Abstract
A novel super-complex of photosystem I (PSI)–light-harvesting complex I (LHCI) was isolated from a siphonaceous marine green alga, Bryopsis corticulans. The super-complex contained 9–10 Lhca antennas as external LHCI bound to the core complex. The super-complex was further disintegrated into PSI core and LHCI sub-complexes, and analysis of the pigment compositions by high-performance liquid chromatography revealed unique characteristics of the B. corticulans PSI in that one PSI core contained around 14 α-carotenes and 1–2 ε-carotenes. This is in sharp contrast to the PSI core from higher plants and most cyanobacteria where only β-carotenes were present, and is the first report for an α-carotene-type PSI core complex among photosynthetic eukaryotes, suggesting a structural flexibility of the PSI core. Lhca antennas from B. corticulans contained seven kinds of carotenoids (siphonaxanthin, all-trans neoxanthin, 9′-cis neoxanthin, violaxanthin, siphonein, ε-carotene, and α-carotene) and showed a high carotenoid:chlorophyll ratio of around 7.5:13. PSI–LHCI super-complex and PSI core showed fluorescence emission peaks at 716 and 718 nm at 77 K, respectively; whereas two Lhca oligomers had fluorescence peaks at 681 and 684 nm, respectively. By comparison with spinach PSI preparations, it was found that B. corticulans PSI had less red chlorophylls, most of them are present in the core complex but not in the outer light-harvesting systems. These characteristics may contribute to the fine tuning of the energy transfer network, and to acclimate to the ever-changing light conditions under which the unique green alga inhabits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Light-energy conversion in oxygenic photosynthesis is driven by four multisubunit membrane protein complexes, photosystem I (PSI), photosystem II (PSII), cytochrome b 6 f complex (Cyt b 6 f), and ATP synthase. PSI mediates light-induced electron transfer from plastocyanin or cytochrome c 6 on the luminal side to ferredoxin on the stromal side. The crystal structures of PSI core complex from a thermophilic cyanobacterium (Jordan et al. 2001) and of the PSI–LHCI super-complex from a higher plant (Ben-Shem et al. 2003; Amunts et al. 2007) have been determined. Cyanobacterial PSI core is a trimer (Jordan et al. 2001), and each monomer is consisted of 12 protein subunits (PsaA, PsaB, PsaC, PsaD, PsaE, PsaF, PsaI, PsaJ, PsaK, PsaL, PsaM, and PsaX), 128 cofactors [96 chlorophylls (Chls), 2 phylloquinones, 3 Fe4S4 clusters, 22 carotenoids, 4 lipids, and a Ca2+ ion], with a total molecular mass of 356 kDa (Jordan et al. 2001). On the other hand, higher plant PSI–LHCI super-complex (and PSI core complex) exists in a monomeric form, and contains 14 core subunits and 4 Lhca antenna subunits (Ben-Shem et al. 2003; Amunts et al. 2007). Among the 14 core subunits, 10 (PsaA, PsaB, PsaC, PsaD, PsaE, PsaF, PsaI, PsaJ, PsaK, and PsaL) are similar to the corresponding subunits in the cyanobacterial PSI core, whereas the other 4 subunits (PsaG, PsaH, PsaN, and PsaO) are unique to the higher plant PSI. Lhca proteins are encoded in the nucleus by the lhc multigene family (Jansson1994; Scheller et al. 2001), and all LHC apoproteins show structural homology in that each has three transmembrane α-helices connected by both stroma and lumen-exposed loops. Structural comparison between cyanobacterial and higher plant PSI revealed a high degree of conservation of the polypeptide backbone structure, as well as chlorophyll arrangement in the core complex over more than one billion years of evolution (Ben-Shem et al. 2003; Nelson and Ben-Shem 2005).
In contrast to the core complex of PSI, different light-harvesting systems of PSI have been observed for various groups of photosynthetic organisms. In cyanobacteria, phycobilisomes act as peripheral antenna systems for PSI under some growth conditions; however, PSI is encircled with a ring of 18 copies of IsiA or other ring-shaped IsiA super-complexes for harvesting light under conditions of iron limitation (Bibby et al. 2001; Boekema et al. 2001;Yeremenko et al. 2004). In higher plants and algae, LHCI is associated with monomeric PSI core with various numbers of Lhca proteins depending on the species of the organisms. Although Lhca proteins share a similar transmembrane helix structure, they may differ greatly in the oligomerization states, association pattern with PSI core complex, and pigment composition (Busch and Hippler 2011), which is largely due to the wide variety of different habitats photosynthetic organisms live in.
As a eukaryotic alga closely related to higher plants, the green alga Chlamydomonas reinhardtii (C. reinhardtii) has been widely used as a model organism to investigate the structural characteristics of PSI. The green alga has the same PSI core subunit composition as that of higher plants, whereas it has a much larger number of Lhca proteins associated with the PSI core than that of higher plants (Stauber et al. 2003). The number of LHCI subunits associated with PSI core has been reported from 9 to 14, and some disagreements have been reported regarding the position of these LHCI subunits within the PSI–LHCI super-complex (Germano et al. 2002; Kargul et al. 2003, 2005; Takahashi et al. 2004). A recent report showed that C. reinhardtii PSI–LHCI was composed of 9 Lhca antennas, and these Lhcas were associated on one side of the core complex with a double-ring arrangement (Drop et al. 2011). This suggests that the green algal PSI has more than double of the LHCI subunits compared with that of higher plant PSI. The C. reinhardtii PSI–LHCI super-complex binds Chl a, Chl b, and 5 kinds of carotenoids (β-carotene, lutein, violaxanthin, neoxanthin, and loroxanthin), among which β-carotene, lutein, and violaxanthin are the main carotenoids, and the number of violaxanthin varies with light intensity (Pineaua et al. 2001). However, higher plant PSI–LHCI super-complex binds only 3 kinds of carotenoids, namely β-carotene, lutein, and violaxanthin, indicating apparent differences in the pigment composition of PSI–LHCI super-complex between higher plants and green algae (Schmid et al. 2002; Qin et al. 2006). In addition, low-temperature fluorescence emission of C. reinhardtii PSI–LHCI super-complex has a maximum at 712–715 nm, which is in contrast to the 730 nm peak in higher plant PSI–LHCI super-complex (Lam et al. 1984; Jansson et al. 1996; Kargul et al. 2003; Takahashi et al. 2004; Drop et al. 2011), suggesting a large difference in the molecular organization of the low-energy chlorophyll molecules (Melkozernov et al. 2004). Except C. reinhardtii, however, little is known about the number, pigment composition, and characteristics of PSI from other green algae.
Bryopsis corticulans is a unique, siphonaceous marine green alga living in the intertidal zone, with its life cycle adapted to a complicated light environment changing between strong sunlight and low, underwater-light. Therefore, the antenna system of B. corticulans must have been evolved to achieve the more distinguished double roles; one is to capture light energy efficiently under dim light, and the other one is to protect the photosynthetic apparatus from strong light. Indeed, light-harvesting complex of photosystem II (LHCII) isolated from this green alga has been shown to contain the unique siphonaxanthin and siphonein pigments instead of lutein and violaxanthin found in the higher plant LHCII (Wang et al. 2013), and these pigments were suggested to be important for the unique light-harvesting features of the green alga (Strain 1951; Anderson 1985; Yokohama et al. 1992; Wang et al. 2013).
In the present work, we isolated a PSI–LHCI super-complex and its sub-fractions from B. corticulans, and characterized them based on their protein and pigment composition, absorption spectroscopy, and 77 K steady-state fluorescence emission spectroscopy. The results obtained were discussed in relation to the habitat environment of this alga.
Methods and materials
Isolation of the PSI–LHCI super-complex
Bryopsis corticulans was collected from intertidal zones around seashore of Qingdao city, China, washed with distilled water, and then homogenized with an ice-cold buffer (30 mM Tricine-NaOH pH 7.8, 0.3 M sucrose, 15 mM NaCl). The homogenized materials were filtered through eight layers of cheesecloth, followed by centrifugation at 4,000×g for 7 min to pellet the chloroplasts. The chloroplasts obtained were broken by suspension in a hypotonic solution (5 mM Tricine-NaOH pH 7.8), and the thylakoid membranes were collected by centrifugation at 20,000×g for 10 min and resuspended in a buffer containing 5 mM Tricine-NaOH (pH 7.8) and 150 mM NaCl. The last step was repeated twice, and the thylakoid membranes were finally precipitated and resuspended in an STN buffer (20 mM Tricine-NaOH pH 7.8, 0.3 M sucrose). The membranes obtained were first frozen in liquid nitrogen and then stored at −80 °C until use.
