Abstract
The bioenergetic processes of photosynthesis and respiration are mutually beneficial. Their interaction extends to photorespiration, which is linked to optimize photosynthesis. The interplay of these three pathways is facilitated by two major phenomena: sharing of energy/metabolite resources and maintenance of optimal levels of reactive oxygen species (ROS). The resource sharing among different compartments of plant cells is based on the production/utilization of reducing equivalents (NADPH, NADH) and ATP as well as on the metabolite exchange. The responsibility of generating the cellular requirements of ATP and NAD(P)H is mostly by the chloroplasts and mitochondria. In turn, besides the chloroplasts, the mitochondria, cytosol and peroxisomes are common sinks for reduced equivalents. Transporters located in membranes ensure the coordinated movement of metabolites across the cellular compartments. The present review emphasizes the beneficial interactions among photosynthesis, dark respiration and photorespiration, in relation to metabolism of C, N and S. Since the bioenergetic reactions tend to generate ROS, the cells modulate chloroplast and mitochondrial reactions, so as to ensure that the ROS levels do not rise to toxic levels. The patterns of minimization of ROS production and scavenging of excess ROS in intracellular compartments are highlighted. Some of the emerging developments are pointed out, such as model plants, orientation/movement of organelles and metabolomics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photosynthesis and respiration are the most important primary metabolic processes, being involved in energy supply, besides assimilation/metabolism of carbon, nitrogen and even sulfur in plants. The first phase of photochemical reactions convert solar energy into ATP and NADPH (or reduced ferredoxin), while the second phase consists of biochemical reactions which utilize ATP/NADPH/reduced ferredoxin to reduce inorganic carbon or nitrogen or sulfur. The photochemical reactions, which involve the transfer of electrons and protons, operate at a much higher speed than the biochemical reactions. A balance of these processes is essential not only to optimize, but also to sustain photosynthesis under challenging environments, such as high light.
The importance of mitochondrial oxidative metabolism for the regulation of photosynthetic carbon assimilation has been emphasized in several reviews (Raghavendra and Padmasree 2003; Nunes-Nesi et al. 2011; Millar et al. 2011). The components of bioenergetic metabolism of mitochondria (which include oxidative phosphorylation, NAD(P)H dehydrogenases, cytochrome oxidase pathway, alternative oxidase (AOX) pathway, and uncoupling proteins) are all essential for efficient functioning of chloroplastic photosynthesis (Noguchi and Yoshida 2008; Nunes-Nesi et al. 2008). We present a comprehensive overview of the interactions between chloroplasts and mitochondria, extending to peroxisomes and cytoplasm. The interorganelle interactions are based on two major principles: (i) sharing of energy and metabolite resources, backed by transporters, and (ii) maintenance of cellular redox state at an optimal level. A few of the emerging trends are highlighted.
Sharing of energy and metabolite resources
Besides being the primary source of biological energy, photosynthesis facilitates the conversion of inorganic forms of C, N and S into organic substrates required for metabolic pathways located in different compartments of cell. ATP/NAD(P)H are required for several metabolic reactions, located in compartments, such as chloroplasts, cytosol, mitochondria and peroxisomes (Table 1). Dynamic interactions between organelles ensure the sharing of these energy resources and metabolite use. The result is the optimization of photosynthesis and sustenance at high rates, even under unfavorable conditions.
Cellular requirements of ATP/NAD(P)H: integrated view
The ATP and/or NADPH generated during the photosynthetic electron transport are used in a variety of metabolic processes in the chloroplasts, including the assimilation of CO2, nitrate and sulfate (Table 1). ATP and NADPH are required also for the biosynthesis of lipids, nucleic acids and pigments (Kramer and Evans 2011). Besides chloroplasts, mitochondria through their oxidative metabolism also provide significant amounts of ATP required for metabolic processes. Role of cytosolic glycolysis in plant cells cannot be ignored even in the light, as triose-P continues to be metabolized to phosphoenolpyruvate (PEP) and pyruvate. ATP produced in glycolytic reactions is an important source of energy for cellular metabolism (Givan 1999).
Under sub-optimal or supra-optimal environmental conditions, there is usually an imbalance in the generation and use of reductants in photosynthesis during illumination. It is essential to dissipate excess redox equivalents to prevent over-reduction of electron transport and thereby to avoid damage to thylakoid membranes (Foyer et al. 2012; Harbinson 2012). Cyclic electron transport by generation of ATP can help balance the requirement for ATP/NADPH, and the Mehler reaction can consume NADPH in thylakoids, but these processes are not sufficient. The excess reducing equivalents are transported from chloroplasts (in the form of DHAP and malate) to the cytosol and can be used to generate NADH. In parallel, the reduction of nitrite to ammonia and oxoglutarate to glutamate within the chloroplasts facilitates the consumption of not only ammonia but also the reduced ferredoxin. Similarly, the mitochondria are capable of either directly oxidizing external NADH and NADPH or indirectly through the shuttles of related metabolites (described in “Metabolite transporters”). Another site of NADH consumption is peroxisomes, wherein hydroxypyruvate reduction is supported by the supply of malate from either mitochondria or chloroplasts.
Metabolite exchange during primary metabolism
Many metabolic processes in plant tissues, such as the assimilation of carbon as well as nitrogen or sulfur, are stimulated by light, due to elevated supply of ATP and NADPH from thylakoid electron transport. These assimilatory pathways are integrated through cross-talks between organelles, to optimize the utilization of energy and metabolite resources (Figs. 1, 2). Such optimization involves shuttling of carbon, nitrogen and sulfur compounds between the organelles of chloroplasts, mitochondria, peroxisomes and cytoplasm (Takahashi et al. 2011; Foyer et al. 2011; Taniguchi and Miyake 2012).
