars is significantly less-studied. Several studies investigated EET from BChl Qx to Car molecules (Cong et al. 2008; Macpherson et al. 2001; Frank and Polívka 2008). Excitation energy transfer from LH2 Qy (BChl B800) to the S1 (21A g −) level of Car molecules with n ≤ 12, where n is the number of conjugated double bonds, is also theoretically possible. But, according to the data of Thiess et al. (2008), EET between BChl B800 and the S1 (21A g −) level of rodopin (n = 11) in LH2 of Allochromatium minutissimum is absent. Also, there are only a few papers that mention EET from the BChl Soret band to Cars (Götze et al. 2014; Amarie et al. 2010; Limantara 1998). Götze et al. (2014) proposed that xanthophylls, and Cars in general, may mediate EET from the chlorophyll Soret band to the Qx at least in LHCII. Excited state dynamics of wild-type and mutant of Rh. rubrum following excitation of the BChl Soret band (388 nm) were reported (Amarie et al. 2010). Excitation energy transfer from the BChl Soret band to spirilloxanthin is shown in Fig. 7 of that paper, but the efficiency of EET via this pathway and its time constant was not reported. BChl-to-Car singlet-energy transfer, which is based on instantaneous interaction between the transition dipoles of the BChl and Car molecules, was used to explain the rapid generation of triplet states and fast decay of singlet states of BChl a and spheroidene upon photoexcitation of the Car-containing LH2 complex (Limantara 1998).
Steady-state absorption and fluorescence excitation spectra allow calculation of the efficiency of EET between pigment bands down to the fluorescence emitting Qy band (Magdaong et al. 2014, 2016). Although the authors of those papers do not consider the issue of EET from the BChl Soret to Cars, the results of their measurements indicate the absence of such EET (Fig. 5 in (Magdaong et al. 2014) and Fig. 3 in (Magdaong et al. 2016), consistent with our results in this article, see below). The quite opposite opinion was expressed by authors of a theoretical paper (Tretiak et al. 2000). They stated that “the energy transfer pathway from the Soret states of BChl a to the 1B u state of lycopene should be extremely efficient and may effectively compete with the internal conversion on the individual BChls-a”. However, experimental evidence of this transfer is scarce. In this study, we investigated the potential involvement of Cars in EET from the BChl Soret band using LH2 from wild-type cells of the purple bacterium Ectothiorhodospira haloalkaliphila.
Materials and methods
Preparation of the wild-type light-harvesting LH2 complexes
Ect. haloalkaliphila cells (Imhoff and Süling 1996) were grown phototrophically under anaerobic conditions in flat glass bottles on modified Pfenning medium (Imhoff and Trüper 1977) at 26 ± 2 °C. As the light source, 75 W incandescent lamps were used. The average light intensity was about 10 W/m2 as described earlier (Makhneva et al. 2008). The collected cells were disintegrated by sonication, membranes were isolated by ultracentrifugation (Moskalenko et al. 1997), and LH2 complex was isolated with preparative electrophoresis in polyacrylamide gel (Moskalenko and Erokhin 1974). Dodecyl maltoside at concentration 2–2.5% was used to solubilize the membranes. After electrophoresis, the LH2 complex was dissolved and concentrated by centrifugation in an Amicon Ultra 50 K filter (Millipore, USA).
The spectrum of the total carotenoids in n-hexane was measured after their extraction from the LH2 sample as reported (Ashikhmin et al. 2014). The molar Car content in the LH2 complex from Ect. haloalkaliphila (Table 3, control column) (Ashikhmin et al. 2014) was the following: lycopene 17.2% (n = 11, where n is the number of conjugated double bonds), rhodopin 13.5% (n = 11), anhydrorhodovibrin 37.1% (n = 12), spirilloxanthin 29.8% (n = 13), and didehydrorhodopin 2.2% (n = 12).
