Abstract
Photosynthetic organisms change the quantity and/or quality of their pigment–protein complexes and the interactions among these complexes in response to light conditions. In the present study, we analyzed light adaptation of the unicellular red alga Cyanidioschyzon merolae, whose pigment composition is similar to that of cyanobacteria because its phycobilisomes (PBS) lack phycoerythrin. C. merolae were grown under different light qualities, and their responses were measured by steady-state absorption, steady-state fluorescence, and picosecond time-resolved fluorescence spectroscopies. Cells were cultivated under four monochromatic light-emitting diodes (blue, green, yellow, and red), and changes in pigment composition and energy transfer were observed. Cells grown under blue and green light increased their relative phycocyanin levels compared with cells cultured under white light. Energy-transfer processes to photosystem I (PSI) were sensitive to yellow and red light. The contribution of direct energy transfer from PBS to PSI increased only under yellow light, while red light induced a reduction in energy transfer from photosystem II to PSI and an increase in energy transfer from light-harvesting chlorophyll protein complex I to PSI. Differences in pigment composition, growth, and energy transfer under different light qualities are discussed.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Oxygenic photosynthetic organisms have two photosystems, photosystem I (PSI) and photosystem II (PSII), which convert light energy to electron flow at their reaction centers. PSI and PSII cooperate, with PSII providing the electrons required for NADPH production in PSI. Hence, maintaining the excitation energy balance between PSI and PSII is extremely important. Because the reaction centers respond to limited wavelengths of light (around 440 and 680 nm), photosynthetic organisms extend their available wavelength region by assembling unique antenna pigment–protein complexes. Cyanobacteria and red algae have phycobilisome (PBS) on thylakoid membrane surfaces, which absorbs light energy in the visible region and efficiently transfers this energy to chlorophyll (Chl) in the thylakoid membrane (Gantt 1981). PBS comprises a core of allophycocyanin (APC) and rods containing phycocyanin (PC) only or a combination of phycoerythrin (PE) with PC (Gantt 1981; Mimuro and Kikuchi 2003). It is reported that the structure of PBS in red algae is different from that in cyanobacteria (Gantt 1981; Arteni et al. 2008). Unlike cyanobacteria, red algae possess an antenna for PSI in thylakoid membrane: light-harvesting chlorophyll protein complex I (LHCI). Red algal LHCI contains Chl a and carotenoid (Car), exhibiting a 671-nm absorption peak at room temperature (Wolfe et al. 1994).
Photosynthetic organisms adapt to light quality by changing the quantity and/or quality of their pigment–protein complexes, and the interactions among these complexes. For example, the PC to Chl a ratio in the cyanobacterium Anacystis nidulans reduces under strong orange light and increases under strong red light (Ghosh and Govindjee, 1966). The cyanobacterium Fremyella diplosiphon, which can control its PE content, exhibits the short-wavelength form of PC (PC615) under red-light cultivation without changing its PC and APC energy-transfer processes (Yokono et al. 2008). In studies on the relationship between light quality and gene expression control mechanism, the cyanobacterium F. diplosiphon contains RcaE, which is related to the chromatic adaptation (Kehoe and Grossman 1996). Subsequent genetic analysis suggested that RcaE controls the expression of PC genes via phosphorylation of two response regulators, RcaF and RcaC, under red light (Gutu and Kehoe 2012). On the other hand, as for adaptation of red algae to light quality, changes in pigment content and ultrastructure of PBS have been reported (Cunningham et al. 1990; Abe et al. 1994; Tsekos et al. 2002), while the gene expression control mechanism and changes in energy transfer are still unknown. The red alga Porphyridium cruentum increases and reduces its PSI/PSII ratio under red-light and green-light cultivation, respectively (Cunningham et al. 1990). In the red alga Porphyra yezoensis, orange light (which excites PSII) induces an increase in PE and a decrease in PC; the reverse response occurs under red light (which excites PSI) (Abe et al. 1994). Monochromatic light alters the structure, shape, and size of PBS in the red alga Porphyra leucosticta; the PBS density is much higher under blue-light cultivation than under red- or green-light cultivation (Tsekos et al. 2002).