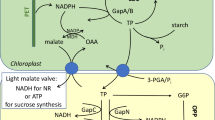
The assimilatory metabolism of carbon in plant cells involving exchange of metabolites across different organelles. The transporters in the inner envelope membranes of chloroplasts or mitochondria facilitate the metabolite movement (indicated by arrows). In Calvin cycle, 3-PGA is converted to triose-P (GAP/DHAP), utilizing ATP and NADPH generated from photochemical reactions. Triose-P is exported out to cytosol through Pi transporter, TPT (1). A part of triose-P is metabolized to sucrose, utilizing mitochondrial respiratory ATP, exported to cytosol by adenylate translocator, AT (2). Within chloroplasts, ATP is required to convert glycerate coming in from peroxisomes, by plastidial glycolate/glycerate transporter, PLGG1 (3) into PGA. Glycolate from Rubisco oxygenase reaction is metabolized to glycine in peroxisomes, which moves into mitochondria by glycine/serine translocator (4). Glycine is decarboxylated in mitochondria to yield serine and NH3, the latter moving into chloroplasts, where it is reduced by the synthesis of glutamate. The reductants in chloroplasts can be moved into cytosol also in the form of malate, via a dicarboxylate translocator, DiT (5). The exchange of reductants between cytosol and mitochondria is facilitated by DTC. Mitochondrial oxidative electron transport (MET) generates ATP, accepting NADH on both sides of the inner membrane. Photorespiration is an internal source of CO2, which may be partially refixed by the Calvin cycle. The reactions involving ATP and NADPH are indicated with specific colors of pink and blue, respectively. Further details are described in Raghavendra and Padmasree (2003), Linka and Weber (2010) and Weber and Bräutigam (2013). BPGA bisphosphoglycerate, DTC dicarboxylate/tricarboxylate transporter, GAP glyceraldehyde-3-phosphate, OAA oxaloacetate, PGA phosphoglycerate
Nitrogen metabolism involving integrated functioning of chloroplasts, mitochondria, peroxisomes and cytosol. The reactions of N assimilation require ATP and reduced equivalents (in the form of NAD(P)H or reduced ferredoxin), and are facilitated by transporters. Nitrate is reduced to nitrite, by nitrate reductase (NR) localized in the cytosol. Nitrite enters chloroplasts, via a nitrite transporter, NiTr (1), and converted to ammonium by nitrite reductase (NiR). Glycine synthesized in the glycolate pathway, moves from peroxisomes into mitochondria, though a glycine/serine translocator. Ammonium is assimilated into glutamine/glutamate and other amino acids by GS and GOGAT, utilizing reduced ferredoxin or NAD(P)H and ATP, generated in photosynthetic metabolism. 2-OG required for GS–GOGAT in chloroplasts can come from either mitochondria, through a modified TCA cycle or peroxisomes. The exchange of 2-OG and glutamate, between chloroplasts and cytosol, is facilitated by DiT1/2 (2), while the movement of 2-OG and citrate between mitochondria and cytosol occurs via DTC (3). Mitochondria import pyruvate and OAA/malate by DTC (3) and PyrT (4), respectively. The reactions involving ATP and NADPH are indicated with specific colors of pink and blue, respectively. Further details are in the reviews of Palmieri et al. (2009), Foyer et al. (2011) and Taniguchi and Miyake (2012). DHAP dihydroxyacetone phosphate, DiT1/2 plastidic 2-oxoglutarate-malate transporter/glutamate-malate transporter, DTC mitochondrial dicarboxylate/tricarboxylate carrier, Fd ferredoxin, GOGAT glutamate synthase, GS glutamine synthetase, OAA oxaloacetate, 2-OG 2-oxoglutarate, PEP phosphoenol pyruvate, PyrT pyruvate transporter
Carbon
Carbon compounds produced from photosynthesis are essential for providing carbon skeletons for whole plant metabolism, growth and development (Stitt 2012). The major end products of carbon assimilation in chloroplasts are triose phosphates (GAP and DHAP, together termed triose-P) and starch (Fig. 1). Triose-P is exported from the chloroplast stroma to the cytosol, and converted into sucrose, to be exported to different sink tissues. A part of carbon from the synthesis of P-glycolate by RuBP oxygenase activity is recovered through the glycolate pathway in the form of glycerate, which enters chloroplasts, is converted to phosphoglycerate and fed into the Calvin cycle.
The role of mitochondrial ATP to sustain the conversion of triose-P into sucrose is now well documented (Raghavendra and Padmasree 2003). Mitochondrial respiration can also be a significant source of internal CO2 for photosynthetic carbon fixation (Riazunnisa et al. 2006; Busch et al. 2013). In illuminated plant cells, the substrates for mitochondrial oxidation include malate and glycine, being supplied from recently assimilated carbon from chloroplasts. In turn, mitochondria provide C skeletons for amino acid biosynthesis. For example, mitochondria operate a modified TCA cycle in the light and export isocitrate to the cytosol, which is converted to oxoglutarate and sent to chloroplasts, to support glutamate biosynthesis (Hanning and Heldt 1993; Sweetlove et al. 2010; Foyer et al. 2011). Cytosolic reactions in glycolysis (particularly PEP carboxylase and pyruvate kinase) provide carbon skeletons needed for mitochondrial respiration and amino acid biosynthesis, involving also chloroplasts.
Nitrogen
Plant growth and development are dependent on C and N, as well as on the interactions between them. A deficiency of N decreases C assimilation, while C starvation reduces N utilization in plants. Thus, C and N metabolism are tightly coordinated and are modulated by the signals from C, N and C/N ratio (Nunes-Nesi et al. 2010). The mutual dependence of plants on carbon and nitrogen assimilation is observed even under elevated CO2 conditions (Kant et al. 2012).
The uptake and assimilation of inorganic N depends on the supply of C skeletons from chloroplasts and mitochondria. Further, the process of nitrogen assimilation is highly energy dependent and requires reduced compounds, such as NADH, NADPH, reduced ferredoxin and ATP (Fig. 2). These energy-rich compounds are provided by either photosynthesis or mitochondrial oxidative metabolism or even the cytosolic reactions. Significant supply of reductants can also come from mitochondrial photorespiratory reactions through glycine decarboxylase (Szal and Podgorska 2012). A striking dependence of N assimilation on mitochondrial oxidative electron transport has been demonstrated in Nicotiana sylvestris CMSII mutant, deficient in complex I. The enhanced availability of NADH in these mutants upregulates their AOX pathway and N assimilation into amino acids (Dutilleul et al. 2005). Thus, the reactions of N assimilation form a dynamic link among chloroplasts, mitochondria, peroxisomes and cytosol (Nunes-Nesi et al. 2010; Foyer et al. 2011). The novel role of photorespiration as an adaptive mechanism against abiotic stress, by virtue of forming a sink for N metabolism, has been emphasized (Voss et al. 2013).
