Abstract
The maximum quantum yield (Φ max), calculated from the maximum chlorophyll a specific photosynthetic rate divided by the quantum absorption per unit chlorophyll a, is 8 photons or 0.125 mol C per mol Quanta light energy. For the average solar radiation that reaches the earth’s surface this relates to a photosynthetic yield of 1.79 g(dw) m−2 day−1 per percentage photosynthetic efficiency and it could be doubled for sunny, dry and hot areas. Many factors determine volumetric yields of mass algal cultures and it is not simply a question of extrapolating controlled laboratory rates to large scale outdoor production systems. This is an obvious mistake many algal biotechnology start-up companies make. Closed photobioreactors should be able to outperform open raceway pond cultures because of the synergistic enhancement of a reduced boundary layer and short light/dark fluctuations at high turbulences. However, this has not been shown on any large scale and to date the industrial norm for very large production systems is open raceway production ponds. Microalgal biomass production offers real opportunities for addressing issues such as CO2 sequestration, biofuel production and wastewater treatment, and it should be the preferred research emphasis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In many aspects microalgae (used pragmatically because it has no taxonomic significance) and photosynthesis are analogous. Although it could be argued that photosynthesis research started some 350 years ago (Huzisige and Ke 1993; Gest 1997), it was with Chlorella as model organism in the laboratories of Warburg in the early twentieth century that photosynthesis, as a process, became unravelled. Applied research and the production of algal biomass became realities in the 1940s and towards the end of World War II microalgae were grown in larger quantities for purposes such as the production of lipids for energy using flue-gasses, anti-microbial substances, the production of various bio-chemicals and plans were made to use algae for sewage treatment (Burlew 1953; Oswald 1980).
The interest in producing algal biomass for various applications essentially started at Stanford (USA) (Burlew 1953) and was then taken up in Tokyo (Japan), Essen (Germany), Israel, Czech Republic and Taiwan (Soeder 1980). The first full-scale research and production facility for the mass production of microalgae started around 1960 at Trebon in the Czech Republic (Setlik et al. 1970). About this time it was reported that naturally growing Spirulina was harvested and eaten by local people at lake Tchad, Africa, Nostoc species in Mongolia, China and Spirulina in the Andes Mountains of Peru (Soeder 1980).
Microalgae biomass production has come a long way since these early studies and this overview deals mainly with photosynthetic efficiencies in mass algal cultures, how this could be influenced in the specific reactor type, challenges and realities.
Photosynthetic efficiency
Maximum photosynthetic efficiency (energetic efficiency) and the upper limits of photosynthetic productivity have, since the early days been central to the mass cultivation of microalgae (Wassink et al. 1953). However, from the early times photosynthetic efficiencies have been the subject of heated debate and differences. Otto Warburg’s extremely high quantum yields were challenged by his own student Robert Emerson, the latter who eventually established the accepted value of 0.12 quanta per mol O2 (Huzisige and Ke 1993). Pirt et al. (1980) as Warburg some 70 years earlier claimed extremely high photosynthetic efficiencies and values as high as 46.8% were reported. This was substantially reduced when Pirt (1986) reported maximum photosynthetic efficiencies being 12–13% based on total incident solar radiation. Bolton and Hall (1991) also stressed the importance of expressing efficiencies in terms of total incident solar radiation, because calculations that are based on photosynthetic active radiation (PAR, 400–700 nm) will be 2.3 times higher. They calculated the maximum photosynthetic efficiency to be 13%, but that 8–9% would be the maximum possible under optimal field conditions. As Grobbelaar (2009a) pointed out, this agrees with the theoretical treatment of photosynthetic efficiencies as done by Gebhardt (1986), who defined the total efficiency of photosynthesis (η); as the Gibbs free energy ∆G of the produced biomass divided by the energy E of the incident radiation. An undisputed fact today, is that the maximum quantum yield (Φ max), calculated from the maximum chlorophyll a specific photosynthetic rate divided by the quantum absorption per unit chlorophyll a, is 8 photons or 0.125 mol C per mol Quanta light energy (Woźniak et al. 2002), which is very similar to the value of Emerson.
The structure and functioning of PSII have been elucidated in recent years but of greater importance in terms of photosynthetic efficiencies and theoretical maxima, are the timescales of forward electron transfer within PSII. This is the spillover rate of excited electrons to the primary electron acceptors and along the electron transport chain. Using Electron Paramagnetic Resonance (EPR), time resolved X-ray and absorbance measurements it was shown that most individual reactions take place within 300 μs, but that about 1 ms is required for an oxygen molecule to evolve (Burda 2007). Thus, the restriction in photosynthetic efficiencies is the turnover rate of 1 ms and excess electrons (energy) must simply be dissipated.