Previously, we analyzed the energy-transfer processes in the cyanobacterium Arthrospira platensis grown under different light qualities (Akimoto et al. 2012, 2013). Both the energy transfers from PBS to PSI and from PSII to PSI were modified to maintain the excitation balance between PSI and PSII (Akimoto et al. 2013). On the other hand, in response to altered PE levels, the red algae P. cruentum, Bangia fuscopurpurea, P. yezoensis, Chondrus giganteus, and Prionitis crispata maintain their excitation balance by adjusting only the energy transfer from PSII to PSI (Yokono et al. 2011). Therefore, it was suggested that the light adaptation mechanism in cyanobacteria differs from that in red algae. In the present study, we examine how the unicellular red alga C. merolae responds to light quality by investigating the energy-transfer processes in cells grown under different light qualities. The pigment composition of C. merolae (Chl a, Car, and PBS composed of PC and APC) is similar to those of typical cyanobacteria. The differences in pigment composition, growth, and energy transfer under different light qualities are analyzed by steady-state absorption, steady-state fluorescence, and time-resolved fluorescence spectroscopy techniques.
Materials and methods
Cultivation conditions
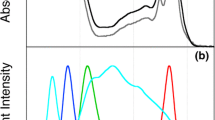
Cyanidioschyzon merolae cells were initially cultured in 200-mL Erlenmeyer flasks containing 100 mL Allen medium adjusted to pH 3.1 (Enami and Fukuda 1975) at 40 °C with 100 rpm agitation. The pre-cultivation was performed under 40 μmol photons m−2 s−1 continuous illumination by white light-emitting diodes (LED) in air in an incubator (SLI-450 N, EYELA, Tokyo, Japan). The cultured cells were repeatedly inoculated into fresh medium at an optical density at 750 nm (OD750) of 0.04 at 7-day intervals (a control). C. merolae cells inoculated in fresh medium were then transferred and cultivated under different light qualities (four LED colors) for 6 days (40 μmol photons m−2 s−1 continuous illumination). Figure 1 shows the spectral profiles of the LEDs used for cultivation and an absorption spectrum of the control cells at 77 K (−196 °C). Blue, green, yellow, and red LEDs emit a single peak at 460, 515, 590, and 666 nm, respectively. From the cellular absorption spectrum, we observe that blue (B) light is absorbed by Car and Chl (Soret), green (G) light is absorbed by Car, yellow (Y) light is absorbed mainly by PBS (PC) and slightly by Car, and red (R) light is absorbed by Chl (Qy) and PBS (APC).
Spectral profiles of light-emitting diodes (LED) used for cultivation and an absorption spectrum of the control cells: a white LED (W), b four single-color LEDs (B, blue; G, green; Y, yellow; R, red), and c absorption spectrum of the control cells (Car, carotenoid; Chl, chlorophyll; PBS, phycobilisome)
Measurements and analyses
Steady-state absorption spectra were measured by a spectrometer equipped with an integrating sphere (JASCO V-650/ISVC-747) at 77 K. Steady-state fluorescence spectra were measured by a spectrofluorometer (JASCO FP-6600/PMU-183), also at 77 K. The excitation wavelength was 600 nm, which mainly excites PC. Time-resolved fluorescence spectra (TRFS) were measured with a time-correlated single-photon counting system at 77 K (Akimoto et al. 2012). The excitation wavelength was 400 nm, which simultaneously excites all pigments. Homogenous ice was obtained by adding polyethylene glycol (average molecular weight 3,350, final concentration 15 % (w/v), Sigma-Aldrich, St Louis, MO, USA) to a sample solution at 77 K. The time interval for data acquisition was set to 2.4 or 24.4 ps/channel. The repetition rate of the pulse train was 2.9 MHz, which does not affect measurements up to 100 ns (24.4 ps/channel × 4,096 channels). Fluorescence rise and decay curves were measured at 1 nm intervals (620–770 nm). The fluorescence behaviors were unchanged in repeated measurements, confirming constant sample conditions throughout the TRFS measurements.
The fluorescence decay-associated spectra (FDAS) were constructed from a global analysis of the fluorescence kinetics (Akimoto et al. 2012). The fluorescence rise and decay curves were fitted by sums of exponentials with common time constants as follows:
The amplitudes (A n (λ)) specify the FDAS for each time constant. Positive and negative amplitudes indicate fluorescence decrease and increase over time, respectively. The fluorescence quantum yield differs between the PSII and PSI fluorescences; to remove this effect, the PSII and PSI fluorescence intensities were divided by their corresponding mean lifetimes (Yokono et al. 2011).