Spectroscopic methods
Transient absorption spectroscopy measurements were done using a spectrometer based on a TOPAS optical parametric amplifier pumped at 790 nm by a Spitfire Ti:Sa regenerative amplifier (Spectra Physics, Mountain View, CA). Pulses from the optical parametric amplifier with 1 kHz repetition rate and tunable wavelength were used for sample excitation. Part of light from the regenerative amplifier was also focused into a sapphire plate to generate a white-light continuum used for probing absorption changes. In addition to the probe beam, a probe-reference beam was passed through the sample outside the area of excitation. The absorption changes spectra ΔA(λ) were recorded as ΔA = lg[(Iprobe/Iref)ex off/(Iprobe/Iref)ex on]. The polarization between the pump and probe pulses was set to the magic angle (54.7°). The duration of the pump pulse was about 70 fs and remained constant over the entire wavelength range.
The sample was diluted to optical density ~0.3 in a 1.5-mm cell at wavelength 850 nm. During transient absorption measurements, the sample was pumped/probed through a 1.5-mm quartz rotating cell. After each transient absorption measurement, the steady-state absorption spectrum was recorded to make sure that there was no degradation of the sample.
All steady-state spectroscopic experiments were conducted at room temperature in a 1-cm pathlength quartz cell. Absorption spectra were recorded using a Hitachi 557 UV–Visible spectrophotometer. When necessary, the absorption spectra were converted to 1-T (where T is transmittance) spectra.
Fluorescence emission and excitation spectra were obtained using a Fluorolog-3 fluorimeter (Horiba Jobin Yvon) equipped with an Osram XBO 450 W xenon arc lamp, a Hamamatsu R5509 PMT detector, and double excitation and emission monochromators having 600 grooves/mm gratings. The fluorescence spectra were recorded with samples having optical density of 0.03 measured in a 1 cm cell at the maximum of the BChl Qy band. The spectra were recorded using excitation into the BChl Soret band at 340 nm. The slit widths of the emission and excitation monochromators were set to correspond to bandpasses of 2 and 8 nm, respectively. Fluorescence excitation spectra were recorded by monitoring the emission intensity at the wavelength corresponding to the emission maxima, which was 865 nm for the wild-type LH2 complex. For this experiment, the slit widths of the emission and excitation monochromators corresponded to bandpasses of 8 and 2 nm, respectively.
Results and discussion
A simplified diagram of possible EET pathways of LH2 complexes is shown in Fig. 1. The energy of a light quantum absorbed in the BChl Soret band can reach BChl Qx via two pathways. First, by intramolecular conversion Soret → Qx; second, by EET Soret → S2 (11B u −) level of the nearest carotenoid molecule followed by transfer from Car S2 to Qx. To test for excitation energy transfer from BChl (Soret band) to Car molecules, pump–probe measurements with femtosecond time resolution and steady-state measurements were carried out on the LH2 complexes from wild-type Ect. haloalkaliphila cells.
A simplified diagram of EET pathways of LH2 complexes. Excitation in the Soret band (S0 → By,Bx) and transitions S1 → Sn shown by solid arrows. The EET pathways from the Soret band to Car S2 and then to Qx are shown by bold solid arrows, and intersystem crossing transitions are shown by dashed arrows. The optical absorption spectrum of LH2 complex of Ect. haloalkaliphila is shown on the right
Transient absorption spectroscopy
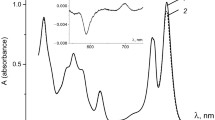
The spectrum of transient absorption changes of the LH2 complex from wild-type Ect. haloalkaliphila upon excitation by 70-fs 340-nm laser pulses is shown in Fig. 2. A bleaching band with extremum around 590 nm (Qx), a bleaching band within 450–550 nm (Car S0 → S2 transition), and a significant increase in absorption over the whole range due to transitions from the first excited singlet state of BChl to higher excited states. For this reason, bleaching bands of Cars and BChl are displaced upwards. Therefore, we determined the magnitude of bleaching in the Car band and Qx band as shown by arrows in Fig. 2.
Spectrum of transient absorption changes in LH2 complex from wild-type Ect. haloalkaliphila cells induced by 340-nm pump pulses (red curve), The spectrum corresponds to maximum absorption changes in the LH2 complex at time delay ~1 ps. The downward arrows show ΔA values in the Car band and the BChl Qx band. Magenta lines show the zero levels in the Car and Qx bands. Concave curves show the calculated spectra in the Car band and in the BChl Qx band for EET efficiencies: black 3%, green 5%, blue 7%
The observed Car band bleaching can be caused both by direct excitation of Car molecules at 340 nm and by EET from the BChl Soret band. In the former case, it is necessary to determine the fraction of light absorbed by the Cars at 340 nm, and in the latter—the efficiency of the EET.