Sulfur
Sulfur, an essential element for plants, has a variety of functions. The uptake, reductive assimilation of sulfate, and integration into cysteine and methionine constitute the conversion of inorganic form of sulfur into organic form. The reactions of sulfur assimilation/metabolism take place in chloroplasts, mitochondria, cytoplasm and even in the vacuole. Sulfate is utilized to form cysteine in chloroplasts, methionine in mitochondria and cytosol, but their oxidation is restricted to peroxisomes (Takahashi et al. 2011).
The process of sulfate assimilation into cysteine is heavily dependent on the supply of ATP and reducing power in chloroplasts and thus forms an important sink for energy produced in the chloroplasts. The activity of mitochondrial electron transport as well as other proteins is modulated by cysteine (Juszczuk and Ostaszewska 2011), mitochondrial AOX, being a good example. Cysteine is a precursor of methionine, which can be synthesized in chloroplasts as well as cytoplasm (Wirtz and Droux 2005). Further, cysteine and methionine are incorporated into glutathione (GSH), another sulfur containing metabolite, which is an integral part of antioxidant components in plant cells. The synthesis and metabolism of GSH occur in chloroplasts and cytosol. Further details of the role of GSH as antioxidant are described in the following sections.
Photorespiration
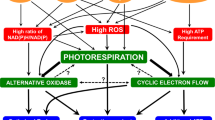
Photorespiration is intimately connected with C as well as N metabolism and involves high metabolite flux (Bauwe et al. 2012). The biochemical reactions, participating enzymes and the carbon flux through photorespiration have been studied extensively (Stitt 2012; Bauwe et al. 2012). The carbon from 2-phosphoglycolate, a toxic product of RuBP oxygenase activity, is recycled back into chloroplasts to form PGA and enter the Calvin cycle. When CO2 is limiting, the utilization of ATP, NADH and reduced ferredoxin in photorespiration helps preventing the over-excitation of electron transport. Photorespiratory reactions protect photosynthesis against glycolate toxicity and even recover part of carbon from glycolate as glycerate (Figs. 1, 2). Although it was once considered to be an unavoidable process, photorespiration may be an adaptation to oxygenic atmosphere. Photorespiration is beneficial under CO2 limiting conditions, by preventing the accumulation of toxic products and allowing photochemistry to function (Voss et al. 2013). Photorespiration can help also to minimize the intracellular reactive oxygen species (ROS) levels in multiple ways: providing intracellular CO2 for chloroplasts (Fig. 1), promotion of AOX pathway in mitochondria by NADH generated from glycine decarboxylase and upregulation of cyclic electron transport in chloroplasts.
Metabolite transporters
The rapid exchange of metabolites among chloroplasts, cytosol, peroxisomes and mitochondria is essential for interorganelle communication and maintenance of metabolic equilibrium, as the redox-rich compounds such as NAD(P)H or ATP are not freely permeable. Metabolite translocators mediate a dynamic and coordinated metabolite flux between chloroplasts and other cellular compartments, facilitating the movement of metabolites as well as movement of NAD(P)H/ATP-related compounds (Figs. 1, 2). There are excellent reviews on the transporters located in the inner envelope membranes of chloroplasts and mitochondria (Palmieri et al. 2009; Linka and Weber 2010; Weber and Linka 2011). Recent articles indicate the operation of transport proteins in peroxisomal membranes to facilitate metabolite movement (Eisenhut et al. 2012; Linka and Esser 2012).
The triose phosphate translocator (TPT) located in the inner chloroplast membrane facilitates the simultaneous exchange of triose-P generated in the chloroplast into cytoplasm and import of cytosolic Pi (Fig. 1). The triose-P in cytoplasm is metabolized to sucrose. Glycerate, an important product of photorespiration in peroxisomes, is moved into chloroplasts by glycolate/glycerate transporter (PLGG1) (Pick et al. 2013). The dicarboxylate transporter displays a high affinity for OAA, can function also as a malate-valve and is quite effective in balancing redox equivalents between the cytosol and the stroma (Scheibe et al. 2005; Kinoshita et al. 2011). The chloroplastic redox shuttle systems, based on dicarboxylate and Pi translocators, help in the optimization of C/N assimilation and balance the stromal ATP/NADPH ratio (Taniguchi and Miyake 2012).
Mitochondrial oxidative phosphorylation is an important source of energy for sucrose biosynthesis as well as other reactions (Raghavendra and Padmasree 2003). Mitochondrial AT transporter transports ATP into cytoplasm in exchange for ADP (Fig. 1). The TCA cycle provides reducing equivalents for oxidative electron transport and carbon skeletons, with the help of metabolite transporters in the inner mitochondrial membrane (Linka and Weber 2010). A typical example of mitochondrial transporter is dicarboxylate/tricarboxylate carrier (DTC), which mediates the transport of both dicarboxylates (such as oxoglutarate, malate or oxaloacetate) and tricarboxylate (such as citrate). Recent reports indicate the operation of NAD carrier proteins (Palmieri et al. 2009).
Maintenance of optimal ROS levels within the cell
The metabolic pathways in plant organelles tend to produce ROS, which can be toxic at high concentrations. However, low levels of ROS act as key signaling molecules during the regulation of many metabolic and growth/development processes in plants. Such dual role of ROS depends on their concentration as well as on the status of the environment (Mullineaux and Baker 2010). The major forms of ROS are hydrogen peroxide (H2O2), singlet oxygen (1O2), superoxide radical (O ·−2 ) and hydroxy radical (OH·); they are produced mainly in chloroplasts, mitochondria and peroxisomes, along with the production of ROS via NADPH oxidases located in the plasma membrane (Mittler et al. 2011; Suzuki et al. 2012). Increased accumulation of ROS can induce oxidative stress, leading to oxidation of cellular components, hindering metabolic activities and affecting organelle integrity (Mittler et al. 2004). Since the molecules are diffusible, ROS can disturb multiple compartments, irrespective of the site of production. It is therefore not surprising that all the cellular organelles have means of controlling, to some extent, the levels of ROS. This can be done by either minimizing ROS production or elevating scavenging systems, or both (Fig. 3).