Results
Absorption spectra of C. merolae grown under different light qualities
Figure 2 shows the absorption spectra of the C. merolae cells grown under different light qualities. All spectra were normalized by the Chl Qy band. Chl exhibited the Soret band (410–440 nm) and the Qy band (670–680 nm), while PC peaked in the 600–640 nm range. APC did not peak in the absorption spectra of the cells, but has a transition energy between the Chl Qy band and the PC band (Mimuro and Kikuchi 2003). The two peaks at 470 and 500 nm were assigned to Car. The light quality affected the relative Car and PC contents of the cells (see Table 1). The relative amount of PC varied from 0.93 (R-light grown cells) to 1.29 (B-light grown cells). Compared with the control cells, the relative PC amount increased in the B-light and G-light grown cells, and decreased in the Y-light and R-light grown cells. On the other hand, the relative amount of Car ranged from 0.94 (Y-light grown cells) to 1.11 (G-light grown cells). The relative Car content was reduced only in Y-light grown cells.
Steady-state fluorescence spectra of C. merolae grown under different light qualities
Figure 3 shows the steady-state fluorescence spectra of the C. merolae cells grown under different light qualities. All fluorescence spectra were normalized by the PC fluorescence peak. The spectra are divided into three fluorescence regions: PBS, PSII, and PSI. In the PBS fluorescence region, the two peaks around 655 and 663 nm were assigned to PC and APC, respectively. The core antenna complexes of the PSII reaction center, CP43 and CP47, exhibited two peaks around 685 and 695 nm, respectively, while red-Chl in PSI peaked around 730 nm. The light quality altered the relative intensities of the pigments. In particular, the relative intensity of APC decreased in cells grown under monochromatic LEDs. The PSI/PSII intensity ratio was elevated in the SF of the Y-light grown cells, but was almost invariant in the other samples (Table 2).
Steady-state fluorescence spectra of C. merolae cells grown under different light qualities. Black line indicates the control. The colored lines indicate the color of the light source used for cultivation. The spectra are normalized by the PC fluorescence peak around 655 nm. The excitation wavelength is 600 nm
Fluorescence decay-associated spectra of C. merolae grown under different light qualities
To precisely examine the energy transfer, we obtained the FDAS thorough a global analysis (Eq. 1) of TRFS (Supplementary Fig. S1). Figure 4 shows the FDAS of C. merolae cells grown under different light qualities. In the first FDAS (45–70 ps), a positive and negative peak (~641 and ~667 nm, respectively) appeared in the PBS region of all samples except the R-light grown cells. Such a positive–negative pair of peaks indicates energy transfer from PC to APC (PC → APC energy transfer). Moreover, the contribution of the positive and negative peaks was larger in the B- and G-light grown cells than in the control cells. The R-light grown cells alone exhibited a positive APC band. In all other samples, a negative peak appeared around 686 nm in the PSII region; the control and R-light grown cells exhibited an additional positive peak around 680 nm. The 686 and 680 nm peaks were assigned to PSII and to LHCI (Marquardt and Rhiel 1997), respectively. However, the positive 680 nm peak was not evident in cells grown under blue, green, or yellow light. The FDAS of all cells showed a negative peak around 725 nm and an upward curve around 700 nm in the PSI region. The amplitudes around 700 nm were positive in the control, R- and Y-light grown cells, and negative in the B- and G-light grown cells. The peak around 700 nm is contributed by the antenna Chls in PSI (Mimuro et al. 2010) and the negative peak around 725 nm arises from red-Chl in PSI. The time constant was shorter in cells grown under single-color LEDs than in the control cells, indicating that growth under single-colored light accelerates energy transfer in the cells. In the second FDAS (140–170 ps), fluorescence in the PSII and PSI regions was observed in all cells