To evaluate the contribution of direct excitation of Car molecules at 340 nm, we compared the optical absorption spectrum of LH2 complex from wild-type Ect. haloalkaliphila cells (Fig. 3, black curve) and the total absorption spectrum of Cars extracted from this complex by n-hexane (Fig. 3, red curve). The spectra in Fig. 3 allowed us to estimate the Car absorption as ~5% of the total absorption at 340 nm.
The value of bleaching in the absorption band of Cars and the Qx band of BChl of LH2 from Ect. haloalkaliphila can be calculated (see detailed calculation in "Appendix" Section). Figure 2 shows the calculated curves of the expected bleaching in the Cars band and the Qx band at 3, 5, and 7% contribution of Cars to the total absorbance at 340 nm. The experimentally measured bleaching was close to the 5% contribution. This value is consistent with the estimate of the contribution of Cars to the total absorption at 340 nm (see Fig. 3).
Although these data convincingly indicate the direct excitation of Car molecules at 340 nm, let us suppose that the total Car absorption curve for this complex (red curve in Fig. 3) overestimates the Cars contribution to the total absorption at 340 nm. Then the observed bleaching in the Car band would be due to transfer of the energy absorbed by BChl molecules in the Soret band with efficiency of about 5%. It is important to stress that in any case the efficiency of EET from the BChl Soret band to Cars does not exceed 5%.
The data presented in Fig. 4 support the hypothesis of direct excitation of Cars. If changes in the Car absorption band are due to direct excitation of Car molecules, then its wavelength dependence must correlate with the absorption spectrum of Cars within the spectral range of the excitation. If, however, Car absorption changes are due to EET from BChl to S2 of Cars, then there must be a correlation with the absorption spectrum of BChl. The experimental points tend, at least qualitatively, to correspond to the absorption spectrum of Cars and thus to the assumption of direct Cars excitation by femtosecond pulses (Fig. 4).
Steady-state absorption and fluorescence spectroscopy
Excitation energy transfer from the BChl Soret band to the Cars band in the LH2 complex can be examined based on the steady-state optical absorption spectrum, A(λ), and BChl fluorescence excitation spectra, Fex(λ). The wavelength dependence of EET efficiency is \({\text{E}}(\lambda )\, = \,{\text{F}}_{{{\text{ex}}}} (\lambda )/[1 - {\text{T}}(\lambda )]\) where T(λ) = 10−A(λ) − sample transmittance.
The efficiency of EET from Car molecules to BChl depends on the type of bacteria (depending on the carotenoid composition). This efficiency varies over a wide range from 20 to 100%. Frank and Polívka 2008) estimated the values of efficiency in LH2 to be within 30–70%. According to Koyama et al. (2004) for LH2 of Rhodobacter sphaeroides G1C, Rba. sphaeroides 2.4.1, and Rsp. molischianum the transfer efficiency is 88–92, 84–89, and 51–53%, respectively. It is ~40% in LH2 from Allochromatium vinosum (Magdaong et al. 2016) and 26% in LH2 from Thermochromatium tepidum (Niedzwiedzki et al. 2011).
Reduced transfer efficiency is seen as a trough on the curve E(λ) in the Car absorption region 425–570 nm. The depth of this trough for the LH2 complex Ect. haloalkaliphila (Fig. 5, green curve) is about 55%, which corresponds to E Car−BChl ~45%. The fact that 55% of the excitation energy is not transferred from the Cars to BChl allows evaluation of the efficiency of EET from high levels of BChl (Bx, By) to the S2 level of Cars, E Soret−Car . Let us assume 100% efficiency of this transfer. Then the E(λ) curve must have the same 45% level in both Soret and Car S2 bands. If the transfer is completely blocked, the curve E(λ) will be at the same 100% level in the Soret and Qx bands and at 45% in the Car band. All the intermediate values of E Soret−Car will give E(λ) levels from 45 to 100% in the BChl Soret region. The E(λ) curve for LH2 from Ect. haloalkaliphila passes approximately at 100% level in Soret band, decreases to the level of 40–50% in the Car S2 band, and then rises again to the level of 100% in the Qx band (Fig. 5).