The sites of generation and scavenging of reactive oxygen species (ROS) in plants. Majority of the ROS is generated during either the electron transport in chloroplasts/mitochondria, or conversion of glycolate to glyoxylate in peroxisomes. Plasma membrane-bound NADPH oxidases also produce ROS (not shown in figure for convenience). When in excess, ROS are scavenged by antioxidant systems in chloroplasts, mitochondria, peroxisomes and cytosol. Cyclic electron transport (CET) minimizes the ROS production in chloroplasts by transferring electrons from Fd to PQ. In mitochondria, two major sources of NADH are TCA cycle and glycine decarboxylation. High amounts of ROS are produced in peroxisomes, during glycolate oxidation, but are scavenged by CAT. Peroxisomes contain also xanthine oxidase (XO), which produces ROS. The reactions of ROS production are indicated in red, while those minimizing or scavenging are indicated in green. More details can be found in the reviews of Mittler et al. (2004) and Mhamdi et al. (2010). AOX alternate oxidase pathway, ASC-GSH cycle ascorbate–glutathione cycle, APX ascorbate peroxidase, CAT catalase, Fd ferredoxin, FNR Fd-NADP + reductase, GDC glycine decarboxylase, GO glycolate oxidase, GPX glutathione peroxidase, MET mitochondrial electron transport, PC plastocyanin, PQ plastoquinone, PrxR peroxiredoxins, SOD superoxide dismutase, TCA cycle tricarboxylic acid cycle
Minimization of ROS production
The production and scavenging of ROS in mitochondria and chloroplasts are interlinked. Electron transport in both chloroplasts and mitochondria tend to generate ROS (Fig. 3). Under normal conditions, when electron acceptors (Fd and NADP in chloroplasts and O2 in mitochondria) are insufficient, the generation of ROS increases (Mubarakshina et al. 2010). High amounts of ROS in the form of H2O2 are generated during glycolate oxidation in peroxisomes, but are scavenged by catalase. Noncyclic PS II-mediated electron transport has the potential to generate high amounts of ROS (acceptance of electrons from PS I by O2, resulting in the formation of O ·−2 and H2O2). Therefore, chloroplasts employ water–water cycle and cyclic electron transport to keep the ROS levels low (Miyake 2010). During illumination, the difference in redox potential between chloroplast stroma and cytosol is high (Nunes-Nesi et al. 2007), posing a risk of oxidative damage to thylakoid membranes and other cellular components.
When there is limitation on the utilization of ATP produced by the cytochrome pathway, the AOX pathway can be induced, preventing the donation of electrons to O2 from other parts of the pathway to form O ·−2 . The predominance of AOX pathway in mitochondrial respiration helps dissipate excess of photosynthetic reducing power and minimizing ROS production (Noguchi and Yoshida 2008; Dinakar et al. 2010). Photorespiratory glycine decarboxylation generates considerable NADH, which can upregulate not only AOX pathway, but also chloroplastic cyclic electron transport (Voss et al. 2013). In turn, ROS can be signals to coordinate and co-regulate multiple compartments (Mittler et al. 2011).
Scavenging ROS: antioxidants
Ascorbate (ASC) and GSH are among the most important antioxidants in plant cells. Besides its antioxidant function, ASC is a key component of interorganelle cross-talk between mitochondria and chloroplasts (Talla et al. 2011; Szarka et al. 2013). The biosynthesis of ascorbate occurs mostly in cytoplasm, with the terminal step in mitochondria. Yet, ASC biosynthesis appears to be dependent on photosynthetic electron transport (Yabuta et al. 2007). The levels and oxidized/reduced status of ascorbate can influence the overall photosynthetic performance and photoinhibition (Chen et al. 2003; Talla et al. 2011). The ascorbate synthesis is shown to be coupled with mitochondrial electron transport at complex IV and also influences the activity of AOX (Bartoli et al. 2006). Thus, changes in either mitochondria or chloroplasts can influence each other, through ascorbate (Szarka et al. 2013). Similarly, GSH is mostly synthesized in chloroplasts, but is required for redox balancing reactions in mitochondria and cytoplasm, besides chloroplasts themselves (Noctor et al. 2012). The GSH-based redox state operating in different organelles becomes an essential part of optimization of photosynthesis, respiration and even N metabolism (Szalai et al. 2009).
Emerging trends and concluding remarks
Model plants and mutants
Arabidopsis has evolved to be the best model plant for elucidating not only photosynthesis but also different metabolic pathways and their interactions (Stitt et al. 2010; Koornneef and Meinke 2010). Availability of a wide spectrum of mutants/transgenics of Arabidopsis, as well as established protocols for isolation of protoplasts, transient gene expression analysis and metabolite analysis using Arabidopsis offer a wide scope for diverse experiments in photosynthesis research (Riazunnisa et al. 2007; Yoo et al. 2007). There have been extensive reports on the modulation of photosynthesis and mitochondrial respiration in Arabidopsis mutants, deficient in specific components of chloroplasts and mitochondria, for example, NADP-MDH in chloroplasts (Hebbelmann et al. 2012) and AOX in mitochondria (Yoshida et al. 2011). Besides Arabidopsis, other important experimental models that have been used widely are: spinach, pea and green algae (Chlamydomonas and Synechocystis) (Harris 2001; Sunil et al. 2008; Nogales et al. 2012). It is essential to identify and exploit the use of other model plants. For example, maize has a high potential, in view of the availability of mutants and C4 photosynthesis. Similarly, Setaria viridis is being considered as model of C4 grass, Cleome as a C4 dicot and Brachypodium as a model C3 grass (Brown et al. 2005; Brutnell et al. 2010; Brkljacic et al. 2012).
Organelle proximity and movement
The strong biochemical interactions between the organelles in plant cells depend on dynamic metabolite exchange, which can be quite effective, where there is physical proximity between them. The importance of the close association of chloroplasts, mitochondria and peroxisomes is being considered as an important component of functional anatomy of leaf photosynthesis (Terashima et al. 2011). Chloroplasts are known to reorient within the cell, either horizontally or vertically, depending on the intensity of incident light. The reorientation and movement of chloroplasts as well as mitochondria is mediated by phototropins and facilitated by cytoskeleton (Kong and Wada 2011). Similar movement of mitochondria and even peroxisomes has also been reported (Islam et al. 2009; Mano et al. 2002). The reorientation of chloroplasts and mitochondria can modulate the intercellular conductance of CO2, increase light use efficiency and may improve the chance of refixation of CO2 by Rubisco (Terashima et al. 2011). The significance and consequences of such organellar movement on the cross-talk between chloroplasts and other organelles are highly interesting and need validation.