grown under monochromatic light (appearing as positive peaks around 687 and 720 nm, respectively). In contrast, the control cells exhibited negative amplitudes at wavelengths exceeding 731 nm, indicating slower energy transfer between red-Chls of different energies in the control cells than in cells grown under monochromatic LEDs. This inference is confirmed by the longer time constant in the first FDAS of the control cells than in any of the experimental cells (Fig. 4). In the third and fourth FDAS (590–800 ps and 1.6–2.0 ns, respectively), the peaks in each fluorescence region were red-shifted under all light conditions. In the fifth component (2.5–5.4 ns), all samples peaked in the PSI region (around 733 nm). On the other hand, a clear APC peak (at 669 nm) appeared in the PBS fluorescence region of all cells grown under monochromatic light. This peak was absent in the control sample. Long-lived (delayed) fluorescence is evident in both the PSI and PSII regions of the final FDAS (15–21 ns). Delayed fluorescence originates from charge recombination at the PSII reaction center (Mimuro et al. 2007), which occurs over a lifetime exceeding that of isolated Chl (~5 ns). Whereas the fluorescence lifetime of isolated PSII is 15–25 ns (Mimuro et al. 2007), that of isolated PSI is below 1 ns (Mimuro et al. 2010). Therefore, if PSI and PSII exist as independent entities, PSII alone contributes to the delayed fluorescence. On the other hand, when PSII and PSI combine into PSII–PSI supercomplexes, delayed fluorescence occurs in the PSI region (Yokono et al. 2011). As energy transferring from PSII to PSI (PSII → PSI energy transfer) makes a greater contribution than energy remaining within PSII, the relative intensity of PSI to PSII increases in the final FDAS. Therefore, by comparing the PSI/PSII intensity ratios, we can estimate the contribution of the PSII → PSI energy transfer. Among the examined light conditions, the PSI/PSII ratio was lowest in the R-light grown cells and highest in the Y-light grown cells (Table 2). These results indicate that PSII → PSI energy transfer makes its smallest contribution in the R-light grown cells, and its largest in the Y-light grown cells. The spectral shapes of each pigment in the third and fourth FDAS (590–800 ps and 1.6–2.0 ns) did not show clear cultivation light dependence, suggesting that adaptation to light quality occurs by altered energy transfer in the first and second FDAS (the 45–70 and 140–170 ps), and in the fifth and final FDAS (2.5–5.4 and 15–21 ns).
Discussion
Cultivation light induced changes in pigment composition and growth
The relative PC content differed between cells grown under white light and monochromatic LEDs (Table 1). It appears that cells balance their harvesting of light energy by increasing their relative amount of PC under blue and green light (which does not excite the PBS band), and decreasing it under yellow and red light (which excites the PBS band). On the other hand, the cyanobacterium A. platensis increases the relative PC content under low red light (Akimoto et al. 2013). This difference might come from the presence of LHCI in red algae; only red algae possess LHCI which can absorb red light. The relative Car content was also affected by light quality (Table 1), but the two factors are not simply correlated, as is the case for the cyanobacterium A. platensis (Akimoto et al. 2013). Car functions as a quencher or an energy donor of Chl depending on the light conditions. Therefore, light quality might alter the function as well as the content of the cellular pigments.
The OD750 values (which correlate with growth) differed among the samples, being smallest in the B-light grown cells (80 % of the control cells). However, these differences were considerably smaller than those found in the cyanobacterium A. platensis (Akimoto et al. 2012), whose OD750 under blue light was reduced to 16 % that of white-LED grown cells. This suggests quenching of the PSI Chls in the B-light grown cells. However, the quenching characteristics were not analyzed in the FDAS of C. merolae cells grown under blue light (Fig. 4).