We conclude that the results of our measurements show EET from the BChl Soret band to the second singlet excited state of Cars, E Soret−Car , is blocked or occurs with efficiency not exceeding a few percent. The same very low E Soret−Car can be obtained on the basis of Fex(λ) and (1-T) curves for LH2 from Allochromatium vinosum (Magdaong et al. 2016) and LH2 from Rhodoblastus acidophilus (Magdaong et al. 2014).
As is well known, one of the main functions of Cars in photosynthesis is improvement of light harvesting. Carotenoids increase the absorption cross section of photosynthetic organisms by absorbing light in the region where BChl is not efficient, and transfer the excitation energy to neighboring BChl molecules. However, the efficiency of EET from Car molecules to BChl in the LH2 complex of many bacteria is rather low. Thus, about half or more of the light energy absorbed by carotenoids does not reach BChl. The same energy loss will occur in the case of EET from the BChl Soret band via Car S2. Therefore, this pathway must be either disabled or strongly limited. Our experimental data show that the efficiency of EET from the BChl Soret band to Cars does not exceed a few percent of the energy absorbed in the Soret band. Carotenoid and BChl molecules are located very close to each other in the light-harvesting complexes. Very low efficiency of energy transfer from BChl Soret to Cars raises a question about the mechanism preventing the transfer, which would seem to be a disadvantage for the bacterium.
Abbreviations
- BChl:
-
Bacteriochlorophyll a
- Car(s):
-
Carotenoid(s)
- EET:
-
Excitation energy transfer
- E Car−BChl :
-
Efficiency of EET from carotenoids to BChl
- E Soret−Car :
-
Efficiency of EET from BChl (Soret) to carotenoids
- LH2:
-
Light-harvesting complex 2
- RC:
-
Reaction center
References
Amarie S, Lupo D, Lenz MO, Saegesser R, Ghosh R, Wachtveitl J (2010) Excitation energy pathways in the photosynthetic units of reaction center LM- and H-subunit deletion mutants of Rhodospirillum rubrum. Photosynth Res 103:141–151
Ashikhmin A, Makhneva Z, Moskalenko (2014) The LH2 complexes are assembled in the cells of purple sulfur bacterium Ectothiorhodospira haloalkaliphila with inhibition of carotenoid biosynthesis. Photosynth Res 119:291–303
Cong H, Niedzwiedzki DM, Gibson GN, LaFountain AM, Kelsh RM, Gardiner AT, Cogdell RJ, Frank HA (2008) Ultrafast time-resolved carotenoid-to-bacteriochlorophyll energy transfer in LH2 complexes from photosynthetic bacteria. J Phys Chem B 112:10689–10703
Frank HA, Polívka T (2008) Energy transfer from carotenoids to bacteriochlorophylls. In: Hunter CN, Daldal F, Thurmauer MC, Beatty JT (eds) The purple phototrophic bacteria: Advances in photosynthesis and respiration, vol 28, Springer, Dordrecht, pp 213–230
Fujii R, Shimonaka S, Uchida N, Gardiner A, Cogdell R, Sugisaki M, Hashimoto H (2008) Construction of hybrid photosynthetic units using peripheral and core antennae from two different species of photosynthetic bacteria: detection of the energy transfer from bacteriochlorophyll a in LH2 to bacteriochlorophyll b in LH1. Photosynth Res 95:327–337
Götze JP, Kroner D, Banerjee S, Karasulu B, Thiel W (2014) Carotenoids as a shortcut for chlorophyll soret-to-Q band energy flow. Chem Phys Chem 15:3392–3401
Gradinaru CC, Kennis JTM, Papagiannakis E, van Stokkum IHM, Cogdell RJ, Fleming GR, Niederman RA, van Grondelle R (2001) An unusual pathway of excitation energy deactivation in carotenoids: singlet-to-triplet conversion on an ultrafast timescale in a photosynthetic antenna. Proc Natl Acad Sci USA 98:2364–2369