Average areal production rates
The average expected production rates can be calculated from photosynthetic efficiencies. Using the 0.125 mol C per mol Quanta light energy one can calculate that on average 1.79 g(dw) m−2 day−1 per percentage photosynthetic efficiency can be produced, assuming that the average solar radiation reaching the earth’s surface is 1,104 μmol quanta m−2 s−1, that algal biomass consists of 40% C and that the diurnal cycle is 12 h:12 h (see also Grobbelaar 2009a). Thus, the maximum areal productivity on average for the earth is 14.31 g(dw) m−2 day−1 at a photosynthetic efficiency of 8%. Many areas of the world would receive double the irradiance and these would be the obvious areas for algal mass cultivation. As shown by Tredici (2010), areas where the highest areal productivities should be attained are; south-western USA, northern and southern areas of Africa, southern areas of Asia, central Australia and the Caribbean Islands.
However, Grobbelaar et al. (1996) and Grobbelaar (2006), as several others, have shown that light energy influences photosynthetic rates on a number of ways, namely:
-
(1)
Available photosynthetic active radiation.
-
(2)
The frequency of the light/dark (L/D) fluctuations.
-
(3)
The ratio of L:D.
-
(4)
The light acclimated state of the microalgae.
-
(5)
The light history, being either continuous or fluctuating.
The impact can be as a single factor or a combination of all five.
Light intensity and quality
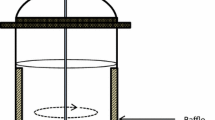
The photosynthetic versus irradiance response curve (P/I) has been used extensively to describe the response of algae to photosynthetic active irradiation (Fig. 1c). Three distinct regions are discernable; i.e. an initial light-limited region at low light intensities where photosynthetic rates increase with increasing irradiance, a light-saturated region where photosynthetic rates are independent of irradiance, and a region of photoinhibition in which photosynthetic rates decrease with an increase in irradiance. In the light-limited region, the rate of photon absorption is correlated with the rate of electron transport from water to CO2, with the liberation of O2. This initial slope is usually donated by the symbol α (Jassby and Platt 1976) and when normalised with chlorophyll a or biomass it represents the maximum quantum yield of photosynthesis (see also Fig. 3). Not only does the intensity determine the rates, but also the quality where blue and red light dominates the action spectra of photosynthesis. Paradoxically as light penetrates a dense algal suspension, both the blue and red wavelengths are attenuated first, leaving the photosynthetically inactive green light to penetrate the deepest.
Light zones in a dense algal culture, where a simplistically divides the optical cross section into an upper photoinhibited layer, overlying a photo-saturated layer, followed by a photo-unsaturated or light dependant layer, and a dark layer at the bottom. The circle with arrows indicates mixing. b gives the attenuation of light with depth and c represents the P/I curve. I is the light intensity, P B is the biomass specific productivity and z is the culture depth or optical cross section
At light saturation, the rate of photon absorption exceeds the rate of electron turnover in PS II and eventually this leads to a light-induced depression of photosynthesis, commonly referred to as photoinhibition. Important are to consider the time scales for the manifestation of photoinhibition. Over the short term, light-induced photoinactivation of PS II could be viewed as a survival strategy by reducing the number of redundant PS II units. Long-term exposures eventually results in PS II reaction centres not being repaired, through the continuous replacement of the D1 protein. The damage then becomes permanent resulting in PS II inactivation (Prasil et al. 1992). Although it is often suggested that photoinhibition does not occur in dense mass algal cultures, Congming and Vonshak (1999) reported a midday maximum quantum yield depression of dark adapted Spirulina platensis as a result of reaction centre inactivation, which they ascribed to photo-inhibition.
Behrenfeld et al. (1998) found for natural phytoplankton assemblages sampled in the south Pacific and laboratory grown Thalassiosira weissflogii, that P max was unaffected even at a reduction in PSII reaction centres of almost 50%, due to increased electron turnover in the remaining functional PSII centres. The reason for this, being that the acceptor side of photosynthesis is limited by the capacity of the Calvin-cycle and the impact of photo-inhibition would only become apparent when the numbers of reaction centres are reduced to such a level that the capacity of the Calvin-cycle cannot be met.
Photoprotection could take place during prolonged exposure to high irradiancies and the distinction or interaction between damage (photo-oxidation) and photoprotection is difficult (Demmig-Adams and Adams 1992). Synthesis of photoprotective pigments such as β-carotene and zeaxanthin is common in algae allowing them to tolerate high irradiances, which is typical of high light-acclimated algae. Temperature, salinity and nutrient (especially nitrogen) stress could severely influence the over-excitation of PS II and synthesis of auxiliary pigments, with the concomitant depression of photosynthetic rates (e.g. Bongi and Long 1987; Vonshak et al. 2001; Herzig and Falkowski 1989).