Energy transfer in PBS
Compared with white-light cultivation, the PC content was elevated under the B- and G-light cultivations, and reduced under the Y- and R-light cultivations. On the other hand, the steady-state fluorescence spectra (Fig. 3) revealed that the relative intensity of APC decreased in all cells grown under single-color LEDs (Table 2). In the first FDAS (Fig. 4) of B- and G-light grown cells, the amplitudes of the positive and negative peaks were larger than in the control cells, suggesting greater contribution of energy transfer in PBS at higher relative amounts of PC (Table 1). A positive APC band was observed only in the R-light grown cells. These results suggest that the PC → APC energy transfer was the dominant fast-phase energy transfer (40–70 ps) in all cells except the R-light grown cells, in which PC → APC energy transfer was accompanied by APC → Chl energy transfer. Cells grown under colored light (but not the control cells) displayed a clear APC fluorescence peak in the PBS region of their fifth FDAS. These results indicate that APC pigments, which transfer energy to Chl with low efficiency, existed in non-negligible quantities in cells grown under single-color LEDs. Conversely, in the control cells, excitation energy was transferred directly from PBS to Chl without loss. Therefore, it seems that the unicellular red alga C. merolae undergoes defective APC → Chl energy transfer under monochromatic light. In contrast, the cyanobacterium A. platensis exhibits long-lived PC fluorescence but no long-lived APC fluorescence, indicating no APC → Chl energy transfer defect in this organism (Akimoto et al. 2012). These differences might originate from structural differences in the PBS between the alga and cyanobacterium (Misumi et al. 2005) and/or the special environment inhabited by C. merolae (Ciniglia et al. 2004).
Energy transfer processes to PSI
In red algae, PBS is integrated into antenna pigment–protein complexes for PSII (Gantt 1981; Mimuro and Kikuchi 2003). However, PBS is also known to transfer energy to PSI. Therefore, we may conceive two energy transfer pathways after PBS excitation: PBS → PSI energy transfer (Mullineaux 1992) and PBS → PSII energy transfer (Butler and Kitajima 1975; Bruce et al. 1985). Figure 5 plots the PSI/PSII intensity ratios in the SF versus those in DF for cells grown under different light qualities. Liu et al. (2013) detected no energy transfer from PBS to PSI via PSII (PBS → PSII → PSI transfer) in the supercomplex containing PBS, PSII, and PSI (the PBS–PSII–PSI supercomplex). Therefore, the PSI/PSII intensity in the SF excited at the PBS band reflects the contributions of both PBS → PSI and PBS → PSII energy transfers. As more of the PSI interacts with PBS, the PSI/PSII intensifies in the SF. On the other hand, the PSI/PSII intensity in the DF reflects only the contribution of the PSII → PSI energy transfer. In this case, as more of the PSII interacts with PSI, the PSI/PSII intensifies in the DF. The PSI/PSII value was almost invariant in the SF (0.67–0.68) of all cells except those grown under yellow light (where it increased to 0.89), while in the DF, it varied from 0.64 (R-light grown cells) to 1.04–1.18 (other cells) (Table 2). These results indicate that the PBS → PSI and PSII → PSI energy transfers are sensitive to yellow and red light, respectively. The Y-light grown cells modified their PBS → PSI energy transfer more than their PSII → PSI energy transfer, whereas the R-light grown cells modified only their PSII → PSI energy transfer. In contrast, cyanobacteria grown under different light qualities alter both their PBS → PSI and PSII → PSI energy transfers (Akimoto et al. 2013). In red algae closely related to C. merolae, the PSII and PBS interact more strongly than in typical cyanobacteria and other red algae; hence, PBS is moved between two photosystems only under temperature stress (suboptimal temperature conditions) (Kaňa et al. 2014). Therefore, it appears that C. merolae modifies the excitation energy balance between PSII and PSI mainly by altering the PSI–PSII interaction. If this modification fails to balance the excitation energy between PSII and PSI, more energy can be transferred to PSI through the excitation energy of PBS (as observed in Y-light grown cells).
PSI/PSII intensity ratio in steady-state fluorescence (PSI/PSII in SF) versus PSI/PSII intensity ratio in delayed fluorescence (PSI/PSII in DF) in C. merolae cells grown under LEDs of different colors: blue (B), green (G), yellow (Y), and red (R). G overlaps with the control (indicated by C). The vertical and horizontal broken lines are the PSI/PSII intensity ratios in the SF (1.04) and the DF (0.68) of the control cells, respectively
In red algae, unlike cyanobacteria, LHCI is incorporated into antenna pigment–protein complexes for PSI (Wolfe et al. 1994). No positive peak in the LHCI fluorescence region appeared in the FDAS of the B-, G-, and Y-light grown cells (Fig. 4), suggesting greater contribution from the PBS → PSII energy transfer than from LHCI to PSI (LHCI → PSI energy transfer) in the early time region of the present study. On the other hand, a positive peak assigned to LHCI fluorescence (~680 nm) appeared in the spectra of control and R-light grown cells. The enhancement of this peak in the spectrum of R-light grown cells indicates that energy is predominantly transferred to PSI via light harvesting by LHCI under red light cultivation. Therefore, the R-light grown cells modify both their PSII → PSI (Fig. 5) and LHCI → PSI energy transfers, whereas the contribution of the PSII → PSI energy transfer was decreased, that of the LHCI → PSI energy transfer was increased. Neither the PBS → PSI nor PBS → PSII energy transfers were affected by red light (Fig. 5). Therefore, under red light cultivation, it appears that excitation energy transferred from LHCI to PSI compensates for the reduced energy transferred from PSII, while the energy transferred from PBS is unaltered.