Role of proline and GABA
Plants are known to accumulate compatible solutes (e.g., proline, polyols, glycine betaine, trehalose), which seem to serve a dual purpose of stabilizing cellular proteins/nucleic acids and even scavenging ROS, which can protect photosynthesis and other processes. These osmolytes can also provide a source of carbon, nitrogen and energy, when the stress is relieved. Among these osmolytes, proline and γ-aminobutyric acid (GABA), may have a role in redox-state mechanisms. Proline is considered as a potent non-enzymatic antioxidant that can mitigate the adverse effects of ROS (Verslues and Sharma 2010). Similarly, GABA, which has until now been treated as a plant metabolite, may provide signaling to modulate metabolic activities in response to stress and carbon/nitrogen metabolism.
Two key enzymes involved in the biosynthesis of proline—Δ1-pyrroline-5-carboxylate synthase (P5CS or P5C synthase) and P5C reductase (P5CR)—are localized in cytosol and chloroplast, whereas two key catabolic enzymes—proline dehydrogenase (PDH) and P5C dehydrogenase (P5CDH)—are located in mitochondria. Such distribution of biosynthetic reactions in chloroplasts and degrading components in mitochondria and cytoplasm makes proline a relevant case for interorganelle interactions. Accumulation of P5CS and P5CR transcripts in chloroplasts (Szekely et al. 2008; Szabados and Savoure 2009) suggests that, under adverse conditions, photosynthesis-derived glutamate could increase proline biosynthesis in plastids. Proline catabolism in the mitochondria is connected to oxidative respiration that provides energy, as the oxidation of one molecule of proline can yield as much as 30 ATPs (Kishor et al. 2005). Proline oxidation can also regulate mitochondrial ROS levels.
GABA, a four-carbon non-protein amino acid and a key player in “GABA-shunt”, is linked to stress and signaling in plants. GABA is derived from glutamate, which is converted to SSA (succinic semialdehyde) and then to succinate, to enter the tricarboxylic acid cycle. These three successive reactions are catalyzed by glutamate decarboxylase (GAD), pyruvate- and 2-oxoglutarate-dependent GABA transaminase (GABA-T) and SSA dehydrogenase (SSADH), respectively. GAD is localized in the cytosol, while GABA-T and SSADH are located in mitochondria (Shelp et al. 2012), implicating the transport of GABA across the mitochondrial membranes. Clark et al. (2009) suggested that photorespiration can interact with GABA metabolism.
GABA and proline could quench the production of O2 ·−, H2O2, and 1O2 in an in vitro system. GABA had a stronger scavenging capacity than proline (Liu et al. 2011). Such quenching capacity can be the basis for the accumulation of these osmolytes in plants. Further experiments are needed to understand the inter-relations of biosynthesis/degradation of proline or GABA by photosynthetic carbon assimilation and mitochondrial respiration.
Retrograde signaling
The ROS-mediated cross-talk between chloroplasts and mitochondria to optimize the function of photosynthesis is reflected also at the level of gene expression in different plant cell compartments (Suzuki et al. 2012). The concept of retrograde signaling in terms of control of nuclear gene expression by mainly chloroplasts in plant cells has long been known. In the last few years, the concept is extended to regulation of nuclear gene expression by mitochondria as well (Leister 2012). The purpose of such dynamic regulation of gene expression in multiple compartments seems to be the need for dynamic coordination of metabolic components in different compartments. The expression of several genes in chloroplasts, mitochondria and the nucleus are highly coordinated, and respond to the energy state of the cell as well as environmental stresses (Leister 2012; Schwarzlander and Finkemeier 2012; Berry et al. 2013).
The exact signal(s) originating from chloroplasts or mitochondria has been a matter of debate. A favored view point is that retrograde signaling is redox-related. For example, the regulations of transcripts encoding components of the photosynthetic and respiratory electron transport chains are regulated by hydrogen peroxide, ascorbate and GSH (Queval and Foyer 2012). The photosynthetic function of chloroplasts is expected to sense and direct intracellular adjustments (Pfannschmidt et al. 2009), but the exact sensor molecule needs to be identified clearly.
Metabolomics
The interactions of chloroplasts with other organelles is a dynamic process and results in marked changes in relative levels of metabolites and their intracellular distribution. Plant metabolomics provide a comprehensive and quantitative analysis of primary and secondary metabolites in plants. Metabolomics, therefore, is a powerful tool to understand the biochemical basis of such metabolic network regulation. The analytical techniques of plant metabolomics are based broadly on either nuclear magnetic resonance (NMR) spectroscopy or mass spectroscopy (MS) or in combination. The combined analysis involving gas chromatography-mass spectrometry (GC–MS) and liquid chromatography (LC–MS) has been the most popular. The use of metabolomics has been helpful in studying the specific roles of selected enzyme/protein components of chloroplasts (NADP-MDH, Hebbelmann et al. 2012), mitochondria (AOX, Strodtkötter et al. 2009) and cytosol (Oliver et al. 2008). The integration of plant metabolomics with conventional information on enzyme activities, metabolite fluxes and modeling is quite promising to understand the synthetic and systems biology of photosynthesis (Arnold and Nikoloski 2011). The plant metabolomics and modeling should be exploited to understand and predict the components of interorganelle cross-talk.
Several reactions of secondary metabolism operate in chloroplasts, as their biosynthesis requires NAD(P)H and ATP, but parts of these metabolic pathways are located also in mitochondria and cytosol. Selmar and Kleinwächter (2013) suggested that biosynthesis of several secondary metabolites may serve to dissipate oxidative stress in plant cells. Some of the secondary metabolites (e.g., isoprenoids) from plastids may serve as signaling molecules in upregulation of mitochondrial transcripts (Hsieh et al. 2008). Further work is warranted on the role of secondary metabolism in integrating the function of chloroplasts with other organelles. Again, metabolomics should be able to reveal the roles of the key secondary metabolites in such interactions.