The thylakoid membranes were solubilized with 0.8–1.2 % (w/v) n-dodecyl-β-d-maltoside (β-DDM) at 1 mg Chl/ml in an ice bath for 20 min. The unsolubilised materials were removed by centrifugation at 40,000×g for 15 min and the supernatant was loaded onto a 0–1.0 M continuous sucrose density gradient prepared by freeze and thawing the centrifuge tubes filled with a buffer containing 20 mM Tricine-NaOH pH 7.8, 0.03 % (w/v) β-DDM and 0.5 M sucrose. Following ultracentrifugation (200,000×g, 3 h, Beckman Vti 50 rotor, 4 °C), three bands appeared. These bands were collected in 0.9 ml fractions, and the protein composition was analyzed by electrophoresis.
Isolation of the PSI core complex and LHCI from the PSI–LHCI super-complex
The PSI–LHCI super-complex was further solubilized into PSI core and LHCI with a procedure similar to that previously reported (Påsllon et al. 1995; Qin et al. 2006). PSI–LHCI super-complex (0.3 mg Chl/ml) was solubilzed with β-DDM at a final concentration of 0.5 % (w/v) and 3-(N,N-dimethylpalmitylammonio) propanesulfonate (zw 3–16) at a final concentration of 0.75 % (w/v) for 30 min in an ice bath. An aliquot of 0.5 ml detergent-treated PSI–LHCI was applied to a 0.3–1.4 M sucrose density gradient tube [containing 20 mM Tricine-NaOH pH 7.8 and 0.06 % (w/v) β-DDM]. Following centrifugation at 200,000×g for 16 h in a Beckman SW 55 Ti rotor at 4 °C, three chlorophyll-containing bands were obtained, which were identified as LHCI, PSI core, and partially solubilized PSI–LHCI from top to bottom, respectively.
Chlorophyll concentrations were determined in 80 % (v/v) acetone according to Arnon (1949).
Electrophoresis and immunoblotting analysis
To analyze the polypeptide composition, samples were treated with an LDS sample buffer containing 2 % (w/v) lithium dodecyl sulfate, 60 mM dithiothreitol, and 60 mM Tris–HCl (pH 8.5) at 60 °C for 10 min, and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) as described by Ikeuchi and Inoue (1988) with a 15–22 % gradient gel containing 7.5 M urea. After electrophoresis, the proteins were transferred to a nitrocellulose membrane, probed with antibodies raised against PsaA (Agrisera AS06172), PsaB (Agrisera AS10695), PsaD (Agrisera AS09461), PsaF (Agrisera AS06104), higher plant Lhca1 (Agrisera AS01005), Lhca 2 (Agrisera AS01006), Lhca 3 (Agrisera AS01007), Lhca 4 (Agrisera AS01008), Lhca 5 (Agrisera AS010082), and C. reinhardtii Lhca5, Lhca6 (provided by Prof. Y. Takahashi), and visualized by enhanced chemiluminescence.
Blue-native (BN)-PAGE was performed essentially as described previously (Schägger et al. 1994). The thylakoid membranes were washed with 50 mM Bis–Tris, pH 7.0, and then solubilized with 1 % (w/v) β-DDM in 25 mM Bis–Tris, pH 7.0 and 20 % (w/v) glycerol at 0.5 mg Chl/ml for 10 min on ice. After centrifugation at 12,000×g for 10 min, the supernatant was combined with 1/10 volume of 5 % Serva blue G in 100 mM Bis–Tris, pH 7.0, 0.5 M 6-amino-n-caproic acid, and 30 % (w/v) glycerol, and then applied to a 1-mm-thick, 6–12 % acrylamide gradient gel.
Green gel electrophoresis was carried out as described by Anderson et al. (1978). Samples were solubilized at a final SDS:Chl (w/w) ratio of 10:1 on ice, and then applied to a 1-mm-thick, 6–10 % gradient gel. After electrophoresis, the bands resolved in the green gel were collected by disk-electrophoresis as described by Wang (1991), and used for the measurements of absorption and 77 K fluorescence spectra.
For two-dimensional electrophoretic analysis, the bands from BN-PAGE or green gels were soaked in the LDS sample buffer at 60 °C for 10 min and loaded onto a 1.5-mm-thick, 15–22 % acrylamide gradient gel containing 7.5 M urea (Ikeuchi and Inoue 1988).
Absorption and fluorescence spectra
Absorption spectra were recorded at room temperature with a Shimadzu UV–Vis 2550 spectrophotometer. Samples were diluted in a buffer containing 20 mM Tricine-NaOH (pH 7.8), 0.03 % (w/v) β-DDM to 10 μg Chl/ml. Low-temperature (77 K) fluorescence spectra were obtained using a Hitachi F-4500 fluorescence spectrophotometer with 440 nm as the excitation wavelength. The spectral sensitivity of the fluorescence spectrophotometer was corrected by using a sub-standard light source with a known radiation profile (Hitachi, Japan). The chlorophyll concentration was adjusted to 5 μg Chl/ml with a buffer containing 20 mM Tricine-NaOH (pH 7.8), 30 % (w/v) glycerol, and 0.015 % (w/v) β-DDM.
Identification and quantification of pigments by high-performance liquid chromatography (HPLC)
Pigments were extracted with 80 % (v/v) cold acetone from isolated PSI–LHCI, PSI core, and LHCI preparations, and analyzed by HPLC as described by Thayer and Björkman (1990) with slight modifications. HPLC was performed with a C-18 reversed-phase column (4.6 × 250 mm2, 5 μm particle size, Grace, USA) in a Waters e2695 separation module equipped with a Waters 2998 photodiode array detector. The pigments were eluted at a flow rate of 1 ml/min using 100 % solvent A (acetonitrile:methanol, 75:25) for the first 9 min followed by a 3 min linear gradient with 0–100 % solvent B (methanol:ethylacetate, 70:30), which was then continued isocratically with 100 % solvent B until the end of the 28 min separation. The column was re-equilibrated in solvent A at a flow rate of 1 ml/min for 10 min prior to the next injection. Pigments were detected by their absorbance at 445 nm. The P700 content and protein concentration were determined as previously described (Qin et al. 2006).
The authentic standards of Chl a, Chl b, α-carotene were purchased from Sigma. For commercially unavailable standards, siphonaxanthin, siphonein, and ε-carotene were extracted from B. corticulans thylakoid membranes, 9′-cis neoxanthin was extracted from pea thylakoid membranes, all-trans neoxanthin was extracted from pumpkin fruit using alumina chromatography, and the isolated carotenoids were further purified by HPLC. Identification of carotenoids was achieved by comparing their retention times and absorption spectra with literature data and/or the authentic standards. The extinction coefficients for the quantification of pigments are shown in Table 1. Standard curves for quantification of carotenoids were obtained by plotting the amount (ng) of the pigments loaded onto the HPLC column against the area of the elution peak, and each data point was obtained from the average of three injections.