Intermittent light
Since the early study of Kok (1953), it was known that photosynthesis is influenced by the intensity of light, L/D fluctuations and the ratio of dark time to light time. Kok (1953) used the term ‘flashing light’ to indicate the light time and it has often been confused with single turnover flashes. He concluded that the light time should be less than 4 ms in order to achieve ‘full’ efficiency and that the dark time should be at least ten times as long as the light time. For several decades, the enhancement of photosynthesis in intermittent light was interpreted as some residual light energy that was captured during the light time and then utilised in the dark until the next flash is received.
Kok (1953) also realised that the pattern of intermittent light required, for high yields of Chlorella was dependant on turbulence manifested in a flowing culture. The value of turbulence enhancement was eloquently demonstrated by Laws et al. (1983) who placed aerofoil type of devices in flowing cultures. These caused vortices of 0.5–1 Hz at a flow rate of 30 cm/s, which resulted in photosynthetic conversion efficiencies increasing from an average of 3.7 to up to 10%.
According to Terry (1986), two conditions are necessary for the enhancement of photosynthesis in a modulating light field, i.e. a longer dark period than the light period and relatively high photon flux densities. In fact, cells that are exposed to intermittent high light intensities in growing algal cultures experience a dilution of the light, because over time they receive less and less light, due to the increase in the volumetric biomass concentrations. A prerequisite for enhancement is that the L/D frequencies are sufficiently short in order to match the turnover time of Q A (the primary electron acceptor of PS II) from a reduced state to an oxidised state and then re-oxidised again (as indicated previously the turnover time is about 1 ms). Should this not be the case, then the excess energy will be dissipated. This could explain why in several studies, both in natural environments and in mass microalgal cultures, no enhancement due to intermittent L/D cycles were found (e.g. Falkowski and Wirick 1981; Grobbelaar, 1989; Jewson and Wood 1975). It was established that the L/D cycles, in these cultures, fell within the medium range of minutes to hours (Grobbelaar 1989) where no enhancement takes place.
Shown in Fig. 2 is the response of Scenedesmus obliquus (TURP.) Kütz. Strain 206 (Bartos, Cuba) to various L/D fluctuations. The material and methods are given in Grobbelaar et al. (1996). The algae were subjected to constant L/D fluctuations of 1 Hz for 24 h (Fig. 2a) and 72 h (Fig. 2b). The specific productivity (P B) at continuous illumination is also shown and it clearly shows that none of the rates in the fluctuating light treatments came near to continuous illumination. The results confirm that specific production rates of microalgae increase with shorter L/D fluctuations. This increase in specific production rates is exponential and it was consistent for all treatments and measurements (see also Grobbelaar et al. 1996). Photosynthetic efficiencies for the equal light/dark periods (1L/1D) was equal to that in continuous illumination at about 50 to 100 ms L/D fluctuations (calculated on the quantity of light received over time). At longer time durations, the photosynthetic efficiency decreased and vice versa. This was also true for the L/D fluctuations where the dark time was double that of the light period (1L/2D).
Legendre et al. (1986) suggested that phytoplankton may acclimate to the dominant L/D frequencies in their environment. It is clear that after 72 h of exposure to L/D frequencies of 1 Hz (Fig. 2), not only was the overall specific production rates lower compared to after 24 h, but also the difference was significantly less between the 1L/1D and the 1L/2D. This was particularly noticeable at the shorter frequencies, while at the longer frequencies essentially the same pattern was seen, as that after an exposure of 24 h (Fig. 2a).
Grobbelaar (1989) pointed out that microalgae grown in dense mass algal cultures are subjected to three ranges of intermittent light, namely:
-
(1)
low frequency cycles of hours to days and years,
-
(2)
medium frequency fluctuations of seconds to minutes, and
-
(3)
high frequency fluctuations of 100 ms (10 Hz) and less.
The low frequency cycles is determined mainly by diurnal and seasonal variations, influencing mainly the periodicity of cell division (Sorokin 1957) and circadian rhythms in metabolism such as photosynthesis (Doty and Oguri 1957). It has also been shown that they influence growth rates, pigment concentrations, and also phytoplankton community composition and diversity (Flöder et al. 2002; Litchman 1998; Nicklisch and Fietz 2001). Medium frequency fluctuations are the dominant range to which most algae in culture and nature are exposed too. Such frequencies would be the norm in open raceway mass algal production systems. High frequency fluctuations would be possible in closed short light path (SLP) photobioreactors (PBR) or in the thin layer open ponds (Grobbelaar et al. 1996) at high mixing rates.