Abbreviations
- 2-OG:
-
2-Oxoglutarate
- 3-PGA:
-
3-Phosphoglycerate
- AOX:
-
Alternative oxidase
- APX:
-
Ascorbate peroxidase
- ASC:
-
Ascorbate
- AT:
-
Adenylate transporter
- BPGA:
-
Bisphosphoglycerate
- CAT:
-
Catalase
- CET:
-
Cyclic electron transport
- DHAP:
-
Dihydroxyacetone phosphate
- DiT1/2:
-
Plastidic 2-oxoglutarate-malate transporter/plastidic glutamate-malate transporter
- DTC:
-
Dicarboxylate/tricarboxylate carrier
- Fd:
-
Ferredoxin
- FNR:
-
Fd-NADP+ reductase
- GABA:
-
γ-Aminobutyric acid
- GAP:
-
Glyceraldehyde 3-phosphate
- GDC:
-
Glycine decarboxylase
- GO:
-
Glycolate oxidase
- GOGAT:
-
Glutamine:oxoglutarate aminotransferase
- GPx:
-
Glutathione peroxidase
- GS:
-
Glutamine synthetase
- GSH:
-
Glutathione
- MET:
-
Mitochondrial electron transport
- NiR:
-
Nitrite reductase
- NiTr:
-
Nitrate transporter
- NR:
-
Nitrate reductase
- OAA:
-
Oxaloacetate
- PC:
-
Plastocyanin
- PEP:
-
Phosphoenolpyruvate
- PLGG1:
-
Plastidic glycolate glycerate transporter
- PQ:
-
Plastoquinone
- PrxR:
-
Peroxiredoxins
- PyrT:
-
Pyruvate transporter
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TPT:
-
Triose phosphate/phosphate translocator
- XO:
-
Xanthine oxidase
References
Arnold A, Nikoloski Z (2011) A quantitative comparison of Calvin-Benson cycle models. Trends Plant Sci 16:676–683
Bartoli CG, Yu J, Gómez F, Fernández L, McIntosh L, Foyer CH (2006) Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. J Exp Bot 57:1621–1631
Bauwe H, Hagemann M, Kern R, Timm S (2012) Photorespiration has a dual origin and manifold links to central metabolism. Curr Opin Plant Biol 15:269–275
Berry JO, Yerramsetty P, Zielinski AM, Mure CM (2013) Photosynthetic gene expression in higher plants. Photosynth Res. doi:10.1007/s11120-013-9880-8
Brkljacic J, Grotewold E, Scholl R, Mockler T, Garvin DF, Vain P, Brutnell T, Sibout R, Bevan M, Budak H, Caicedo AL, Gao C, Gu Y, Hazen SP, Holt BF III, Hong SY, Jordan M, Manzaneda AJ, Mitchell-Olds T, Mochida K, Mur LAJ, Park CM, Sedbrook J, Michelle Watt M, Zheng SJ, Vogel JP (2012) Brachypodium as a model for the grasses: today and the future. Plant Physiol 157:3–13
Brown NJ, Parsley K, Hibberd JM (2005) The future of C4 research—maize, Flaveria or Cleome? Trends Plant Sci 10:215–221
Brutnell TP, Wang L, Swartwood K, Goldschmidt A, Jackson D, Zhu XG, Kellogg E, Eck JV (2010) Setaria viridis: a model for C4 photosynthesis. Plant Cell 22:2537–2544
Busch FA, Sage TL, Cousins AB, Sage RF (2013) C3 plants enhance rates of photosynthesis by reassimilating photorespired and respired CO2. Plant Cell Environ 36:200–212
Chen Z, Young TE, Ling J, Chang SC, Gallie DR (2003) Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc Natl Acad Sci USA 100:3525–3530
Clark SM, Leo RD, Dhanoa PK, Cauwenberghe ORV, Mullen RT, Shelp BJ (2009) Biochemical characterization, mitochondrial localization, expression, and potential functions for an Arabidopsis γ-aminobutyrate transaminase that utilizes both pyruvate and glyoxylate. J Exp Bot 60:1743–1757
Dinakar CH, Abhaypratap V, Yearla SR, Raghavendra AS, Padmasree K (2010) Importance of ROS and antioxidant system during the beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Planta 231:461–474
Dutilleul C, Lelarge C, Prioul JL, De Paepe R, Foyer CH, Noctor G (2005) Mitochondria-driven changes in leaf NAD status exert a crucial influence on the control of nitrate assimilation and the integration of carbon and nitrogen metabolism. Plant Physiol 134:64–78
Eisenhut M, Pick TR, Bordych C, Weber AP (2012) Towards closing the remaining gaps in photorespiration - the essential but unexplored role of transport proteins. Plant Biol. doi:10.1111/j.1438-8677.2012.00690
Foyer CH, Noctor G, Hodges M (2011) Respiration and nitrogen assimilation: targeting mitochondria-associated metabolism as a means to enhance nitrogen use efficiency. J Exp Bot 62:1467–1482
Foyer CH, Neukermans J, Queval G, Noctor G, Harbinson J (2012) Photosynthetic control of electron transport and the regulation of gene expression. J Exp Bot 63:1637–1661
Givan CV (1999) Evolving concepts in plant glycolysis: two centuries of progress. Biol Rev 74:277–309
Hanning I, Heldt HW (1993) On the function of mitochondrial metabolism during photosynthesis in spinach (Spinacia oleracea) leaves partitioning between respiration and export of redox equivalents and precursors for nitrate assimilation products. Plant Physiol 103:1147–1154
Harbinson J (2012) Modeling the protection of photosynthesis. Proc Natl Acad Sci USA 109:15533–15534
Harris EH (2001) Chlamydomonas as a model organism. Annu Rev Plant Physiol Plant Mol Biol 52:363–406
Hebbelmann I, Selinski J, Wehmeyer C, Goss T, Voss I, Mulo P, Kangasjärvi S, Aro EM, Oelze ML, Dietz KJ, Nunes-Nesi A, Do PT, Fernie AR, Talla SK, Raghavendra AS, Linke V, Scheibe R (2012) Multiple strategies to prevent oxidative stress in Arabidopsis plants lacking the malate valve enzyme NADP-malate dehydrogenase. J Exp Bot 63:1445–1459
Hsieh MH, Chang CY, Hsu SJ, Chen JJ (2008) Chloroplast localization of methylerythritol 4-phosphate pathway enzymes and regulation of mitochondrial genes in ispD and ispE albino mutants in Arabidopsis. Plant Mol Biol 66:663–673
Islam MS, Niwa Y, Takagi S (2009) Light-dependent intracellular positioning of mitochondria in Arabidopsis thaliana mesophyll cells. Plant Cell Physiol 50:1032–1040
Juszczuk IM, Ostaszewska M (2011) Respiratory activity, energy and redox status in sulphur-deficient bean plants. Environ Exp Bot 74:245–254