Results
Isolation and characterization of the PSI–LHCI super-complex
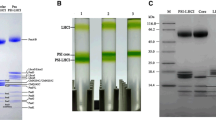
Similar to the isolation of PSI–LHCI from pea (Ben-Shem et al. 2003) and C. reinhardtii (Takahashi et al. 2008), washing of B. corticulans thylakoid membranes with salt (150 mM NaCl) was found to be critical for removal of soluble and peripheral proteins, especially those of ATPase subunits, which interfere with the purity of PSI–LHCI. Using the washed membranes, the concentration of β-DDM used for solubilization was determined to be 1.2 % (w/v), based on the results of sucrose density gradient ultracentrifugation and SDS-PAGE analysis. As shown in Fig. 1a, treatments with 0.8, 1.0, 1.2 % β-DDM all resulted in three bands in the sucrose density gradient, among which, the upper wide dark band (band I) contained mainly LHCII, the middle band (band II) contained mainly PSII and PSI core, and the lowest band (band III) contained mainly PSI–LHCI (Fig. 1b). However, band I and band II became to merge into one broad band if a large amount of DDM-treated thylakoids was loaded onto the sucrose density gradient. The polypeptide composition of PSI–LHCI from the three treatments was analyzed by SDS-PAGE. As can be seen in Fig. 1c, some Lhcb bands were found in the PSI–LHCI sample obtained with 0.8 % β-DDM, whereas these bands became very weak in the PSI–LHCI samples obtained with 1.0 and 1.2 % β-DDM. The coexistence of LHCII in the PSI–LHCI fraction was confirmed by increase in the low-temperature fluorescence emission peak at 683 nm in the samples treated at the lower β-DDM concentrations (Fig. 2a). These results suggest that a small part of aggregated LHCII is mixed with the PSI–LHCI sample and its amount decreased with the increase of β-DDM concentration (Fig. 1c). To decrease the coexisted LHCII, 1.2 % β-DDM was used to prepare the PSI–LHCI super-complex.
a Separation of PSI–LHCI by sucrose density gradient ultracentrifugation. β-DDM was used at different concentrations (0.8, 1.0, 1.2 %) for solubilization of thylakoids before the ultracentrifugation. b Polypeptide composition of fractions from the treatment of 1.2 % β-DDM analyzed by SDS-PAGE. c Polypeptide composition of PSI–LHCI (prepared by 0.8, 1.0, and 1.2 % DDM) analyzed by SDS-PAGE and immunoblot analysis with antibodies against core subunits (PsaA, PsaB, PsaD, and PsaF), higher plant Lhca1-5, and C. reinhardtii (indicated as C.r.) Lhca-5, Lhca-6
a Low-temperature (77 K) fluorescence emission spectra of PSI–LHCI (prepared by 0.8, 1.0, and 1.2 % DDM). Fluorescence emission spectra were normalized at 716 nm. b Absorption spectra at room temperature of PSI–LHCI (prepared by 1.2 % DDM) and spinach PSI–LHCI. Absorption spectra were normalized with respect to their maximal absorption at red region around 679 nm. The differential spectrum of B. corticulans PSI–LHCI minus spinach PSI–LHCI was shown. Inset the differential spectrum in the wavelength region of 680–725 nm
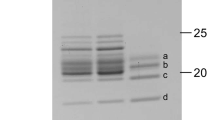
The polypeptide composition of isolated PSI–LHCI is shown in Fig. 1c. B. corticulans PSI core subunits of PsaA, PsaB, PsaD, and PsaF were identified by Western blotting using antibodies against PsaA (Agrisera AS06172), PsaB (Agrisera AS10695), PsaD (Agrisera AS09461), PsaF (Agrisera AS06104). In addition, eight Lhca bands with different apparent molecular masses were resolved on the SDS-PAGE gel, and were named as Lhca-a—Lhca-h, respectively. Immunoblot analysis using antibodies against higher plant Lhca1, Lhca 2, Lhca 3, Lhca 4, and Lhca 5 demonstrated that all Lhca proteins of B. corticulans, except Lhca-b and Lhca-d, reacted with one or two antibodies against Lhca1, Lhca 2, Lhca 4, and Lhca 5, whereas none reacted with the antibodies against Lhca3. On the other hand, Lhca-b and Lhca-e cross-reacted with an antibody against C. reinhardtii Lhca5, and Lhca-d together with Lhca-c cross-reacted with an antibody against C. reinhardtii Lhca6. These results suggested that all of the bands labeled Lhca-a—Lhca-h belong to the Lhca superfamily.
The large number of Lhca subunits found in the B. corticulans PSI–LHCI super-complex suggests that it should be a larger complex than that of higher plants. This was proved by the result of BN-gel analysis of thylakoid membranes from spinach and B. corticulans. (Figure 3), from which, the apparent molecular mass of the PSI–LHCI from B. corticulans and spinach were found to be 740 and 530 kDa, respectively. The crystal structure of pea PSI–LHCI has shown that it contains one copy of each of Lhca 1–4, giving rise to a molecular mass of 150 kDa, and the total molecular mass of the PSI–LHCI super-complex was around 525 kDa (Ben-Shem et al. 2003). Assuming an average molecular mass of 37.5 kDa for an Lhca complex (including that of apoprotein and pigments) and 380 kDa for the PSI core complex, there should be 9.6 Lhcas per B. corticulans PSI–LHCI super-complex.
a BN-gel of thylakoid membranes of spinach and B. corticulans. Molecular masses of high molecular weight protein makers (GE 17-0445-01) were indicated at the left of the gel. b Identification of the pigment–protein complexes of B. corticulans thylakoid membranes resolved from the BN-gel by two-dimensional SDS-PAGE analysis
The absorption spectra of B. corticulans PSI–LHCI prepared with 1.2 % β-DDM was compared with PSI–LHCI of spinach (Fig. 2b). Differential spectrum between the two species revealed that they differed in three aspects. (i) Two major positive peaks were found at 470 and 650 nm, suggesting that PSI–LHCI of B. corticulans contains more Chl b molecules than that of spinach. (ii) Broad positive absorptions were found in the wavelength region of 510–560 nm, which is originated from the characteristic pigments of siphonaxanthin and siphonein bound in B. corticulans PSI–LHCI (see below). (iii) There was an obvious negative peak at around 690 nm and a small shoulder at 700 nm. The component(s) contributed to the negative peak at 690 nm is not known, whereas the smaller shoulder at 700 nm may be caused by the presence of less red Chls (absorbing light at longer wavelengths than P700) in the PSI–LHCI of B. corticulans than that of spinach. This is in agreement with the features of fluorescence emission spectra of PSI–LHCI from B. corticulans, where it showed a wide peak at around 716 nm (Fig. 2a). This peak wavelength was significantly shorter than that of higher plant PSI–LHCI, which usually showed a major emission peak longer than 730 nm (Kuang et al. 1984; Lam et al. 1984; Jansson et al. 1996). These results indicate that the PSI–LHCI of B. corticulans has less red Chls than that of spinach PSI–LHCI.
Isolation and characterization of PSI core and LHCI subcomplexes
In order to separate the PSI core complex and LHCI sub-fractions from B. corticulans PSI–LHCI, we used a combination of β-DDM and zw 3–16 similar to that used for solubilization of higher plant PSI–LHCI. As shown in Fig. 4a, in the presence of 0.5 % β-DDM, increasing the concentration of zw 3–16 led to the effective separation of LHCI from the PSI–LHCI super-complex. When the concentration of zw 3–16 was increased to 0.75 %, most of LHCI (Lhca monomers) was dissociated from the super-complex (Fig. 4a, tube 5). The polypeptide composition of the sub-fractions was analyzed by SDS-PAGE. By comparison between partially solubilized PSI–LHCI with native PSI–LHCI, it became clear that Lhca-e, Lhca-f, and Lhca-h were dissociated from the PSI–LHCI super-complex more easily than other Lhca proteins.
a Separation of LHCI and the PSI core complex by sucrose density gradient ultracentrifugation. A combination of 0.5 % (w/v) β-DDM and zw 3-16 at different concentrations were used for solubilization of PSI–LHCI before the ultracentrifugation. b Polypeptide composition of the sub-fractions obtained from the sucrose density gradient analyzed by SDS-PAGE
The purified PSI core and LHCI complexes were characterized by their absorption spectra and 77 K fluorescence emission spectra. The core complex and LHCI have absorption maxima at 678 and 670 nm, respectively, in the red region (Fig. 5a). Differential spectrum (Fig. 5a) between the PSI core from B. corticulans and spinach revealed that: (i) There were still two small positive peaks at 465 and 650 nm attributable to Chl b, which may be due to slight contaminations of Lhcas (Lhca-a, Lhca-b, Lhca-c, Lhca-d) in the PSI core during the preparation (Fig. 4b). (ii) The absorption spectra of the two complexes in the red region were similar, except the presence of a small minus peak at 690 nm which was also observed in the PSI–LHCI super-complex (Fig. 2b). (iii) A minus peak appeared at 506 nm, suggesting the presence of less carotenes in the B. corticulans PSI core compared with that of spinach PSI. Indeed, our pigment analysis revealed that B. corticulans PSI core bound around 15 carotene molecules, which was less than the 22 carotene molecules bound in the spinach PSI core complex (Qin et al. 2006).