Imhoff JF, Süling J (1996) The phylogenetic relationship among Ectothiorhodospiraceae: a reevaluation of their taxonomy on the basis of 16 S rDNA analyses. Arch Microbiol 165:106–113
Imhoff JF, Trüper HG (1977) Ectothiorhodospira halochloris sp. nov., a new extremely halophilic phototrophic bacterium containing bacteriochlorophyll b. Arch Microbiol 114:115–121
Kosumi D, Maruta S, Horibe T, Fujii R, Sugisaki M, Cogdell RJ, Hashimoto H (2011) Ultrafast energy-transfer pathway in a purple-bacterial photosynthetic core antenna, as revealed by femtosecond time-resolved spectroscopy. Angewandte Chemie International Edition 50:1097–1100
Koyama Y, Rondonuwu FS, Fujii R, Watanabe Y (2004) Light-harvesting function of carotenoids in photo-synthesis: the roles of the newly found 11Bu – state. Biopolymers 74:2–18
Krikunova M, Kummrow A, Voigt B, Rini M, Lokstein H, Moskalenko A, Scheer H, Razjivin A, Leupold D (2002) Fluorescence of native and carotenoid-depleted LH2 from Chromatium minutissimum, originating from simultaneous two-photon absorption in the spectral range of the presumed (optically “dark”) S1 state of carotenoids. FEBS Lett 528:227–229
Limantara L, Fujii R, Zhang J-P, Kakuno T, Hara H, Kawamori A, Yagura T, Cogdell RJ, Koyama Y (1998) Generation of triplet and cation-radical bacteriochlorophyll a in carotenoidless LH1 and LH2 antenna complexes from Rhodobacter sphaeroides. BioChemistry 37:17469–17486
Macpherson AN, Arellano JB, Fraser NJ, Cogdell RJ, Gillbro T (2001) Efficient energy transfer from the carotenoid S2 state in a photosynthetic light-harvesting complex. Biophys J 80:923–930
Magdaong NM, LaFountain AM, Greco JA, Gardiner AT, Carey A-M, Cogdell RJ, Gibson GN, Birge RR, Frank HA (2014) High efficiency light harvesting by carotenoids in the LH2 complex from photosynthetic bacteria: unique adaptation to growth under low-light conditions. J Phys Chem B 118:11172–11189
Magdaong NM, LaFountain AM, Hacking K, Niedzwiedzki DM, Gibson GN, Cogdell RJ, Frank HA (2016) Spectral heterogeneity and carotenoid-to-bacteriochlorophyll energy transfer in LH2 light-harvesting complexes from Allochromatium vinosum. Photosynth Res 127:171–187
Makhneva Z, Bolshakov M, Moskalenko A (2008) Heterogeneity of carotenoid content and composition in LH2 of the sulphur purple bacterium Allochromatium minutissimum grown under carotenoid-biosynthesis inhibition. Photosynth Res 98:633–641
Moskalenko AA, Erokhin YE (1974) Isolation of pigment–lipoprotein complexes from purple photosynthesizing bacteria by the method of preparative polyacrylamide gel electrophoresis. Mikrobiology 43:654–658 (In Russian)
Moskalenko AA, Makhneva ZK (2012) Light-harvesting complexes from purple sulfur bacteria Allochromatium minutissimum assembled without carotenoids. J Photochem Photobiol 108:1–7
Moskalenko AA, Britton G, Connor A, Young A, Toropygina O (1991) The carotenoid content in the chromatophores and pigment–protein complexes isolated from cells of Chromatium minutissimum. Biol Membr USSR 8:249–260 (in Russian)
Moskalenko AA, Toropygina OA, Makhneva ZK (1997) Behavior of carotenoids in Rhodospirillum rubrum cells under cultivation with diphenylamine. Dokl Akad Nauk 355:259–261 (Russian)
Niedzwiedzki DM, Fuciman M, Kobayashi M, Frank HA, Blankenship RE (2011) Ultrafast time-resolved spectroscopy of the light-harvesting complex 2 (LH2) from the photosynthetic bacterium Thermochromatium tepidum. Photosynth Res 110:49–60.
