Abstract
Parachlorella kessleri is a unicellular alga which grows in fresh as well as marine water and is commercially important as biomass/lipid feedstock and in bioremediation. The present study describes the successful transformation of marine P. kessleri with the help of Agrobacterium tumefaciens. Transformed marine P. kessleri was able to tolerate more than 10 mg l−1 hygromycin concentration. Co-cultivation conditions were modulated to allow the simultaneous growth of both marine P. kessleri and A. tumefaciens. For co-cultivation, P. kessleri was shifted from Walne’s to tris acetate phosphate medium to reduce the antibiotic requirement during selection. In the present study, the transfer of T-DNA was successful without using acetosyringone. Biochemical and genetic analyses were performed for expression of transgenes by GUS assay and PCR in transformants. Establishment of this protocol would be useful in further genetic modification of oil-bearing Parachlorella species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The potential of algae as third-generation biofuels is being explored as the most effective alternative to fossil fuels. Mass-scale algal culturing can allow synergistic coupling of fuel generation, carbon sequestration and wastewater treatment resulting in sustainable energy conversion and utilisation. Algae biomass can be used directly as feedstock for biofuels or their high inherent oil content can be converted to biodiesel specifically (Brennan and Owende 2010; Chisti 2007). However, various biological as well as technological challenges need to be overcome to realise the true potential of any large-scale algal cultivation system (Hannon et al. 2010). Genetic modification can be one of the promising approaches to overcome the biological limitations of the algal system. In recent years, considerable advancements have been made on algal genetic modification (Hallmann 2007; Kiliana et al. 2011). However, this has been restricted mostly to the model system Chlamydomonas reinhardtii and few other freshwater species (Kumar et al. 2004; Cha et al. 2012; Kathiresan and Sarada 2009). Marine algal species are advantageous over freshwater ones as they can obviate the use of both freshwater and land for biomass/oil production. Establishment of a genetic manipulation system in potential marine species can provide a platform for further advancements in biofuel production.
Recently, the green algae class Chlorellaceae has been subdivided into two sister groups, i.e. Chlorella clade including ‘true’ Chlorella species and Parachlorella clade based on the nucleotide sequence of 18 S rRNA and ITS 2 region (Krienitz et al. 2004). Many algal species earlier described as Chlorella are hence being reclassified in the Parachlorella clade based on their molecular phylogenetic analysis. Parachlorella is a unicellular algal genus comprising different species of fresh as well as marine origin. Parachlorella algal species has also shown considerable potential in terms of oil accumulation (Ahmad et al. 2013; Li et al. 2013) and bioaccumulation of metals (Mahdavi et al. 2012) like its close relative genus Chlorella. These attributes make the genus Parachlorella an attractive system to develop genetic manipulation tools. However, the genus Parachlorella is comparatively a newly established clade and little information, either genetic or metabolic, is available for this genus. Development of a transformation method is the first basic step for any further genetic improvement for any species of interest. In the recent years, the use of Agrobacterium-mediated transformation has gained momentum after its successful demonstration in the model organism Chlamydomonas (Kumar et al. 2004). There have been reports of Agrobacterium-mediated transformation in freshwater Chlorella vulgaris and marine Schizochytrium sp. (Cha et al. 2012; Cheng et al. 2012). It has also been successfully developed for Haematococcus pluvialis (Kathiresan and Sarada 2009) and Dunaliella bardawil (Anila et al. 2011). This clearly indicates that both fresh and marine water algae are under the natural host range of Agrobacterium tumefaciens like plants. However, the use of Agrobacterium-mediated transformation for marine algal species poses a specific challenge as bacteria fail to grow under high salinity conditions and thus require cautious selection of a culture medium which supports both algal and Agrobacterium growth simultaneously. In the present study, the first successful transformation of T-DNA of A. tumefaciens in the marine Parachlorella kessleri identified as a promising algal species for its high oil content has been reported.
Materials and methods
Algal strain and culture conditions
The marine P. kessleri strain, originally isolated from the Bay of Bengal, India, was a generous gift from the Indian Institute of Technology, Chennai, and was confirmed by DNA barcoding (Ahmad et al. 2013). It was maintained in Walne’s medium (Walne 1970) for the present study. For transformation, it was grown and maintained on tris acetate phosphate (TAP) medium and afterwards transferred back to Walne’s medium for further analysis. The growth profile was studied on both TAP and Walne’s media in triplicates and the average values were taken for comparison. Cells were grown at 100 rpm under continuous light intensity of 75 μmol photons m−2 s−1 at 25 °C. Growth was assessed by measuring optical density at 750 nm.