Absorption spectra at room temperature (a) and low-temperature (77 K) fluorescence emission spectra (b) of the PSI core complex (Fig. 4, tube 5), LHCI (Fig. 4, tube 5), Lhca oligomer-1 (Fig. 6), and Lhca oligomer-2 (Fig. 6). The absorption spectra were normalized with respect to their maximal absorption at the red region, and the differential absorption spectrum of B. corticulans PSI core complex minus spinach PSI core complex was shown. The fluorescence spectra were normalized with respect to their main emission peaks
The PSI core complex of B. corticulans flucroscenced at 718 nm with a shoulder at 685 nm, whereas LHCI flucroscenced at 677 nm (Fig. 5b). Therefore, the isolated B. corticulans LHCI did not show the characteristic emission of red Chls, which is a distinct feature of higher plant LHCI (Kuang et al. 1984).
In order to further characterize its sub-complex organization, PSI–LHCI was treated with a low concentration of SDS and separated by a green gel, which gave rise to six bands. These six bands were identified to be undissociated PSI–LHCI, Lhca oligomer-1, the Lhca oligomer-2, the PSI core complex (mainly the largest protein subunits of the PSI core complex, PsaA, and PsaB), Lhca monomer, and free pigments, respectively, by a second-dimensional SDS-PAGE (Fig. 6). It was notable that two different Lhca oligomers with molecular masses slightly larger than the PSI core complex appeared in the green gel. While both Lhca oligmoers have the same absorption maxima at 670 nm, Lhca oligomer-1 fluorescenced at 684 nm, which was 2.5 nm red-shifted compared to Lhca oligomer-2, and 7 nm red-shifted compared to Lhca monomers (Fig. 5). Significantly, the larger Lhca oligomer-1 contained all Lhca proteins except Lhca-g, whereas the smaller Lhca oligomer-2 showed a further loss of Lhca-h. This indicates that Lhca-g and Lhca-h were dissociated from the PSI–LHCI super-complex more easily than the other Lhca proteins, which seemed to be in contradiction with what has been observed upon solubilization of PSI–LHCI with β-DDM and zw 3–16 described above (Fig. 4), where Lhca-e, Lhca-f, and Lhca-h were released from the PSI–LHCI super-complex more easily than the other Lhca proteins. This discrepancy may be explained as follows. Lhca-e, Lhca-f, Lhca-h may be associated with the outside of the PSI–LHCI complex as proposed for the C. reinhardtii PSI–LHCI, and treatment with a combination of β-DDM and zw 3-16 resulted in their dissociation from the PSI–LHCI super-complex while leaving the other Lhca subunits attached to the PSI core. On the other hand, Lhca-g may bind to LHCI rather weakly in the interface between LHCI and PSI core, and low amount of SDS treatment may have preferentially disrupted the interactions between LHCI and PSI core, leading to the exposure and dissociation of this subunit (and Lhca-h) from the isolated LHCI complex.
Identification of the composition of pigment-protein complexes from B. corticulans PSI–LHCI by green gel (a) and two-dimensional gel electrophoresis (b). Six bands were visualized in the green gel of PSI–LHCI, and they were identified as PSI–LHCI, Lhca oligomer-1, Lhca oligomer-2, the core complex, Lhca monomer, and free pigments, respectively (see text for more details)
Pigment composition of B. corticulans PSI–LHCI and its sub-complexes
The pigment composition of PSI–LHCI, PSI core, and LHCI was analyzed by HPLC (Fig. 7a). In the HPLC chromatogram of B. corticulans PSI–LHCI, nine peaks were observed, among which, peaks number 4, 6, and 7 are violaxanthin, Chl b, and Chl a, respectively. Peak 1 and peak 5 were identified as siphonaxanthin and siphonein, respectively (Wang et al. 2013). Peak 3 was identified as 9′-cis neoxanthin, which was also found in LHCII of higher plants (Liu et al. 2004; Standfuss et al. 2005) and B. corticulans (Wang et al. 2013), by comparing their retention time and absorption spectrum (Fig. 7b) with those of the standard pigment. The component of peak 2, with retention time between siphonaxanthin and 9′-cis neoxanthin, was very unique and was identified as all-trans neoxanthin, because its absorption spectrum (Fig. 7b), retention time, and the specific fine structure of the absorption spectrum (namely, the ratio of the peak heights from the trough between the longest (470.4 nm) and the middle wavelength (441.3 nm) absorption peaks [Davies 1976; Takaichi and Shimada 1992]) were similar to those of an authentic all-trans form of neoxanthin obtained from pumpkin (Cucurbita sp.) fruit (Takaichi and Mimuro 1998). Absorption maxima of the all-trans neoxanthin from the HPLC eluant were located at 441.3 and 470.4 nm (Fig. 7b), whereas those of 9′-cis neoxanthin were located at 437.7 and 465.5 nm, indicating that absorption of the all-trans neoxanthin was red-shifted by around 5 nm relative to that of the 9′-cis form, which is in agreement with the previous reports (Parry and Horgan 1992; Yoshii et al. 2003). Peaks 8 and 9 should be some kinds of carotenes according to their retention time. Since a small part of ε-carotene has been found in the pigments of B. corticulans (Strain 1951; Chapman and Haxo 1963) and another siphonaceous green alga (Benson and Cobb 1981), and the absorption spectrum of peak 8 (Fig. 7b) was in agreement with that reported for ε-carotene earlier (Strain and Manning 1943; Strain 1951; Chapman and Haxo 1963), the component of peak 8 was identified as ε-carotene. On the other hand, α-carotene usually predominates over β-carotene in siphonaceous green algae (Strain 1951). Therefore, peak 9 with a retention time shorter than β-carotene, was identified as α-carotene by comparing its retention time and absorption spectra with literature data (Strain 1951; Benson and Cobb 1981; Young and Britton 1989).
a HPLC chromatograms of PSI–LHCI, PSI core, and LHCI showing elution peaks of chlorophylls and carotenoids. The pigments were detected by their absorbance at 445 nm. The peaks obtained were identified as follows (see text for more details): 1 Sx (Siphonaxanthin), 2 all-trans neo (neoxanthin), 3 9′-cis neo (neoxanthin), 4 Vio (violaxanthin), 5 Sn (Siphonein), 6 Chl b; 7, Chl a, 8 ε-carotene, 9 α-carotene. b Absorption spectra of pigments of peak 2 (all-trans neoxanthin), peak 3 (9′-cis neoxanthin), peak 8 (ε-carotene), peak 9 (α-carotene) in the HPLC eluate. c Pigment composition of PSI–LHCI, the core complex, and LHCI of B. corticulans. For PSI–LHCI and PSI core, the numbers of pigments are given on the basis of one P700. For LHCI, the numbers of pigments showing average values of all Lhca monomers are given on the basis of one apoprotein. The results are average values (with their SD) from three separate experiments. ND not detected
The above results indicated that B. corticulans PSI–LHCI contained Chl a, Chl b, and 7 kinds of carotenoids (siphonaxanthin, all-trans neoxanthin, 9′-cis neoxanthin, violaxanthin, siphonein, ε-carotene and α-carotene). All of the 7 kinds of carotenoids were present in the purified LHCI complex, whereas only siphonein, ε-carotene and α-carotene were found in the PSI core complex. From the chromatogram of HPLC, however, the peak of siphonein from the PSI core samples was very small, suggesting that it may be due to the slight contamination of LHCI present in the PSI core. This indicates that only ε-carotene and α-carotene were associated with the PSI core. The presence of siphonaxanthin and its ester siphonein in LHCI is characteristic of siphonaceous green algae (Strain 1951), and they have also been found in LHCII from B. corticulans (Wang et al. 2013).
The pigment-to-protein stoichiometry of each isolated complex was determined by protein quantification in combination with pigment analysis. It was shown that PSI–LHCI bound 173.37 ± 15.77 Chl a, 65.15 ± 7.18 Chl b, 103.80 ± 11.88 carotenoids; the PSI core complex bound 97.01 ± 3.72 Chl a, 8.42 ± 0.08 Chl b, 13.84 ± 0.60 α-carotene, 1.47 ± 0.16 ε-carotene, and 2.26 ± 0.26 siphonein; and each Lhca protein bound an average of 8.00 ± 0.50 Chl a, 5.14 ± 0.34 Chl b, and 7.50 ± 1.01 carotenoids. These results are in good agreement with the number of 9.6 Lhca copies present per PSI core estimated by BN-PAGE analysis described above.