Polívka T, Frank HA (2010) Molecular factors controlling photosynthetic light harvesting by carotenoids. Acc Chem Res 43:1125–1134
Polívka T, Sundström V (2004) Ultrafast dynamics of carotenoid excited states–from solution to natural and artificial systems. Chem Rev 104:2021–2072
Rademaker H, Hoff AJ, van Grondelle R, Duysens LNM (1980) Carotenoid triplet yields in normal and deuterated Rhodospirillum rubrum. Biochim et Biophys Acta 592:240–257
Scheer H (2006) An overview of chlorophylls and bacteriochlorophylls: biochemistry, biophysics, functions and applications. In: Grimm В et al. (eds) Chlorophylls and bacteriochlorophylls. Springer, Berlin, Chap. 1, pp 1–26
Slouf V, Fuciman M, Dulebo A, Kaftan D, Koblízek M, Frank HA, Polívka T (2013) Carotenoid charge transfer states and their role in energy transfer processes in LH1–RC complexes. J Phys Chem B 117:10987–10999
Sundström V (2008) Femtobiology. Annu Rev Phys Chem 59:53–77
Theiss C, Leupold D, Moskalenko AA, Razjivin AP, Eichler HJ, Lokstein H (2008) Femtosecond spectroscopy of native and carotenoidless purple-bacterial LH2 clarifies functions of carotenoids. Biophys J 94:4808–4811
Tretiak S, Middleton C, Chernyak V, Mukamel S (2000) Bacteriochlorophyll and carotenoid excitonic couplings in the LH2 system of purple bacteria. J Phys Chem B 104:9540–9553
Acknowledgements
This work has been supported in part by grants from the Russian Foundation for Basic Research (15-04-09289-a; OFI-M 15-29-01167; 15-04-02660-a). We are thankful to Dr. E. A. Kotova for valuable comments.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
We estimate the degree of sample bleaching when short laser pulses λ = 350 nm pass through the rotating cell with L = 1.5 mm layer of LH2 preparation.
Excitation laser pulse energy was measured to be W = 0.24 µJ, it thus including n0 = W/(hc/λ) = 0.41 × 1012 quanta. Since absorption at 340 nm is D340 = 0.350, the number of quanta absorbed in the cell is n = n0·(1–10−D340) = 0.227 × 1012. These quanta were absorbed within the cylindrical volume containing the LH2 sample; since the cylinder diameter d = 0.3 mm, the illuminated cylinder volume V = 1.06 × 10−7 liter. Although the exact composition of the LH2 complex of Ect. haloalkaliphila is not known, it is assumed to be similar to LH2 composition of Rps. acidophila, which includes p = 18 molecules of BChl B850, q = 9 molecules of BChl B800, and r = 9 molecules of Cars.
Only BChl B850 molecules absorb light at 846 nm, so with the known molar extinction coefficient ε = 1.33 × 105 and measured absorbance D846 = 0.457 we calculate the molar concentration of BChl B850 in the sample, CB850 = D846/(ε846·L) = 22.9 × 10−6 M, and the number of BChl B850 and B800 molecules in the illuminated volume: NB850 = CB850·NA·V = 1.46 × 1012, NA being Avogadro’s number, NB800 = NB850·q/p = 0.731 × 1012, total BChl number NBChl = NB850 + NB800 = 2.19 × 1012. Similarly, Car molecules number NC = NB850·r/p = 0.731 × 1012.
Both BChl and Car molecules contribute to the absorbance at 340 nm, the contribution of Car molecules being about β = 0.05 (see Fig. 4). This means that nC = n·β = 1.14 × 1010 quanta were absorbed by Car molecules, while the remaining nB = n·(1−β) = 2.16 × 1011 quanta were absorbed by BChl molecules.
Absorption of excitation quanta by some Car molecules appears as a proportional reduction in the Car absorption band centered at 500 nm, where the LH2 preparation absorbance was measured to be D500 = 0.287. Obviously, the excitation of fC = FC/nC = 0.0155 fraction of Car molecules would result in ΔD500 = fC·D500 = 0.00446 bleaching at 500 nm. Indeed, the calculated bleaching ΔD500 = 0.00446 is close to the experimentally measured value ΔA500 = 0.00416, differing by only 6–7%.
Rights and permissions
About this article
Cite this article
Razjivin, A.P., Lukashev, E.P., Kompanets, V.O. et al. Excitation energy transfer from the bacteriochlorophyll Soret band to carotenoids in the LH2 light-harvesting complex from Ectothiorhodospira haloalkaliphila is negligible. Photosynth Res 133, 289–295 (2017). https://doi.org/10.1007/s11120-017-0341-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-017-0341-7