Summary
We analyzed light adaptation in unicellular red alga C. merolae cells grown under different light qualities (white light, and monochromatic blue, green, yellow, and red). The OD750 values were not significantly altered by the cultivation light color (Table 1). However, cultivation under single-color LEDs induced defective APC → Chl energy transfer (the fifth FDAS in Fig. 4). Compared with the control cells, the B- and G-light grown cells showed elevated PBS content (Table 1), and facilitation of energy transfer in PBS (first FDAS in Fig. 4). The pathway of energy transfer to PSI was sensitive to yellow and red light (Fig. 5); yellow light induced modifications of the PBS → PSI energy transfer (Fig. 5), while red light altered the PSII → PSI and LHCI → PSI energy transfers (Fig. 5; first FDAS in Fig. 4). Blue-light induced quenching, which was found in the cyanobacterium A. platensis (Akimoto et al. 2012), was not recognized for the red alga C. merolae (Fig. 4).
Abbreviations
- APC:
-
Allophycocyanin
- Car:
-
Carotenoid
- Chl:
-
Chlorophyll
- FDAS:
-
Fluorescence decay-associated spectrum (spectra)
- LED:
-
Light-emitting diodes
- LHC:
-
Light-harvesting chlorophyll protein complex
- PBS:
-
Phycobilisome
- PC:
-
Phycocyanin
- PE:
-
Phycoerythrin
- PS:
-
Photosystem
- TRFS:
-
Time-resolved fluorescence spectrum (spectra)
References
Abe S, Murakami A, Ohki K, Aruga Y, Fujita Y (1994) Changes in stoichiometry among PSI, PSII and Cyt b 6 -f complexes in response to chromatic light for cell growth observed with the red alga Porphyra yezoensis. Plant Cell Physiol 35:901–906
Akimoto S, Yokono M, Hamada F, Teshigahara A, Aikawa S, Kondo A (2012) Adaptation of light-harvesting systems of Arthrospira platensis to light conditions, probed by time-resolved fluorescence spectroscopy. Biochim Biophys Acta 1817:1483–1489
Akimoto S, Yokono M, Aikawa S, Kondo A (2013) Modification of energy-transfer processes in the cyanobacterium, Arthrospira platensis, to adapt to light conditions, probed by time-resolved fluorescence spectroscopy. Photosynth Res 117:235–243
Arteni AA, Liu LN, Aartsma TJ, Zhang YZ, Zhou BC, Boekema EJ (2008) Structure and organization of phycobilisomes on membranes of the red alga Porphyridium cruentum. Photosynth Res 95:169–174
Bruce D, Biggins J, Steiner T, Thewalt M (1985) Mechanism of the light state transition in photosynthesis. IV. Picosecond fluorescence spectroscopy of Anacystis nidulans and Porphyridium cruentum in state 1 and state 2 at 77 K. Biochim Biophys Acta 806:237–246
Butler WL, Kitajima M (1975) Energy transfer between photosystem II and photosystem I in chloroplasts. Biochim Biophys Acta 396:72–85
Ciniglia C, Yoon HS, Pollio A, Pinto G, Bhattacharya D (2004) Hidden biodiversity of the extremophilic cyanidiales red algae. Mol Ecol 13:1827–1838
Cunningham FX Jr, Dennenberg RJ, Jursinic PA, Gantt E (1990) Growth under red light enhances photosystem II relative to photosystem I and phycobilisomes in the red alga Porphyridium cruentum. Plant Physiol 93:888–895
Enami I, Fukuda I (1975) Mechanisms of the acido- and thermo-phily of Cyanidium caldarium Geitler I. Effects of temperature, pH and light intensity on the photosynthetic oxygen evolution of intact and treated cells. Plant Cell Physiol 16:211–220
Gantt E (1981) Phycobilisomes. Ann Rev Plant Physiol 32:327–347
Ghosh AK, Govindjee (1966) Transfer of the excitation energy in Anacystis nidulans grown to obtain different pigment ratios. Biophys J 6:611–619