Light acclimation
Algae (and plants) have developed several mechanisms to cope with changes in the quality and intensity of light. In essence, higher plants and algae aim to balance the light and dark photosynthetic reactions. Sukenik et al. (1987) found for Dunaliella tertiolecta, that Rubisco levels remained relatively constant under varying light regimes and they concluded that the major regulation occurs in the light reactions, especially of PS II. This is possible through the modulation of the light-harvesting capacity and/or changes in the number of PS II reaction centres.
Light acclimation in essence means that the microalgae will undergo a number of physiological changes in order to capture as much of the prevailing light energy as possible (Fig. 3, after Grobbelaar 2006). Arbitrarily, the extremes can be termed high light (HL) and low light (LL) acclimation. In any optically dense culture that is completely mixed this would imply the average light intensity for the specific optical depth and light attenuation as determined by the biomass. These changes include altering photosynthetic efficiencies, concentrations of chlorophyll a and accessory pigments, maximum chlorophyll a specific production rates, the transition light intensity from light-limited to light-saturated photosynthesis, light compensation intensities and the light intensity that causes photoinhibition (Falkowski et al. 1994; Grobbelaar et al. 1996; Grobbelaar 2006). Even dark respiration (R d) varies considerably depending on the light history (Grobbelaar and Soeder 1985), where high light-acclimated algae will have higher dark respiration rates than low light acclimated ones.
P/I or the light response curve of photosynthesis versus light intensity. Pmax, the maximum photosynthetic rate measured at saturated I; Rd, dark respiration; α, maximum photosynthetic efficiency; Ik, the transition light intensity between light dependant and light saturated photosynthesis; and Pi, photoinhibition. Solid arrows indicate the affect of dark acclimation and open arrows the response to light acclimation (see also Grobbelaar 2006)
The time scales within which algal cells photoacclimate range from seconds to hours, depending on the parameters measured. Cullen and Lewis (1988) determined that the photoadaptive response for Thalassiosira, of up to 50% of the full response, could take place within 10 min to 21 h. Paradoxically, it appears as if the tendency is for algae to mostly be in the low LL-acclimated state. This is the case in all high density algal cultures (Grobbelaar et al. 1995). The implication of this is that algae can effectively utilise low light intensities, but on the other hand they often have to deal with excess energy. Mostly the excess energy is handled in PS II through either state transitions or dissipation, and these happen within seconds to minutes. The reality is that any excess energy, that is received by a LL-acclimated algal culture is wasted and it contributes nothing to photosynthetic rates and yields. Structural and biochemical changes that take place require longer time scales.
The effect of light acclimation on the P/I curve is shown in Fig. 3, where the open arrows show the direction of the response due to high light and the solid arrows the response due to low light acclimation. This dynamic response of microalgae to the light climate in mass algal cultures directly influences the areal yields of growth reactors. Following inoculation of a culture operated batch-wise, the algae become high light acclimated and as the culture density increases they become low light acclimated (Grobbelaar et al., 1995). Should the turbulence in the reactor remain the same, the increase in biomass has important implications for the overall light regime and light/dark cycles to which the algae are exposed.
It is possible to exploit the photo-acclimation properties of microalgae. Grobbelaar and Kurano (2003) described a multi-layer photobioreactor where the microalgae are forced to acclimate to either HL or LL in the same reactor. In the reactor, the entire light intensity range is optimally used and increases of up to 43% over a single layer photobioreactor were measured.
Turbulence
The in-culture light climate, as discussed above, is directly dependent on mixing. However, Richmond and Becker (1986) stated that, when the nutritional requirements of mass-cultured algae are satisfied and the environmental conditions are not growth-limiting, then mixing designed to create a turbulent flow constitutes the most important requisite for consistently high yields of algal mass. The three major effects of mixing are:
-
(1)
Prevention of algal sedimentation.
-
(2)
To prevent the formation of nutritional and gaseous gradients.
-
(3)
To move the cells through an optically dense gradient, with variations in the quantity and quality of light energy.