Kant S, Seneweera S, Rodin J, Materne M, Burch D, Rothstein SJ, Spangenberg G (2012) Improving yield potential in crops under elevated CO2: integrating the photosynthetic and nitrogen utilization efficiencies. Front Plant Sci 3:162. doi:10.3389/fpls.2012.00162
Kinoshita H, Nagasaki J, Yoshikawa N, Yamamoto A, Takito S, Kawasaki M, Sugiyama T, Miyake H, Weber AP, Taniguchi M (2011) The chloroplastic 2-oxoglutarate/malate transporter has dual function as the malate valve and in carbon/nitrogen metabolism. Plant J 65:15–26
Kishor PBK, Sangam S, Amrutha RN, Laxmi PS, Naidu KR, Rao KRSS, Rao S, Reddy KJ, Theriappan P, Sreenivasulu N (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci 88:424–438
Kong SG, Wada M (2011) New insights into dynamic actin-based chloroplast photorelocation movement. Mol Plant 4:771–781
Koornneef M, Meinke D (2010) The development of Arabidopsis as a model plant. Plant J 61:909–921
Kramer DM, Evans JR (2011) The importance of energy balance in improving photosynthetic productivity. Plant Physiol 155:70–78
Leister D (2012) Retrograde signaling in plants: from simple to complex scenarios. Front Plant Sci. doi:10.3389/fpls.2012.00135
Linka N, Esser C (2012) Transport proteins regulate the flux of metabolites and cofactors across the membrane of plant peroxisomes. Front Plant Sci 3:3. doi:10.3389/fpls.2012.00003
Linka N, Weber AP (2010) Intracellular metabolite transporters in plants. Mol Plant 3:21–53
Liu C, Zhao L, Yu G (2011) The dominant glutamic acid metabolic flux to produce γ-amino butyric acid over proline in Nicotiana tabacum leaves under water stress relates to its significant role in antioxidant activity. J Integr Plant Biol 53:608–618
Mano S, Nakamori C, Hayashi M, Kato A, Kondo M, Nishimura M (2002) Distribution and characterization of peroxisomes in Arabidopsis by visualization with GFP: dynamic morphology and actin-dependent movement. Plant Cell Physiol 43:331–341
Mhamdi A, Queval G, Chaouch S, Vanderauwera S, Van Breusegem F, Noctor G (2010) Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. J Exp Bot 61:4197–4220
Millar AH, Whelan J, Soole KL, Day DA (2011) Organization and regulation of mitochondrial respiration in plants. Annu Rev Plant Biol 62:79–104
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) The reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F (2011) ROS signaling: the new wave? Trends Plant Sci 16:300–309
Miyake C (2010) Alternative electron flows (water–water cycle and cyclic electron flow around PSI) in photosynthesis: molecular mechanisms and physiological functions. Plant Cell Physiol 51:1951–1963
Mubarakshina MM, Ivanov BN, Naydov IA, Hillier W, Badger MR, Krieger-Liszkay A (2010) Production and diffusion of chloroplastic H2O2 and its implication to signaling. J Exp Bot 61:3577–3587
Mullineaux PM, Baker NR (2010) Oxidative stress: antagonistic signaling for acclimation or cell death? Plant Physiol 154:521–525
Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez-Garcia B, Queval G, Foyer CH (2012) Glutathione in plants: an integrated overview. Plant Cell Environ 35:454–484
Nogales J, Gudmundsson S, Knight EM, Palsson BO, Thiele I (2012) Detailing the optimality of photosynthesis in cyanobacteria through systems biology analysis. Proc Natl Acad Sci USA 109:2678–2683
Noguchi K, Yoshida K (2008) Interaction between photosynthesis and respiration in illuminated leaves. Mitochondrion 8:87–99
Nunes-Nesi A, Sweetlove LJ, Fernie AR (2007) Operation and function of the tricarboxylic acid cycle in the illuminated leaf. Physiol Plant 129:45–56
Nunes-Nesi A, Sulpice R, Gibon Y, Fernie AR (2008) The enigmatic contribution of mitochondrial function in photosynthesis. J Exp Bot 59:1675–1684
Nunes-Nesi A, Fernie AR, Stitt M (2010) Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol Plant 3:973–996
Nunes-Nesi A, Araújo WL, Fernie AR (2011) Targeting mitochondrial metabolism and machinery as a means to enhance photosynthesis. Plant Physiol 155:101–107
Oliver SN, Lunn JE, Urbanczyk-Wochniak E, Lytovchenko A, van Dongen JT, Faix B, Schmalzlin E, Fernie AR, Geigenberger P (2008) Decreased expression of cytosolic pyruvate kinase in potato tubers leads to a decline in pyruvate resulting in an in vivo repression of the alternative oxidase. Plant Physiol 148:1640–1654
Palmieri SF, Rieder B, Ventrella A, Blanco E, Do PT, Nunes-Nesi A, Trauth AU, Fiermonte G, Tjaden J, Agrimi G, Kirchberger S, Paradies E, Fernie AR, Neuhaus HE (2009) Molecular identification and functional characterization of Arabidopsis thaliana mitochondrial and chloroplastic NAD+ carrier proteins. J Biol Chem 284:31249–31259
Pfannschmidt T, Bräutigam K, Wagner R, Dietzel L, Schröter Y, Steiner S, Nykytenko A (2009) Potential regulation of gene expression in photosynthetic cells by redox and energy state: approaches towards better understanding. Ann Bot 103:599–607
Pick TR, Bräutigam A, Schulz MA, Obata T, Fernie AR, Weber APM (2013) PLGG1, a plastidic glycolate glycerate transporter, is required for photorespiration and defines a unique class of metabolite transporters. Proc Natl Acad Sci USA 110:3185–3190
Queval G, Foyer CH (2012) Redox regulation of photosynthetic gene expression. Philos Trans R Soc Lond B 367:3475–3485
Raghavendra AS, Padmasree K (2003) Beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Trends Plant Sci 8:546–553
Riazunnisa K, Padmavathi L, Bauwe H, Raghavendra AS (2006) Markedly low requirement of added CO2 for photosynthesis by mesophyll protoplasts of pea (Pisum sativum): possible roles of photorespiratory CO2 and carbonic anhydrase. Physiol Plant 128:763–772