Discussion
In this work, we isolated the PSI–LHCI super-complex from B. corticulans after detergent solubilization of thylakoid membranes, which were further disintegrated into PSI core and Lhca antennas by sucrose density gradient centrifugation after solubilization of PSI–LHCI with β-DDM and zw 3–16. These complexes were characterized by absorption and fluorescence spectra, electrophoresis, pigment analyses, etc. Furthermore, two Lhca oligomers with different molecular masses were obtained by green gel after solubilization of PSI–LHCI with a low concentration of SDS, suggesting that the association between Lhca monomers is flexible and not very tight under some conditions. In the following, we discuss the characteristics of these complexes from the unique green alga in comparison with those of the well-studied green alga C. reinhardtii and higher plants.
Organization of Lhca antennas in PSI–LHCI of B. corticulans
The most recent report on PSI–LHCI from the green alga C. reinhardtii showed that there are 9 Lhca antenna proteins associated at one side of the PSI core arranged in a double half-ring fashion, and the discrepancy reported for the Lhca/PSI core ratio of C. reinhardtii PSI–LHCI may have resulted from a relatively loose association between some of the antenna subunits to the core (Drop et al. 2011). B. corticulans belongs to the same green algae group, and we found that there were 9.6 copies of Lhca proteins per PSI core in its PSI–LHCI super-complex according to the BN-gel, protein and pigment quantification analyses, which is very similar to the number reported for the C. reinhardtii PSI–LHCI. SDS-PAGE, however, revealed 8 Lhca apo-proteins with different apparent molecular masses in the B. corticulans PSI–LHCI. This may be due to either the presence of more than one copy of some Lhca proteins in the B. corticulans PSI–LHCI, or overlap of some Lhca bands in the SDS-PAGE gel, resulting in the failure of complete identification of the Lhca proteins. In any case, it is clear that green algal PSI contained a much larger number of Lhca subunits than that found in higher plant PSI.
Solubilization of PSI–LHCI with β-DDM and zw 3–16 led to the dissociation of Lhca from PSI–LHCI, giving rise to the PSI core complex. In addition, partially solubilized PSI–LHCI complexes were obtained, from which Lhca-e, Lhca-f, and Lhca-h were found to be lost preferentially. This suggests that these 3 subunits may be located at the outside of the PSI–LHCI assuming LHCI is arranged in a double half-ring fashion as that in C. reinhardtii (Drop et al. 2011). On the other hand, solubilization of the B. corticulans PSI–LHCI with a low concentration of SDS resulted in the separation of LHCI and PSI core and the formation of large Lhca oligomers 1 and 2, where Lhca-g were found to be lost preferentially in addition to Lhca-h. This suggests that Lhca-g is located in the interface between LHCI and PSI core, and the separation of them led to the exposure and preferential loss of this subunit.
Red Chls in the B. corticulans PSI–LHCI super-complex
Red Chls have been found in both the core complex and LHCI of higher plant PSI–LHCI (van Grondelle et al. 1994), with most of them being associated with LHCI (Croce et al. 1998). Although red Chls accounts for a small part (3–10 %) of the total Chls, they have significant effects on the energy transfer and trapping in the whole PSI complex (Gobets and van Grondelle 2011). The fluorescence peak of higher plant PSI core was located at about 720 nm, and binding of LHCI red-shifted the peak to more than 730 nm (Kuang et al. 1984; Lam et al. 1984; Qin et al. 2006). However, this was not the case in B. corticulans PSI–LHCI. The fluorescence peak of B. corticulans PSI core complex was located at 718 nm, and the light-harvesting systems (both Lhca monomers and oligomers) contributed less to the long wavelength fluorescence emission (Fig. 5b), leading to the observation that binding of LHCI blue-shifted the peak to 716 nm (Fig. 2a). In addition, B. corticulans PSI–LHCI exhibited another fluorescence peak at 685 nm. PSI–LHCI from a red alga Cyanidium caldarium has been reported to exhibit two main fluorescence peaks at 677 and 725 nm, and the former was originated from LHCI. These characteristics are somewhat analogous to the fluorescence features of B. corticulans PSI–LHCI. It is worth to mention that the red alga has been reported to adapt to different light intensities by changing the size of PSI antennas (Gardian et al. 2007). Since higher plant PSI–LHCI does not have a distinct peak at around 685 nm, and C. reinhardtii PSI–LHCI usually shows a main peak at 712–715 nm and sometimes a shoulder at 685 nm depending on preparations (Kargul et al. 2003; Takahashi et al. 2004; Drop et al. 2011), the observation of the peak at 685 nm from the B. corticulans PSI–LHCI may imply that some Lhca antennas lack the red Chls, and at 77 K the energy absorbed by them could not be transferred to any red Chls within the PSI–LHCI pigment network. Additionally, the absorption of spinach PSI–LHCI at around 700 nm was higher than that of B. corticulans PSI–LHCI (Fig. 2b), whereas the absorption of spinach PSI core complex in the same wavelength region was similar to that of B. corticulans PSI core (Fig. 5a). These results suggested that most of the red Chls of B. corticulans PSI–LHCI originate from the core complex, but not the outer light-harvesting systems.
PSI core complex with α-carotenes
The cofactors of the electron transport chain, antenna chlorophylls, and structure of the PSI core complex have been highly conserved during the long period of evolution (Ben-Shem et al. 2003; Nelson and Ben-Shem 2005), and the functional flexibility of PSI–LHCI to acclimate to a wide range of environmental conditions has been considered to rely mainly on the flexible antenna systems (Ben-Shem et al. 2004). However, the present results showed that the PSI core complex of B. corticulans contained a large amount of α-carotene as well as a small amount of ε-carotene, instead of β-carotenes found in higher plant and most cyanobacterial PSI core, indicating the variability of carotenoids in the PSI core among different organisms. This was the first report for an α-carotene-type PSI core complex among photosynthetic eukaryotes. Among photosynthetic prokaryotes, only cyanobacteria, Acaryochloris, and Prochlorococcus have been reported to synthesize α-carotene (Chisholm et al. 1988; Miyashita et al. 1996; Takaichi et al. 2012). Both Acaryochloris PSI and Prochlorococcus PSI contain significant amounts of α-carotene, but not β-carotene. However, the former contained Chl d (Hu et al. 1998; Tomo et al. 2008) and the latter contained divinyl-Chl a and divinyl-Chl b (Garczarek et al. 1998), indicating great differences in the pigment composition of PSI core between these cyanobacteria and B. corticulans.
Carotenoids perform two crucial functions in photosynthetic organisms. One is the absorption of light in the blue-green region where chlorophylls display little absorption, and transfer of the energy to neighboring chlorophylls to increase the absorption cross-section for photosynthesis (Siefermann-Harms 1985; Frank and Cogdell 1993). The other one is protection of the photosynthetic apparatus under excess light conditions by quenching triplet-state chlorophyll and singlet oxygen (Griffiths et al. 1955; Foote et al. 1970; Fraser et al. 2001). The β-carotene, α-carotene, and ε-carotene, also known as β, β-carotene, β, ε-carotene, and ε, ε-carotene, respectively (Davies 1976), differ in the configuration of the end groups and, therefore, in the degree of conjugations. In the ε end groups, the 4, 5 double bond is not in conjugation with the other double bonds of the polyene chain, which means that the number of conjugated double bond is decreased by one for the change of each β end group to the ε form. Thus, the numbers of conjugated double bonds of β-carotene, α-carotene, and ε-carotene are 11, 10, and 9, respectively, which resulted in the shift of absorption peaks to shorter wavelengths in the same order.