Plasmid constructs and bacterial strains
A binary vector—pCAMBIA1301, which has a bacterial hpt and a GUS gene each under the 35S CaMV promoter, was used for transformation (Gift from ICGEB, Delhi). Plasmid was maintained and amplified in the Escherichia coli strain DH5α. pCAMBIA1301 was transferred to A. tumefaciens strain LBA4404 (octopine type) by electroporation using standard protocol and used for algal transformation studies.
Antibiotic sensitivity analysis for marine P. kessleri and A. tumefaciens
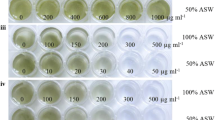
Hygromycin sensitivity study of marine P. kessleri was done on TAP agar medium with a concentration range of 2–20 mg l−1. 107 cells ml−1 were spread plated on TAP medium with different concentrations of hygromycin and kept in continuous light with 75 μmol m−2 s−1 at 25 °C for 2 weeks.
Cefotaxime sensitivity study for A. tumefaciens was done on TAP agar medium with a concentration range of 100–700 mg l−1. 106 cells ml−1 were spread plated on TAP medium with different concentrations of cefotaxime and incubated in the dark for 2 days at 28 °C.
Transformation
Transformations were done by co-cultivating marine P. kessleri with A. tumefaciens on TAP medium twice in triplicates. A cell density of 107 ml−1 of marine Parachlorella was plated onto TAP agar medium and incubated in light for 3 days to allow a lawn of cells to be grown. In the present study, acetosyringone (AS), an inducer of vir gene, was not used. An overnight grown A. tumefaciens culture in liquid YEM medium was pelleted and further resuspended in liquid TAP medium. Two hundred microlitres of this overnight grown bacterial suspension (A600 − 0.5) was plated onto the lawn of P. kessleri cells growing on agar plates for co-cultivation. Following co-cultivation for 72 h, cells were harvested and washed twice with liquid TAP medium containing 500 mg l−1 cefotaxime by centrifugation at 3,000 rpm for 2 min. P. kessleri cells were resuspended in liquid TAP and plated onto TAP agar medium containing 15 mg l−1 hygromycin + 500 mg l−1 cefotaxime. Cells were incubated till the appearance of transformed colonies at 25 °C under continuous light intensity of 75 μmol m−2s−1. Transformed colonies were maintained on TAP agar medium containing 15 mg l−1 hygromycin. Transformed cells were grown in liquid Walne’s medium for the GUS assay and PCR analysis.
Detection of reporter gene expression
For the GUS histochemical assay, transformed algal cells were centrifuged, washed with distilled water, resuspended in sonication buffer (Na2HPO4 − 0.06 M, KH2PO4 − 0.007 M, KCl − 0.1 M) and sonicated for 10 min at 10 kHz frequency. The cell lysate was mixed with GUS staining solution and incubated for 3 days at 37 °C (Jefferson 1987) and checked for the appearance of a blue colour. Wild-type algal cell lysate and distilled water were used as negative controls.
Confirmation and analysis of T-DNA transformation
Genomic DNA of the transformants was isolated using a GeneiPure™ Plant Genomic DNA Purification Kit (Genei™, Bangalore, India). PCR was carried out with forward primer 5′-CTATTTCTTTGCCCTCGGA-3′ and reverse primer 5′-AAAGCCTGAACTCACCGCGA-3′ specific to hpt gene to amplify a 1 kb fragment. Genomic DNA of wild-type marine P. kessleri and plasmid DNA of pCAMBIA1301 were used as negative and positive controls, respectively. The PCR products were run on 1 % agarose gel along with a 1 kb ladder to confirm the transformation.
Confirmation of A. tumefaciens’ absence in transformants
Genomic DNA of transformants and wild-type P. kessleri was isolated using the GeneiPure™ Plant Genomic DNA Purification Kit (Genei™, Bangalore, India). PCR was carried out with forward primer 5′-AGGTCGTTCGCTCCAAGCTG-3′ and reverse primer 5′-AGGAAAGCTGCCTGTTCCAAAG-3′ specific to nptII gene to amplify the 1,040 bp fragment. PCR was also carried out to detect the presence of VirC gene using VirC forward primer 5′-ATCATTTGTAGCGACT-3′ and reverse primer 5′-AGCTCAAACCTGCTTC-3′ to amplify a 730 bp fragment to confer the absence of A. tumefaciens in transformed cells. pCAMBIA 1301 and A. tumefaciens LBA 4404 strain were used as positive control for nptII and VirC genes, respectively. The PCR products were run on 1 % agarose gel along with a 1 kb ladder.