Algal sedimentation and accumulation of algal biomass in ‘dead’ zones (sections of the growth reactor where mixing is low) could severely inhibit algal production, because of the development of anoxic biomass decay products, bacterial growth and zones where invertebrates and protozoa proliferate. Such infections can cause pond failure in the matter of days (Grobbelaar 1981). Mixing implies moving liquid in a tube, channel or container (e.g. plate reactors). The degree of mixing directly determines the resultant turbulence in the reactor, which again is a function of energy input. Several studies have investigated different stirring devices, energy transfer efficiencies and impact on algal growth (Richmond 2004). There is a delicate balancing act that needs to be followed in the mass production of microalgae between; mixing, hydrodynamic stress, turbulent flow and energy input. Hydrodynamic or shear stress can reduce productivities significantly and can lead to cell breakage. Turbulent flow will only manifest at Reynolds numbers greater than 3,300 (Grobbelaar 1989), which could be attained in thin (<25 mm diameter) tubular reactors at a high energy input, but for all practical purposes impossible in cultures with optical depths >100 mm. If high turbulences could be achieved, Grobbelaar (1994) showed that there is a synergistic relationship between the above points (2) and (3) resulting in higher productivities (see below).
Nutrients
Nutrients, culture depth (optical depth), rate of mixing and growth reactor type are variables that are not only chosen, but they can be modified, controlled and manipulated. Many studies have been conducted to determine the optimal nutrient concentrations for various algal species. Initially, soil water extracts formed the basis of culture solutions (Pringsheim 1950). Vonshak (1986) summarised the requirements for developing nutrient recipes for algal cultivation as follows:
-
(i)
The total salt content, which is determined by the habitat from where the algae originates.
-
(ii)
The composition in terms of the major ionic components such as K+, Mg2+, Na+, Ca2+, SO =4 and Cl−.
-
(iii)
The nitrogen sources, especially nitrate, ammonium and urea.
-
(iv)
Carbon source either CO2 or HCO3 −.
-
(v)
pH.
-
(vi)
Trace elements and some chelating agent such as EDTA.
-
(vii)
Vitamins.
Additionally, the specific trophic route and the introduction of nutrient stress should also be considered (Grobbelaar 2004). For example, nitrogen stress is important for carotogenesis in Dunaliella salina (Ben-Amotz and Avron 1989) and increased lipid production in Chlorella vulgaris (Stephenson et al. 2010).
The adequate supply of nutrients is a prerequisite for high production rates, but the range of concentrations is fairly wide for macronutrients, but much narrower for micronutrients. Not only is growth retarded in the deficient zone, but it can lead to alien species becoming dominant, increased infections by bacteria, fungi, viruses, invertebrates and protozoa, and finally this could lead to the total collapse of the cultures. Furthermore, microalgae are adapted to scavenge their environments for resources, be it through structural changes, storage or increased resource utilisation efficiencies. Internal adjustments involve biochemical and physiological acclimation, whilst externally they can excrete a variety of compounds to amongst others render nutrients available or limit the growth of competitors (Grobbelaar 2004). They are also capable of ‘luxury uptake’ especially for phosphorus, that will allow them to survive for several generations in a phosphorus deplete medium. This has led to the development of growth predictive models that rely on the internal cellular concentrations (Droop 1983) as opposed to those that only take the nutrient content of the growth medium into consideration.
Growing microalgae in the real world
The previous sections dealt with the growth requirements of microalgae, photosynthetic efficiencies and the potential of growing microalgae. Yet, many obstacles remain and in general it can be stated that most promises and claims made to date, of producing extreme quantities of biomass (e.g. >1,200 l oil ha−1 day−1) were misleading and totally unrealistic. Unfortunately, many investors lost large amounts of money (see also Tredici 2010) and Walker (2009) make the point that all green organisms photosynthesise at the same rate at dim light, with some differences at high light intensities.
In essence, growing microalgae appears to be simple and easy, especially when observing natural blooms of phytoplankton. Microalgae require an energy source, either light energy for autotrophic growth or an organic compound for heterotrophic growth. Between these two extremes there are several intermediate trophic routes (Grobbelaar 2004). Growth in a photobioreactor implies autotrophic growth and for this they require plant growth nutrients, a carbon source being either dissolved CO2 or HCO3 −, a favourable temperature and pH, and water (moist soil or surfaces are also suitable). In nature, microalgal growth rates reaching 1 g (dw) m−2 day−1 and more would be exceptional. For biomass production to satisfy the demands of the so-called ‘green revolution’ much higher rates are obviously needed.
All organisms have a minimum, optimal and maximum requirement for the factors that influence their growth rates. As Grobbelaar (2009b) pointed out, growing an alga at its optimal temperature, light intensity, nutrient concentrations and CO2 levels will significantly increase the yield, but the rates will be low. In fact increased rates are what algal biotechnologists strive for and not yields per se (yield defined as the biomass produced from a given set of resources). The object, therefore, is to realise the highest yields in the shortest possible time, or in terms of algal biotechnology high volumetric and areal production rates. According to Grobbelaar (2009b), the questions applied phycologists need to resolve are:
-
(1)
How to improve the capture of light energy, uptake of nutrients and CO2?