According to the 2.5 Å crystal structure of cyanobacterial PSI, 22 β-carotene molecules are found in the core complex. These carotenes were bound at the surfaces of PsaA, PsaB, and several small subunits (Jordan et al. 2001) and are suggested to play a structural role as a “hydrophobic glue” in stabilizing the multi-subunit PSI core complex by interactions with their surrounding chlorophylls and aromatic residues (Fromme et al. 2003; Wang et al. 2004). Two important implications for the replacement of β-carotene by α-carotene and ε-carotene can be considered. First, it indicates that the carotenes of PSI core complex were not completely conserved as expected previously, and the microenvironments of the binding sites for carotenes, as well as the interactions with surrounding chlorophylls and aromatic residues, may have been changed. Secondly, compared with higher plant PSI, the presence of more Chl b and siphonaxanthin, siphonein in B. corticulans PSI–LHCI (Fig. 2b) facilitates absorption in the blue-green and green light region, which is crucial for B. corticulans to live in the intertidal zone, where visible light is largely attenuated except those in the blue-green and green light region. In addition, photosynthetic apparatus has to be protected from strong light when the tide recedes. The PSI core complex containing α-carotenes may have an advantage of light-harvesting and photoprotection in the unique light conditions, although its exact mechanism is still unknown.
The regulation of light harvesting and photoprotection by carotenoids requires a close proximity and proper orientation of the carotenoids to chlorophyll molecules. Carotenoids have the ground state, S0, and two excited states S1 and S2. The energy levels of the S1 and S2 states depend on the number of conjugated double bonds (Polívka and Frank 2010). Generally, Car(S2)-to-(B)Chl(Qx) energy transfer operates in almost all light-harvesting systems regardless of the length of the conjugation; whereas Car(S1)-to-(B)Chl(Qy) energy transfer depends on the conjugation length of carotenoids, and the S1 pathway happens only when carotenoids have a short conjugation or the acceptor chlorophyll has a lower energy (Polívka and Frank 2010). In LH2 complexes from Rhodospirillum molischianum, Rhodobactor sphaeroides 2.4.1, and Rb. sphaeroides G1C, the rate and efficiency of the Car(S1)-to-BChl(Qy) energy transfer increase systematically with the decrease in the number of conjugated double bonds (Zhang et al. 2000). In the PSI core complex that contains β-carotene and some red Chls, the efficiency of the S1 pathway did not exceed 20 % (de Weerd et al. 2003). The present results may suggest that the efficiency of Car(S1)-to-Chl(Qy) energy transfer in the PSI core complex from B. corticulans may be higher than that from cyanobacteria and higher plants. Changes in the conjugation length of carotenes in the B. corticulans PSI core may therefore optimize the harvest of light energy, and/or photoprotection under the complicated environment where the organism inhabits. Future research may elucidate the physiological importance of the replacement of β-carotene by α-carotene and ε-carotene.
The unique pigment composition of B. corticulans LHCI
The evolution of light-harvesting antennas enabled photosynthetic organisms to utilize light energy efficiently under a wide range of light conditions (Amunts et al. 2008). In the crystal structure of higher plant LHCII, 8 Chl a, 6 Chl b, 4 carotenoids (2 luteins, 1 9′-cis neoxanthin, and 1 mixed xanthophyll-cycle carotenoids) were found in each monomer of LHCII (Liu et al. 2004; Standfuss et al. 2005). However, we recently reported that the siphonaxanthin-type LHCII from B. corticulans contained 6 Chl a, 8 Chl b, and 4 carotenoids (1 neoxanthin, and 3 siphonaxanthin and siphonein, but no lutein and violaxanthin) in a monomer (Wang et al. 2013), indicating significant differences in the pigment composition of LHCII between the siphonaxanthin-type green algae and higher plants. Analysis of pigment composition of Lhca antenna from higher plants revealed that each Lhca antenna contained 2–4 carotenoids, including lutein, violaxanthin, and β-carotene, but no 9′-cis neoxanthin (Schmid et al. 2002; Qin et al. 2006). On the other hand, 7 kinds of carotenoids (siphonaxanthin, all-trans neoxanthin, 9′-cis neoxanthin, violaxanthin, siphonein, ε-carotene, and α-carotene) were found in the Lhca antennas of B. corticulans, which was far beyond our expectation.
Notably, it is the first time to show that both all-trans neoxanthin and 9′-cis neoxanthin are present in photosynthetic pigment-protein complexes. The 9′-cis neoxanthin had been considered to be the only form of neoxanthin in photosynthetic organs (Takaichi and Mimuro 1998) until Yoshii et al. (2003) reported that all-trans neoxanthin, but not 9′-cis neoxanthin, was one of the photosynthetic pigments of Mesostigma Viride, a primitive green alga. In the carotenogenesis pathway, 9′-cis neoxanthin may be synthesized from all-trans neoxanthin by cis-isomerase, or from 9-cis violaxanthin by neoxanthin synthase (Takaichi and Mimuro 1998). B. corticulans probably retains an intermediate form of the neoxanthin biosynthesis pathway between primitive green algae and higher green algae and plants. However, the physiological importance of coexistence of both carotenoids remains to be elucidated.
Another feature of the B. corticulans Lhca antennas was the high carotenoid:Chl ratio of around 7.5:13. Although there were four carotenoid binding sites resolved in the higher plant LHCII structure, some members of the LHC superfamily that are predicted to have the same three transmembrane helices and to fold similarly with higher plant LHCII, indeed contained more carotenoids than four. Lhcf (FCP), the intrinsic LHC of haptophyte and heterokont algae, and Lhcd (iPCP), the intrinsic LHC of dinoflagellates are known for their high carotenoid:Chl ratio (Macpherson and Hiller 2003). FCP uses fucoxanthin as the main carotenoid; for example, FCP from Laminaria saccharina contained Chl a, Chl c, and fucoxanthin in a ratio of 6:2:7 (Martino et al. 2000), whereas iPCP uses peridinin as the primary carotenoid, and the ratio of peridinin to Chl a is typically 0.9–2.0 (Macpherson and Hiller 2003). One common point among these LHC antennas with high carotenoid:Chl ratio is that all of the carotenoids have conjugated carbonyl groups, such as peridinin, fucoxanthin, and siphonaxanthin. These carotenoids have special photophysical characteristics and may contribute to expand the absorption region of the light-harvesting systems (Zigmantas et al. 2004; Polívka and Sundström 2004; Premvardhan et al. 2005, 2008; Polívka et al. 2006), which is indispensable for algae living in complicated environments (Wang et al. 2013). The presence of so many kinds of carotenoids in the B. corticulans Lhca antennas may suggest that they are required to fine tune the energy harvesting and transfer networks in order to acclimate to the ever-changing light conditions; and future research may elucidate the functions of these carotenoids in the energy transfer processes of B. corticulans Lhca antennas.
Conclusions
The present study revealed a number of unique features for the PSI–LHCI and its sub-complexes PSI core and LHCI from B. corticulans, which can be summarized as follows: (1) B. corticulans PSI–LHCI contained 9–10 Lhca subunits, which is much more than that found in higher plant PSI–LHCI but similar to that found for another green alga C. reinhardtii, suggesting that the large number of Lhca subunits is a common feature for green algal PSI–LHCI. (2) Among the Lhca subunits, Lhca-e, Lhca-f, and Lhca-h were found to be lost preferentially from the PSI–LHCI super-complex, suggesting that they may be associated at the outer surface of the super-complex, presumably in the outer ring if the LHCI is arranged in a double half-ring fashion as proposed for C. reinhardtii. On the other hand, Lhca-g (together with Lhca-h) was lost in a LHCI oligomer when it is detached from the PSI core, suggesting that Lhca-g is associated in the interface between LHCI and PSI core. (3) B. corticulans PSI–LHCI contained less red Chls compared with that of spinach, most of them are originated from the PSI core and almost no red Chls were found in its LHCI. (4) Importantly, the B. corticulans PSI core contained a large amount of α-carotenes and a small amount of ε-carotenes, but no β-carotenes. This is the first report for an α-carotene-type PSI core from eukaryotic photosynthetic organisms. In addition, Lhca antennas bound 7 kinds of carotenoids (siphonaxanthin, all-trans neoxanthin, 9′-cis neoxanthin, violaxanthin, siphonein, ε-carotene, and α-carotene) and show a high carotenoid:Chl ratio of around 7.5:13. These features may be related with the requirement for fine tuning of the energy transfer network under ever-changing light conditions that this alga inhabit, and also suggest structural flexibilities of the PSI core complex and Lhca antennas. Future researches may elucidate the characteristics of energy transfer processes happening in the core complex, Lhca antennas and the whole PSI–LHCI super-complex of B. corticulans.