Growth study of transformed cells
Transformed cells were grown in liquid Walne’s medium to study the growth pattern in triplicates and average values were taken for comparison with wild-type P. kessleri. Cells were incubated at 100 rpm under continuous light intensity of 75 μmol m−2s−1 at 25 °C. Samples were withdrawn at regular intervals and optical density was measured at 750 nm to assess growth.
Results and discussion
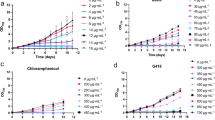
For Agrobacterium-mediated transformation, it is essential that algae and bacteria grow together on a medium which supports the growth of both the organisms during the co-cultivation period. This poses a technical hurdle for the transformation of marine algal species as A. tumefaciens and marine algal species may not be able to survive in marine and freshwater media, respectively. Therefore, either A. tumefaciens needs to be adapted on marine medium or algae need to be grown in freshwater medium. According to previous reports, A. tumefaciens has been grown on TAP, a freshwater medium, which also supports algal growth (Kumar et al. 2004). Therefore, marine P. kessleri was inoculated in TAP medium and the effect of transferring from marine medium (Walne’s) to freshwater medium (TAP) on growth was assessed. The marine P. kessleri was able to grow on TAP medium and no significant difference in growth pattern was observed in both marine and freshwater media (Fig. 1). Based on these results, TAP medium was used for co-cultivation purpose. Our result indicates that TAP can be used as co-cultivation medium for the transformation of marine algal species which can survive in TAP medium. In a similar manner, the salinity of the co-cultivation medium was reduced to 0.2 M NaCl instead of 1 M for A. tumefaciens-mediated transformation of D. bardawil, a halophilic alga (Anila et al. 2011). Hence, it can be concluded that establishing the common growth conditions for both the organisms is an essential step for the transformation of marine/salt-tolerant algal species when using the Agrobacterium-mediated method.
Ti-plasmid-based pCAMBIA series of A. tumefaciens binary vectors have long been used as the vectors of choice for plants and are increasingly being exploited for algal transformation. This is due to the inclusion of antibiotics as well as a diversity of reporter genes in the constructs (www.pcambia.org). Therefore, pCAMBIA1301 was selected for this study. This vector has a hygromycin phosphotransferase (hpt) gene and a GUS gene under the CaMV promoter for the selection of transformants. It is essential to determine the minimum inhibitory concentration (MIC) of hygromycin for species of interest as different algal species might respond distinctively towards the antibiotics. The growth of marine P. kessleri was inhibited beyond 12 mg l−1 of hygromycin concentration (Table 1) and thus 15 mg l−1 hygromycin was used for the selection of transformants. The MIC of hygromycin for P. kessleri was found to be higher as compared to freshwater algae C. reinhardtii (10 mg l−1) and H. pluvialis (2 mg l−1) (Kumar et al. 2004; Kathiresan and Sarada 2009), but much lower than marine algal species such as D. bardawil, Porphyra yezoensis and Schizochytrium sp. (Anila et al. 2011; Takahashi et al. 2011; Cheng et al. 2012). This may be because of the growth of P. kessleri on TAP medium (freshwater) as opposed to the marine water medium. It has been reported that a higher concentration of salt leads to the use of higher amount of hygromycin for complete inhibition of D. salina cell growth due to the involvement of H+-ATPase (Anila et al. 2012). However, the exact mechanism of antibiotic tolerance for other marine algal species has not been studied in detail and needs further investigation. Cefotaxime concentration of 500 mg l−1 was found to be inhibitory for Agrobacterium; however, this concentration did not affect the P. kessleri cells and thus was used for elimination of bacteria after co-cultivation.
The DNA transfer events were studied by monitoring the expression of hpt and GUS genes present in transformed marine P. kessleri. Colonies of transformed P. kessleri cells appeared on the hygromycin-containing TAP plate after 3–4 weeks of incubation. No growth was observed in control P. kessleri cells even after 6 weeks of incubation. The transformation was done twice in triplicates to ensure the reproducibility of the method. Transformation frequencies were found to be 246 ± 5.77 and 251 ± 5.54 cells per 107 cells ml−1 (Table 2). The transformed colonies were maintained on TAP agar medium with hygromycin as a selection pressure. Individual transformed colonies, which exhibited proliferate growth, were inoculated in liquid Walne’s medium for confirmation of transformation. It is important to note that efficient transformation occurred even without using AS, a common inducer of vir genes.