Riazunnisa K, Padmavathi L, Schiebe R, Raghavendra AS (2007) Preparation of Arabidopsis mesophyll protoplasts with high rates of photosynthesis. Physiol Plant 129:679–686
Scheibe R, Backhausen JE, Emmerlich V, Holtgrefe S (2005) Strategies to maintain redox homeostasis during photosynthesis under changing conditions. J Exp Bot 56:1481–1489
Schwarzlander M, Finkemeier I (2012) Mitochondrial energy and redox signaling in plants. Antioxid Redox Signal. doi:10.1089/ars.2012.5104
Selmar D, Kleinwächter M (2013) Stress enhances the synthesis of secondary plant products: the impact of stress-related over-reduction on the accumulation of natural products. Plant Cell Physiol 54:817–826
Shelp BJ, Mullen RT, Waller JC (2012) Compartmentation of GABA metabolism raises intriguing questions. Trends Plant Sci 17:57–59
Stitt M (2012) Progress in understanding and engineering primary plant metabolism. Curr Opin Biotechnol 24:229–238
Stitt M, Lunn J, Usadel B (2010) Arabidopsis and primary photosynthetic metabolism: more than the icing on the cake. Plant J 61:1067–1091
Strodtkötter I, Padmasree K, Dinakar C, Speth B, Niazi PS, Wojtera J, Voss I, Do PT, Nunes-Nesi A, Fernie AR, Linke V, Raghavendra AS, Scheibe R (2009) Induction of the AOX1D isoform of alternative oxidase in A. thaliana T-DNA insertion lines lacking isoform AOX1A is insufficient to optimize photosynthesis when treated with antimycin A. Mol Plant 2:284–297
Sunil B, Riazunnisa K, Krishna TS, Schansker G, Strasser RJ, Raghavendra AS, Mohanty P (2008) Application of fast chlorophyll a fluorescence transient (OJIP) analysis to monitor functional integrity of pea (Pisum sativum) mesophyll protoplasts during isolation. Ind J Biochem Biophys 45:37–43
Suzuki N, Koussevitzky S, Mittler R, Miller G (2012) ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ 35:259–270
Sweetlove LJ, Beard KF, Nunes-Nesi A, Fernie AR, Ratcliffe RG (2010) Not just a circle: flux modes in the plant TCA cycle. Trends Plant Sci 15:462–470
Szabados L, Savoure A (2009) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Szal B, Podgorska A (2012) The role of mitochondria in leaf nitrogen metabolism. Plant Cell Environ 35:1756–1768
Szalai G, Kellos T, Galiba G, Kocsy G (2009) Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J Plant Growth Regul 28:66–80
Szarka A, Bánhegyi G, Asard H (2013) The inter-relationship of ascorbate transport, metabolism and mitochondrial, plastidic respiration. Antioxid Redox Signal. doi:10.1089/ars.2012.5059
Szekely G, Abraham E, Cseplo A, Rigo G, Zsigmond L, Csiszar J, Ayaydin F, Strizhov N, Jasik J, Schmelzer E, Koncz C, Szabados L (2008) Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J 53:11–28
Takahashi H, Kopriva S, Giordano M, Saito K, Hell R (2011) Sulfur assimilation in photosynthetic organisms: molecular functions and regulations of transporters and assimilatory enzymes. Annu Rev Plant Biol 62:157–184
Talla S, Riazunnisa K, Padmavathi L, Sunil B, Rajsheel P, Raghavendra AS (2011) Ascorbic acid is a key participant during the interactions between chloroplasts and mitochondria to optimize photosynthesis and protect against photoinhibition. J Biosci 36:163–173
Taniguchi M, Miyake H (2012) Redox-shuttling between chloroplast and cytosol: integration of intra-chloroplast and extra-chloroplast metabolism. Curr Opin Plant Biol 15:252–260
Terashima I, Hanba YT, Tholen D, Niinemets U (2011) Leaf functional anatomy in relation to photosynthesis. Plant Physiol 155:108–116
Verslues PE, Sharma S (2010) Proline metabolism and its implications for plant-environment interaction. Arabidopsis Book. doi:10.1199/tab.0140
Voss I, Sunil B, Scheibe R, Raghavendra AS (2013) Emerging concept for the role of photorespiration as an important part of abiotic stress response. Plant Biol 15:713–722
Weber APM, Bräutigam A (2013) The role of membrane transport in metabolic engineering of plant primary metabolism. Curr Opin Biotechnol 24:256–262
Weber AP, Linka N (2011) Connecting the plastid: transporters of the plastid envelope and their role in linking plastidial with cytosolic metabolism. Annu Rev Plant Biol 62:53–77
Wirtz M, Droux M (2005) Synthesis of the sulfur amino acids: cysteine and methionine. Photosynth Res 86:345–362
Yabuta Y, Mieda T, Rapolu M, Nakamura A, Motoki T, Maruta T, Yoshimura K, Ishikawa T, Shigeoka S (2007) Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J Exp Bot 58:2661–2671
Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2:1565–1572
Yoshida K, Terashima I, Noguchi K (2011) How and why does mitochondrial respiratory chain respond to light? Plant Signal Behav 6:864–866
Acknowledgments
The study is supported by a J.C. Bose National Fellowship (No. SR/S2/JCB-06/2006) to A.S.R, from the Department of Science and Technology, India; Council of Scientific and Industrial Research (New Delhi)—Research Associateship to B. S. and University Grants Commission-Junior Research Fellowship to V. A. We also thank DBT-CREBB, DST-FIST and UGC-SAP-CAS, for support to Department/School.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sunil, B., Talla, S.K., Aswani, V. et al. Optimization of photosynthesis by multiple metabolic pathways involving interorganelle interactions: resource sharing and ROS maintenance as the bases. Photosynth Res 117, 61–71 (2013). https://doi.org/10.1007/s11120-013-9889-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-013-9889-z