Gutu A, Kehoe DM (2012) Emerging perspectives on the mechanisms, regulation, and distribution of light color acclimation in cyanobacteria. Mol Plant 5:1–13
Kaňa R, Kotabová E, Lukeš M, Papáček Š, Matonoha C, Liu L-N, Prášil O, Mullineaux CW (2014) Phycobilisome mobility and its role in the regulation of light harvesting in red algae. Plant Physiol 165:1618–1631
Kehoe DM, Grossman AR (1996) Similarity of a chromatic adaptation sensor to phytochrome and ethylene receptors. Science 273:1409–1412
Liu H, Zhang H, Niedzwiedzki DM, Prado M, He G, Gross ML, Blankenship RE (2013) Phycobilisomes supply excitations to both photosystems in a megacomplex in cyanobacteria. Science 342:1104–1107
Marquardt J, Rhiel E (1997) The membrane-intrinsic light-harvesting complex of the red alga Galdieria sulphuraria (formerly Cyanidium caldarium): biochemical and immunochemical characterization. Biochim Biophys Acta 1320:153–164
Mimuro M, Kikuchi H (2003) Antenna systems and energy transfer in Cyanophyta and Rhodophyta. In: Green BR, Parson WW (eds) Light-harvesting antennas in photosynthesis. Kluwer Academic Publishers, Dordrecht, pp 281–306
Mimuro M, Akimoto S, Tomo T, Yokono M, Miyashita H, Tsuchiya T (2007) Delayed fluorescence observed in the nanosecond time region at 77 K originates directly from the photosystem II reaction center. Biochim Biophys Acta 1767:327–334
Mimuro M, Yokono M, Akimoto S (2010) Variations in photosystem I properties in the primordial cyanobacterium Gloeobacter violaceus PCC 7421. Photochem Photobiol 86:62–69
Misumi O, Matsuzaki M, Nozaki H, Miyagishima S, Mori T, Nishida K, Yagisawa F, Yoshida Y, Kuroiwa H, Kuroiwa T (2005) Cyanidioschyzon merolae genome. A tool for facilitating comparable studies on organelle biogenesis in photosynthetic eukaryotes. Plant Physiol 137:567–585
Mullineaux CW (1992) Excitation energy transfer from phycobilisomes to photosystem I in a cyanobacterium. Biochim Biophys Acta 1100:285–292
Tsekos I, Niell FX, Aguilera J, Figueroa FL, Delivopoulos SG (2002) Ultrastructure of the vegetative gametophytic cells of Porphyra leucosticta (Rhodophyta) grown in red, blue and green light. Phycol Res 50:251–264
Wolfe GR, Cunningham FX, Durnford D, Green BR, Gantt E (1994) Evidence for a common origin of chloroplasts with light-harvesting complexes of different pigmentation. Nature 367:566–568
Yokono M, Akimoto S, Koyama K, Tsuchiya T, Mimuro M (2008) Energy transfer processes in Gloeobacter violaceus PCC 7421 that possesses phycobilisomes with a unique morphology. Biochim Biophys Acta 1777:55–65
Yokono M, Murakami A, Akimoto S (2011) Excitation energy transfer between photosystem II and photosystem I in red algae: larger amounts of phycobilisome enhance spillover. Biochim Biophys Acta 1807:847–853
Acknowledgments
The authors thank Prof. Y. Kashino and Dr. N. Kashino for providing technical information about the culturing procedure. This work was supported in part by a grant from the Kurita Water and Environment Foundation to S. Aikawa (No. 13A021).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ueno, Y., Aikawa, S., Kondo, A. et al. Light adaptation of the unicellular red alga, Cyanidioschyzon merolae, probed by time-resolved fluorescence spectroscopy. Photosynth Res 125, 211–218 (2015). https://doi.org/10.1007/s11120-015-0078-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-015-0078-0