-
(2)
The role of excreted metabolites and auto-inhibition?
-
(3)
The differences/advantages/disadvantages between “open” and “closed” photobioreactors, if any?
-
(4)
The requirements of the specific application and the resources at hand.
When growing algae in a photobioreactor the number of variables that can be controlled are limited. These are:
-
(1)
Culture depth or optical cross section. In open ponds light is attenuated with depth (Fig. 1a, b), while the light attenuation and distribution become more complicated in closed vertically placed and transparent tube reactors.
-
(2)
Mixing and the resultant turbulence. This differs markedly between open and closed systems, where much higher rates of turbulence can be achieved in closed tubular reactors, while laminar flow is often a problem in open raceway ponds.
-
(3)
Supply of nutrients and CO2, and the prevention of deficit zones.
-
(4)
The biomass concentration or areal density. This determines the in-culture light climate, where a higher biomass will attenuate light energy more and vice versa. This also determines the light acclimated state of the microalgae.
-
(5)
The culture operation being either batch, semi-continuous or continuous.
-
(6)
Temperature within limits as well as the O2 build up in closed reactors.
Several of these variables are interrelated, e.g. the in-culture light climate is determined by the biomass concentration and mixing. When considering all these variables and options, it might explain why areal productivity rates are on average about 12–14 g(dw) m−2 day−1 (Benemann 2008). As mentioned above, many unrealistic claims about productivities and the ownership of some secret novel ‘super’ growth reactors are rife in recent times. Tredici (2010) lists some of these start-up companies that have failed in recent times.
Another major problem facing the industry is the large variance in areal production rates (Fig. 4). There is an urgent need to rigorously generate long-term data for various growth reactor types, under comparable field conditions and operating processes. Until this is done, many more claims will be made and many more investors will lose their money.
Maximum areal productivities against optical culture depth of several researchers as quoted by Lee (2001). Circles are for closed photobioreactors and the triangles for open raceway ponds
Growth reactor
In general, a distinction is made between open raceway reactors and photobioreactors for the mass production of microalgae. Grobbelaar (2009b) argued against this practice and also classifies open raceway production systems as photobioreactors. The major reason for the distinction is whether cultures are ‘open’ (portion of the culture is in contact with the atmosphere) or ‘closed’ (no direct contact between the culture and the atmosphere) (Tredici 2004).
As mentioned before, major differences and problems between ‘open’ and ‘closed’ photobioreactors are the degree of turbulence achieved in the cultures, oxygen accumulation in closed cultures (open thin layer sloping ponds are excluded from this generalisation) and fouling in closed reactors. Since the mass transfer rates between the nutrient solution and the algal cells, as well as the transfer of metabolites (e.g. oxygen, excreted organic compounds) from the cells to the growth medium, are affected by turbulence, this would have a direct influence on growth rates. This is because turbulence determines the mass transfer rates between the liquid phase and the microalgal cell (Grobbelaar 1994), where this boundary layer is low at low turbulences and vice versa. A thin boundary layer enhances the uptake of nutrients and the release of metabolites, because adequate mixing essentially prevents the formation of nutritional and gaseous gradients.
Mixing also moves the cells through an optically dense gradient, with variations in the quantity and quality of light energy and Grobbelaar (1994) showed that there is a synergistic relationship between nutrient uptake, release of metabolites, L/D cycles and growth rates. Increased turbulence would result in increased exchange rates of nutrients and metabolites between the cells and their growth medium. Together with the increased light/dark frequencies this increases productivity synergistically (Grobbelaar 1994). He also pointed out that this will probably only be possible in tubular cultures with an optical depth of <50 mm. Hanging plastic bags, plate reactors and raceway ponds will be excluded from such possible enhancement.
The advantages and disadvantages of open raceway ponds versus closed PBRs have been discussed in detail (Pulz 2001; Grobbelaar 2009b; Tredici 2010). An important trend is the number of published articles in the scientific literature that deals with photobioreactors (Fig. 5), many of them claiming some unique property or some undisclosed competitive advantage. For most, the question of scale finally proves insurmountable and they end up being ‘nice toys’. For any photobioreactor that will fulfil the requirements of producing large masses of biomass over time, they need to be scalable to many hectares. At this stage the only truly very large production systems (excluding the extensive systems such as the shallow dams for producing Dunaliella on the west coast of Australia, Borowitzka and Borowitzka 1989) are raceway ponds with surface areas of up to 1,000 m2 and more, arranged side by side and covering many hectares, e.g. Cyanotech Corporation, Hawaii and Earthrise Nutritionals, California. There are many options available for improving the designs of open raceway ponds, of which mixing and turbulence are the most important. No long-term data have yet been generated for any large scale closed reactor (PBR).