Abbreviations
- Chl:
-
Chlorophyll
- β-DDM:
-
n-Dodecyl-β-d-maltopyranoside
- HPLC:
-
High-performance liquid chromatography
- LHCI:
-
Light-harvesting complex of photosystem I
- LHCII:
-
Light-harvesting complex of photosystem II
- PAGE:
-
Polyacrylamide gel electrophoresis
- PSI:
-
Photosystem I
- SDS:
-
Sodium dodecyl sulfate
- zw 3–16:
-
3-(N,N-dimethylpalmitylammonio) propanesulfonate
References
Amunts A, Drory O, Nelson N (2007) The structure of a plant photosystem I supercomplex at 3.4 Å resolution. Nature 447:58–63
Amunts A, Drory O, Nelson N (2008) A glimpse into the atomic structure of plant photosystem I. In: Fromme P (ed) Photosynthetic protein complexes: a structural approach. Wiley, Weinheim, pp 65–81
Anderson JM (1985) Chlorophyll-protein complexes of a marine green alga, Codium species (Siphonales). Biochim Biophys Acta 806:145–153
Anderson JM, Waldron JC, Thorne SW (1978) Chlorophyll-protein complexes of spinach and barley thylakoids. FEBS Lett 92:227–233
Arnon DI (1949) Copper enzymes in isolated chloroplasts: polyphenyloxidase in Beta vulgaris. Plant Physiol 14:1–15
Ben-Shem A, Frolow F, Nelson N (2003) Crystal structure of plant photosystem I. Nature 426:630–635
Ben-Shem A, Frolow F, Nelson N (2004) Light-harvesting features revealed by the structure of plant Photosystem I. Photosynth Res 81:239–250
Benson E, Cobb AH (1981) The separation, identification and quantitative determination of photopigments from the siphonaceous marine alga Codium fragile. New Phytol 88:627–632
Bibby TS, Nield J, Barber J (2001) Iron deficiency induces the formation of an antenna ring around trimeric photosystem I in cyanobacteria. Nature 412:743–745
Boekema EJ, Hifney A, Yakushevska AE, Piotrowski M, Keegstra W, Berry S, Michel KP, Pistorius EK, Kruip J (2001) A giant chlorophyll–protein complex induced by iron deficiency in cyanobacteria. Nature 412:745–748
Busch A, Hippler M (2011) The structure and function of eukaryotic photosystem I. Biochim Biophys Acta 1807:864–877
Chapman DJ, Haxo FT (1963) Identity of ε-carotene and ε 1-carotene. Plant Cell Physiol 4:57–63
Chisholm SW, Olson RJ, Zettler ER, Goericker R, Waterbury JB, Welschmeyer NA (1988) A novel free-living prochlorophyte abundant in the oceanic euphotic zone. Nature 334:340–343
Croce R, Zucchelli G, Garlaschi FM, Jennings RC (1998) A thermal broadening study of the antenna chlorophylls in PSI-200, LHCI, and PSI core. Biochemistry 37:17355–17360
Davies BH (1976) Carotenoids. In: Goodwin TW (ed) Chemistry and biochemistry of plant pigments, vol 2. Academic Press, London, pp 38–165
de Weerd FL, Kennis JTM, Dekker JP, van Grondelle R (2003) β-Carotene to chlorophyll singlet energy transfer in the photosystem I core of Synechococcus elongatus proceeds via the β- carotene S2 and S1 states. J Phys Chem B 107:5995–6002
Drop B, Webber-Birungi M, Fusetti F, Kouřil R, Redding KE, Boekema EJ, Croce R (2011) Photosystem I of Chlamydomonas reinhardtii contains nine light-harvesting complexes (Lhca) located on one side of the core. J Biol Chem 286:44878–44887
Foote CS, Chang YC, Denny RW (1970) Chemistry of singlet oxygen. X. Carotenoid quenching parallels biological protection. J Am Chem Soc 92:5216–5218
Frank H, Cogdell RJ (1993) Photochemistry and function of carotenoids in photosynthesis. In: Young AJ, Britton G (eds) Carotenoids in photosynthesis. Chapman and Hall, London, pp 253–326
Fraser NJ, Hashimoto H, Cogdell RJ (2001) Carotenoids and bacterial photosynthesis: the story so far. Photosynth Res 70:249–256
Fromme P, Schlodder E, Jansson A (2003) Structure and function of the antenna system in photosystem I. In: Green BR, Parson WW (eds) Light-harvesting antennas in photosynthesis. Kluwer Academic Publishers, Dordrecht/Boston/London, pp 253–279
Garczarek L, van der Staay GWM, Thomas JC, Partensky F (1998) Isolation and characterization of photosystem I from two strains of the marine oxychlorobacterium Prochlorococcus. Photosynth Res 56:131–141
Gardian Z, Bumba L, Schrofel A, Herbstova M, Nebesarova J, Vacha F (2007) Organisation of Photosystem I and Photosystem II in red alga Cyanidiumcaldarium: encounter of cyanobacterial and higher plant concepts. Biochim Biophys Acta 1767:725–731
Germano M, Yakushevska AE, Keegstra W, van Gorkom HJ, Dekker JP, Boekema EJ (2002) Supramolecular organization of photosystem I and light-harvesting complex I in Chlamydomonas reinhardtii. FEBS Lett 525:121–125
Gobets B, van Grondelle R (2011) Energy transfer and trapping in photosystem I. Biochim Biophys Acta 1507:80–99
Griffiths M, Sistrom WR, Cohen-Battire G, Stanier RY (1955) Function of carotenoids in photosynthesis. Nature 176:1211–1214
Hu Q, Miyashita H, Iwasaki I, Kurano N, Miyachi S, Iwaki M, Itoh S (1998) A photosystem I reaction center driven by chlorophyll d in oxygenic photosynthesis. Proc Natl Acad Sci USA 95:13319–13323
Ikeuchi M, Inoue Y (1988) A new 4.8-kDa polypeptide intrinsic to the PSII reaction center, as revealed by modified SDS-PAGE with improved resolution of low-molecular-weight proteins. Plant Cell Physiol 29:1233–1239
Jansson S (1994) The light-harvesting chlorophyll a/b binding-proteins. Biochim Biophys Acta 1184:1–19
Jansson S, Andersen B, Scheller HV (1996) Nearest-neighbor analysis of higher-plant photosystem I holocomplex. Plant Physiol 112:409–420
Jordan P, Fromme P, Witt HT, Klukas O, Saenger W, Krauβ N (2001) Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution. Nature 411:909–917
Kargul J, Nield J, Barber J (2003) Three-dimensional reconstruction of a light-harvesting complex I-photosystem I (LHCI-PSI) supercomplex from the green alga Chlamydomonas reinhardtii. J Biol Chem 278:16135–16141
Kargul J, Turkina MV, Nield J, Benson S, Vener AV, Barber J (2005) Light-harvesting complex II protein CP29 binds to photosystem I of Chlamydomonas reinhardtii under state 2 conditions. FEBS J 272:4797–4806
Kuang TY, Argyroudi-Akoyunoglou JH, Nakatani HY, Watson J, Arntzen CJ (1984) The origin of the long-wavelengthfluorescence emission band (77 K) from photosystem I. Arch Biochem Biophys 235:618–627
Lam E, Ortiz W, Malkin R (1984) Chlorophyll a/b proteins of photosystem I. FEBS Lett 168:10–14
Liu Z, Yan H, Wang K, Kuang T, Zhang J, Gui L, An X, Chang W (2004) Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 428:287–292
Macpherson AN, Hiller RG (2003) Light-harvesting systems in chlorophyll c-containing algae. In: Green BR, Parson W (eds) Light-harvesting antennas in photosynthesis. Kluwer Academic Publishers, The Netherlands, pp 324–352
Martino AD, Douady D, Quinet-Szely M, Rousseau B, Crépineau F, Apt K, Caron L (2000) The light-harvesting antenna of brown algae, highly homologous proteins encoded by a multigene family. Eur J Biochem 267:5540–5549
Melkozernov AN, Kargul J, Lin S, Barber J, Blankenship RE (2004) Energy coupling in the PSI–LHCI supercomplex from the green alga Chlamydomonas reinhardtii. J Phys Chem B 108:10547–10555
Miyashita H, Ikemoto H, Kurano N, Adachi K, Chihara M, Miyachi S (1996) Chlorophyll d as a major pigment. Nature 383:402
Nelson N, Ben-Shem A (2005) The structure of photosystem I and evolution of photosynthesis. BioEssays 27:914–922