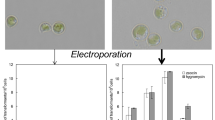
GUS histochemical assay of transformed colonies was conducted to confirm the transformation of pCAMBIA1301 into marine P. kessleri. The cell lysate of transformed cells showed GUS activity as indicated by the development of blue colour (Fig. 2a), which was confirmed by microscopic observation of the cells (Fig. 2b). Hence, it can be concluded that hygromycin (hpt gene) and GUS gene can be used as selection marker and reporter gene respectively and 35S CaMV as a promoter for marine P. kessleri. The final confirmation of transformation was done by confirming the presence of hpt gene in the genomic DNA of transformed cells by polymerase chain reaction with the help of specifically designed primers. A 1 kb band was seen in transformed marine P. kessleri and positive control (pCAMBIA) only, whilst it was absent in wild-type cells (Fig. 3). To rule out the possibility of GUS expression/hpt amplification due to contaminating Agrobacterium, PCR was carried out for nptII gene of 1,040 bp which is present outside the T-DNA region of pCAMBIA 1301 and VirC gene of 730 bp of Agrobacterium plasmid (Sawada et al. 1995). No amplification was observed for both the genes in transformed as well as wild-type P. kessleri, whilst single corresponding bands were obtained in the respective positive controls (Fig. 4a, b). Also, the streaking of transformants on LB medium did not show the growth of Agrobacterium. This proved that the transformants did not harbour A. tumefaciens contamination, and GUS expression/hpt amplification resulted due to transformation of P. kessleri.
a Histochemical detection of GUS activity in P. kessleri wild-type and transformed cells, A transformed marine P. kessleri, B wild-type marine P. kessleri and C distilled water. b Microscopic observation of GUS-positive transformed cells. Arrow indicates the blue coloured cell demonstrating the transfer of pCAMBIA into P. kessleri
Detection of A. tumefaciens contamination in the transformants. a PCR analysis using nptII gene-specific primers of 1,040 bp in length. Lane A 1 kb ladder (bands from bottom to 8th band: 250, 500, 750, 1,000, 1,500, 2,000, 2,500 and 3,000 bp), B pCAMBIA1301 plasmid, C transformed P. kessleri and D wild-type P. kessleri. Arrow indicates the positive control. b PCR analysis using VirC gene-specific primers of 730 bp in length. Lane A 1 kb ladder (bands from bottom to 8th band: 250, 500, 750, 1,000, 1,500, 2,000, 2,500 and 3,000 bp), B A. tumefaciens, C transformed P. kessleri and D wild-type P. kessleri. Arrow indicates the positive control
After transformation, P. kessleri cells were grown on liquid Walne’s medium and their growth was found to be comparable to wild-type cells (Fig. 1). This suggests that transformants had the same biomass growth and co-cultivation in freshwater medium did not affect the growth potential of cells adversely. This is the first detailed report on Agrobacterium-mediated transformation of marine P. kessleri. Recently, Agrobacterium-mediated transformation was found to be successful in freshwater C. vulgaris (Cha et al. 2012) and in marine Chlorella sp. (Cha et al. 2011). In marine Chlorella, the transformation was found to be transient and was achieved by using different concentrations of AS (Cha et al. 2011). In marine algae Schizochytrium sp., the transformation has been achieved with the protoplast rather than with the cell wall containing cells (Cheng et al. 2012). Although cell wall-less mutants can be transformed with electroporation, glass beads or Agrobacterium-based methods, their regeneration to cell wall containing cells is a long and tedious process. In the present study, transformation of marine P. kessleri was achieved without undergoing protoplast formation and was stable for four generations. The amplification of hpt gene by PCR showed that the T-DNA region of pCAMBIA was successfully transformed in P. kessleri. Thus, Agrobacterium-mediated transformation can be used for further molecular manipulation of this important genus for commercial use.
Conclusions
Advancements in molecular phylogenetic analysis of Chlorophyta have resulted in reshuffling of some strains designated as Chlorella into Parachlorella clade. Genus Parachlorella is emerging as a model candidate as oleaginous algae and for bioremediation along with Chlorella. The capability of P. kessleri to produce high biomass/oil content and its marine origin are most suitable for biofuel purposes and may be of considerable interest for bioenergy and carbon sequestration. Here, we report a simple Agrobacterium-mediated transformation protocol which can be exploited for genetic modifications in marine P. kessleri. The establishment of this method will open up opportunities in utilising this economically important genus for fundamental studies as well as for biotechnological applications.