Conclusions
Microalgal biotechnology can be important in providing solutions for CO2 sequestration and global warming, biofuel production, wastewater treatment, production of fine chemicals and many other uses. Chisti (2007) even went so far as to suggest that biodiesel from microalgae could replace petroleum-based fuels. There is no doubt that microalgal biomass could significantly change energy supply patterns, food and feed and Grobbelaar et al. (2000) concluded that microalgae of all the processes available for CO2 sequestration, affords the best possible solution. However, more cooperation and exchange of information are needed. The many unsubstantiated claims pose a serious threat to the industry and a repetition of the solutions to the world protein shortages of the 1970s, that failed dismally as well as the promises made during the energy crisis of the 1980s should be prevented. The real breakthrough in successful algal biotechnology ventures will come when a multi-product approach is followed, whilst utilising some waste product. Ideally, this waste product should be CO2 and the entire contents of the produced algal biomass should be used, e.g. lipids, carbohydrates, proteins, pigments and even cellulose.
References
Behrenfeld MJ, Prasil O, Kolber ZS, Babin M, Falkowski PG (1998) Compensatory changes in photosystem II electron turnover rates protect photosynthesis from photoinhibition. Photosynth Res 58:259–268
Ben-Amotz A, Avron A (1989) The biotechnology of mass culturing Dunaliella for products of commercial interest. In: Cresswell RC, Rees TAV, Shah N (eds) Algal cyanobacterial biotechnology. Longman Scientific & Technical, Essex, pp 91–114
Benemann J (2008) Opportunities and challenges in algae biofuels production: a position paper. www.futureenergyevents.com
Bolton JR, Hall DO (1991) Maximum efficiency of photosynthesis. Photochem Photobiol 53:545–548
Bongi G, Long SP (1987) Light-dependant damage to photosynthesis in olive leaves during chilling and high temperature stress. Plant Cell Environ 10:241–249
Borowitzka MA, Borowitzka LJ (1989) Dunaliella. In: Borowitzka MJ, Borowitzka LJ (eds) Micro-algal biotechnology. Cambridge University Press, New York, pp 27–58
Burda K (2007) Dynamic of electron transfer in photosystem II. Cell Biochem Biophys 47:271–284
Burlew JS (1953) Algal culture: from laboratory to pilot plant. Carnegie Institution of Washington Publication, Washington, p 357
Chisti Y (2007) Biodiesel from microalgae beats bioethanol. Trends Biotechnol 26:126–131
Congming L, Vonshak A (1999) Photoinhibition in outdoor Spirulina platensis cultures assessed by polyphasic chlorophyll fluorescence transients. J Appl Phycol 11:355–359
Cullen JJ, Lewis MR (1988) The kinetics of algal photoadaptation in the context of vertical mixing. J Plankton Res 10:1039–1063
Demmig-Adams B, Adams WW (1992) Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol 43:590–626
Doty MS, Oguri M (1957) Evidence for a photosynthetic daily periodicity. Limnol Oceanogr 2:37–40
Droop MR (1983) 25 years of algal growth kinetics: a personal view. Bot Mar 26:99–112
Falkowski PG, Wirick CD (1981) A simulation model of the effects of vertical mixing on primary productivity. Mar Biol 45:289–295
Falkowski PG, Greene R, Kolber Z (1994) Light utilization and photoinhibition of photosynthesis in marine phytoplankton. In: Baker NR, Bower JR (eds) Photoinhibition of photosynthesis. Bios Scientific Publishers Ltd, Oxford, pp 407–432
Flöder S, Urable J, Kawabata Z (2002) The influence of fluctuating light intensities on species composition and diversity of natural phytoplankton communities. Oecologia 133:395–401
Gebhardt W (1986) Photosynthetic efficiency. Radiat Environ Biophys 25:275–288
Gest H (1997) A ‘misplaced chapter’ in the history of photosynthesis research; the second publication (1796) on plant processes by Dr Jan Ingen-Housz, MD, discoverer of photosynthesis. Photosynth Res 53:65–72
Grobbelaar JU (1981) Infections: experiences in mini-ponds. In: Grobbelaar JU, Soeder CJ, Toerien DF (eds) Wastewater for aquaculture, UOVS Publication Series C, vol 3. UOVS, Bloemfontein, pp 116–123
Grobbelaar JU (1989) Do light/dark cycles of medium frequency enhance phytoplankton productivity? J Appl Phycol 1:333–340