Parry AD, Horgan R (1992) Abscisic and biosynthesis in root, 1. the identification of potential abscisic acid precursors, and other carotenoids. Planta 187:185–191
Påsllon L-O, Tjus SE, Andersson B, Gillbro T (1995) Ultrafast energy transfer dynamics resolved in isolated spinach lightharvesting complex I and the LHCI-730 subpopulation. Biochim Biophys Acta 1230:1–9
Pineaua B, Gérard-Hirne C, Selve C (2001) Carotenoid binding to photosystems I and II of Chlamydomonas reinhardtii cells grown under weak light or exposed to intense light. Plant Physiol Biochem 39:73–85
Polívka T, Frank HA (2010) Molecular factors controlling photosynthetic light harvesting by carotenoids. Accounts Chem Res 43:1125–1134
Polívka T, Sundström V (2004) Ultrafast dynamics of carotenoid excited states-from solution to natural and artificial systems. Chem Rev 104:2021–2071
Polívka T, van Stokkum IH, Zigmantas D, van Grondelle R, Sundström V, Hiller RG (2006) Energy transfer in the major intrinsic light-harvesting complex from Amphidinium carterae. Biochemistry 45:8516–8526
Premvardhan L, Papagiannakis E, Hiller RG, van Grondelle R (2005) The charge-transfer character of the S0 → S2 transition in the carotenoid peridinin is revealed by stark spectroscopy. J Phys Chem B 109:15589–15597
Premvardhan L, Sandberg DJ, Fey H, Birge RR, Buchel C, van Grondelle R (2008) The charge-transfer properties of the S2 State of fucoxanthin in solution and in fucoxanthin chlorophyll-a/c 2 protein (FCP) based on stark spectroscopy and molecular-orbital theory. J Phys Chem B 112:11838–11853
Qin X, Wang K, Chen X, Qu Y, Li L, Kuang T (2006) Rapid purification of photosystem I chlorophyll binding proteins by differential centrifugation and vertical rotor. Photosyn Res 90:195–204
Ricketts TR (1971) The structures of siphonein and siphonaxanthin from codium fragile. Phytochemistry 10:155–160
Rodriguez-Amaya DB (2001) A guide to carotenoid analysis in foods. OMNI Research. ILSI Press, Washington DC, pp 47
Schägger H, Cramer WA, von Jagow G (1994) Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal Biochem 217:220–230
Scheller HV, Jensen PE, Haldrup A, Lunde C, Knoetzel J (2001) Role of subunits in eukaryotic Photosystem I. Biochim Biophys Acta 1507:41–60
Schmid VHR, Potthast S, Wiener M, BergauerV, Paulsen H, Storf S (2002) Pigment binding of photosystem I light harvesting proteins. J Biol Chem 277: 37307-37314
Siefermann-Harms D (1985) The light-harvesting and protective functions of carotenoids in photosynthetic membranes. Physiol Plant 69:561–568
Standfuss J, Terwisscha van Scheltinga AC, Lamborghini M, Kühlbrandt W (2005) Mechanisms of photoprotection and nonphotochemical quenching in pea light harvesting complex at 2.5 Å resolution. EMBO J 24:919–928
Stauber EJ, Fink A, Markert C, Kruse O, Johanningmeier U, Hippler M (2003) Proteomics of Chlamydomonas reinhardtii light-harvesting proteins. Eukaryot Cell 2:978–994
Strain HH (1951) Pigments of algae. In: Smith GM (ed) Manual of phycology. Chronica Botanica, Waltham, pp 243–262
Strain HH, Manning WM (1943) A unique polyene pigment of the marine diatom Navicula torquatum. J Am Chem Soc 65:2258–2259
Takahashi Y, Yasui T, Stauber EJ, Hippler M (2004) Comparison of the subunit compositions of the PSI–LHCI supercomplex and the LHCI in the green alga Chlamydomonas reinhardtii. Biochemistry 43:7816–7823
Takahashi Y, Kodama N, Takahashi H (2008) Purification and characterization of photosystem I complex. Low Temp Sci 67:265–273
Takaichi S, Mimuro M (1998) Distribution and geometric isomerism of neoxanthin in oxygenic phototrophs: 9′-cis, a sole molecular form. Plant Cell Physiol 39:968–977
Takaichi S, Shimada K (1992) Characterization of carotenoids in photosynthetic bacteria. Methods Enzymol 213:374–385
Takaichi S, Mochimaru M, Uchida H, Murakami A, Hirose E, Maoka T, Tsuchiya T, Mimuro M (2012) Opposite chirality of a-carotene in unusual cyanobacteria with unique chlorophylls, Acaryochloris and Prochlorococcus. Plant Cell Physiol 53:1881–1888
Thayer SS, Björkman O (1990) Leaf xanthophyll content and composition in sun and shade determined by HPLC. Photosynth Res 23:331–343
Tomo T, Kato Y, Suzuki T, Akimoto S, Okubo T, Noguchi T, Hasegawa K, Tsuchiya T, Tanaka K, Fukuya M, Dohmae N, Watanabe T, Mimuro M (2008) Characterization of highly purified photosystem I complexes from the chlorophyll d- dominated cyanobacterium Acaryochloris marina MBIC 11017. J Biol Chem 26:18198–18209
van Grondelle R, Dekker JP, Gillbro T, Sundström V (1994) Energy transfer and trapping in photosynthesis. Biochim Biophys Acta 1187:1–65
Wang K-B (1991) The re-electrophoretic method for the eluted collection of chlorophyll–protein complexes. Chin Bull Bot 8:56–57
Wang Y, Mao L, Hu X (2004) Insight into the structural role of carotenoids in the photosystem I: a quantum chemical analysis. Biophys J 86:3097–3111
Wang W, Qin X, Sang M, Chen D, Wang K, Lin R, Lu C, Shen J-R, Kuang T (2013) Spectral and functional studies on siphonaxanthin-type light harvesting complex of photosystem II from Bryopsis corticulans. Photosynth Res 117:267–279
Wintermans JFGM, Mots AD (1965) Spectrophotometric characteristics of chlorophyll a and b and their pheophytins in ethanol. Biochim Biophys Acta 109:448–453
Yeremenko N, Kouřil R, Ihalainen JA, D’Haene S, van Oosterwijk N, Andrizhiyevskaya EG, Keegstra W, Dekker HL, Hagemann M, Boekema EJ, Matthijs HCP, Dekker JP (2004) Supramolecular organization and dual function of the IsiA chlorophyll-binding protein in cyanobacteria. Biochemistry 43:10308–10313
Yokohama Y, Hirata T, Misonou T, Tanaka J, Yokochi H (1992) Distribution of green light-harvesting pigments, siphonaxanthin and siphonein, and their precursors in marine green algae. J Phycol 40:25–31
Yoshii Y, Takaichi S, Maoka T, Inouye I (2003) Photosynthetic pigment composition in the primitive green alga Mesostigma viride (Prasinophyceae): phylogenetic and evolutionary implications. J Phycol 39:570–576
Young AJ, Britton G (1989) The distribution of α-carotene in the photosynthetic pigment–protein complexes of higher plants. Plant Sci 64:179–183
Zhang JP, Fujii R, Qian P, Inaba T, Mizoguchi T, Koyama Y, Onaka K, Watanabe Y (2000) Mechanism of the carotenoid-to-bacteriochlorophyll energy transfer via the S1 state in the LH2 complexes from purple bacteria. J Phys Chem B 104:3683–3691
Zigmantas D, Hiller R, Sharples F, Frank H, Sundström V, Polívka T (2004) Effect of a conjugated carbonyl group on the photophysical properties of carotenoids. Phys Chem Chem Phys 6:3009–3016
Acknowledgments
We thank Prof. Yuichiro Takahashi of Okayama University for providing the antibodies against C. reinhardtii Lhca-5 and Lhca-6. This work was supported by the National Basic Research Program of China (973 Program 2011CBA00901), and the National Natural Science Foundation (30900095).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xiaochun Qin and Wenda Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Qin, X., Wang, W., Chang, L. et al. Isolation and characterization of a PSI–LHCI super-complex and its sub-complexes from a siphonaceous marine green alga, Bryopsis Corticulans . Photosynth Res 123, 61–76 (2015). https://doi.org/10.1007/s11120-014-0039-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-014-0039-z