References
Ahmad I, Fatma Z, Yazdani SS, Kumar S (2013) DNA barcode and lipid analysis of new marine algae potential for biofuels. Algal Res 2:10–15
Anila N, Chandrashekhar A, Ravishankar GA, Sarada R (2011) Establishment of Agrobacterium tumefaciens mediated genetic transformation in Dunaliella bardawil. Eur J Phycol 46(1):36–44
Anila N, Simon DP, Chandrashekar A, Sarada R (2012) Glucose-induced activation of H+-ATPase in Dunaliella salina and its role in hygromycin resistance. J Appl Phycol. doi:10.1007/s10811-012-9845-x
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev 14:557–577
Cha TS, Yee W, Aziz A (2011) Assessment of factors affecting Agrobacterium-mediated transformation of microalgae. UMTAS 2011 Empowering Science, Technology and Innovation Towards a Better Tomorrow, LSP93, pp 633–637
Cha TS, Yee W, Aziz A (2012) Assessment of factors affecting Agrobacterium-mediated genetic transformation of the unicellular green alga, Chlorella vulgaris. World J of Microbiol and Biotechnol 28:1771–1779
Cheng R, Ma R, Li K, Rong H, Lin X, Wang Z, Yang S, Ma Y (2012) Agrobacterium tumefaciens mediated transformation of marine microalgae Schizochytrium. Microbiol Res 167:179–186
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Hallmann A (2007) Algal transgenics and biotechnology. Transgenic Plant J 1(1):81–98
Hannon M, Gimpel J, Tran M, Rasala B, Mayfield S (2010) Biofuels from algae: challenges and potential. Biofuels 1(5):763–784
Jefferson R (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5:387–405
Kathiresan S, Sarada R (2009) Towards genetic improvement of commercially important microalga Haematococcus pluvialis for biotech applications. J Appl Phycol 21:553–558
Kiliana O, Benemanna CSE, Niyogi KK, Vicka B (2011) High-efficiency homologous recombination in the oil-producing alga Nannochloropsis sp. Proc Natl Acad Sci USA 108(52):21265–21269
Krienitz L, Hegewald EH, Hepperle D, Huss VAR, Rohr T, Wolf M (2004) Phylogenetic relationship of Chlorella and Parachlorella gen. nov. (Chlorophyta, Trebouxiophyceae). Phycologia 43(5):529–542
Kumar S, Misquitta R, Reddy V, Rao B, Rajam M (2004) Genetic transformation of the green alga—Chlamydomonas reinhardtii by Agrobacterium tumefaciens. Plant Sci 166(3):731–738
Li X, Pribyl P, Bisova K, Kawano S, Cepak V, Zachleder V, Cizkova M, Branyikova I, Vitova M (2013) The microalga Parachlorella kessleri—a novel highly efficient lipid producer. Biotechnol Bioeng 110(1):97–106
Mahdavi H, Urich AC, Liu Y (2012) Metal removal from oil sands tailings pond water by indigeous micro-alga. Chemosphere 89:350–354
Sawada H, Ieki H, Matsuda I (1995) PCR detection of Ti and Ri plasmids from phytopathogenic Agrobacterium strains. Appl Environ Microbiol 61(2):828–831
Takahashi M, Mikami K, Mizuta H, Saga N (2011) Identification and efficient utilization of antibiotics for the development of a stable transformation system in Porphyra yezoensis (Bangiales, Rhodophyta). J Aquac Res Dev. doi:10.4172/2155-9546.1000118
Walne PR (1970) Studies on the food value of nineteen genera of algae to juvenile bivalves of the genera Ostrea, Crassostrea, Mercenaria, and Mytilis. Fish Invest 26:162
Acknowledgements
This work was supported by the Department of Biotechnology, Ministry of Science and Technology, Government of India (No. BT/PR13796/PBD/26/139/2010). P. kessleri was kindly provided by Mr. Shrikumar Suryanaran (Sea6 Energy Private Limited, India). The Agrobacterium culture and plasmid were generous gifts from Dr. Shashi Kumar and Dr. Syed Shams Yazdani, ICGEB, Delhi. The authors thank Chaitali Vira for fruitful discussions during the work. Jayant Pralhad Rathod is thankful to the Council of Scientific and Industrial Research (CSIR), Govt. of India, New Delhi, for providing the fellowship during the above research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rathod, J.P., Prakash, G., Pandit, R. et al. Agrobacterium-mediated transformation of promising oil-bearing marine algae Parachlorella kessleri . Photosynth Res 118, 141–146 (2013). https://doi.org/10.1007/s11120-013-9930-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-013-9930-2