Grobbelaar JU (1994) Turbulence in mass algal cultures and the role of light/dark fluctuations. J Appl Phycol 6:331–335
Grobbelaar JU (2004) Algal Nutrition. In: Richmond A (ed) Handbook on microalgal culture. Blackwell Science, Malden, pp 97–115
Grobbelaar JU (2006) Photosynthetic response and acclimation of microalgae to light fluctuations. In: Subba-Rao DV (ed) Algal cultures analogues of blooms, applications. Science Publishers, Enfield/Plymouth, pp 671–683
Grobbelaar JU (2009a) Upper limits of photosynthetic productivity and problems of scaling. J Appl Phycol 21:519–522
Grobbelaar JU (2009b) Factors governing algal growth in photobioreactors: the “open” versus the “closed” debate. J Appl Phycol 21:489–492
Grobbelaar JU, Kurano N (2003) A novel photobioreactor for achieving extreme high yields. J Appl Phycol 15:121–126
Grobbelaar JU, Soeder CJ (1985) Respiration losses in planktonic green algae cultivated in raceway ponds. J Plankton Res 7(4):497–506
Grobbelaar JU, Nedbal L, Tichy V, Setlik I (1995) Variations in some photosynthetic characteristics of microalgae cultured in outdoor thin-layered sloping reactors. J Appl Phycol 7:243–260
Grobbelaar JU, Nedbal L, Tichy V (1996) Influence of high frequency light/dark fluctuations on photosynthetic characteristics of microalgae photoacclimated to different light intensities and implications for mass algal cultivation. J Appl Phycol 8(4–5):335–343
Grobbelaar JU, Mohn FH, Soeder CJ (2000) Potential of algal mass cultures to fix CO2 emissions from industrial point sources. Algol Stud 98:169–183
Herzig R, Falkowski PG (1989) Nitrogen limitation in Isochrysis galbana. 1. Photosynthetic energy conversion and growth efficiencies. J Phycol 25:462–471
Huzisige H, Ke B (1993) Dynamics of the history of photosynthesis research. Photosynth Res 38:185–209
Jassby AD, Platt T (1976) Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol Oceanogr 21:540–547
Jewson DH, Wood RB (1975) Some effects on integral photosynthesis of artificial circulation of phytoplankton through light gradients. Verh Int Verein Limnol 19:1037–1044
Kok B (1953) Experiments on photosynthesis by Chlorella in flashing light. In: Burlew JS (ed) Algal culture: from laboratory to pilot plant, vol 600. Carnegie Institution of Washington Publication, Washington. pp 63–75
Laws EA, Terry KL, Wickman J, Chalup MS (1983) A simple algal production system designed to utilize the flashing light effect. Biotechnol Bioeng 25:2319–2335
Lee Y-K (2001) Microalgal mass culture systems and methods: their limitation and potential. J Appl Phycol 13:307–315
Legendre L, Rochet M, Demers S (1986) Sea-ice microalgae to test the hypothesis of photosynthetic adaptation to high frequency light fluctuations. J Exp Mar Biol Ecol 97:321–326
Litchman E (1998) Population and community responses of phytoplankton to fluctuating light. Oecologia 117:247–257
Nicklisch A, Fietz S (2001) The influence of light fluctuations on growth and photosynthesis of Stephanodiscus neoastrea (diatom) and Planktothrix agardhii (cyanobacterium). Arch Hydrobiol 151:141–156
Oswald WJ (1980) Algal production—problems, achievements and potential. In: Shelef G, Soeder CJ (eds) Algae biomass. Elsevier/North Holland Biomedical Press, Amsterdam, pp 1–8
Pirt SJ (1986) The thermodynamic efficiency (quantum demand) and dynamics of photosynthetic growth. New Phytol 102:3–37
Pirt SJ, Lee Y-K, Richmond A, Pirt MW (1980) The photosynthetic efficiency of chlorella biomass growth with reference to solar energy utilization. J Chem Technol Biotechnol 30:25–34
Prasil O, Adir N and Ohad I (1992) Dynamics of photosystem II: mechanisms of photoinhibition and recovery processes. In: Barber J (ed) Topics in photosynthesis, the photosystems: structure, function and molecular biology, vol 11. Elsevier, Amsterdam, pp 295–348
Pringsheim EG (1950) The soil-water culture technique for growing algae. In: Prescott JB, Tiffany LH (eds) Culturing of algae. The Charles F. Kettering Foundation, Dayton, pp 19–26
Pulz O (2001) Photobioreactors: production systems for phototrophic microorganisms. Appl Microbiol Biotechnol 57:287–293
Richmond A (2004) Biological principals of mass cultivation. In: Richmond A (ed) Handbook of microalgal culture: biotechnology, applied phycology. Blackwell Science, London, pp 125–177
Richmond A, Becker EW (1986) Technological aspects of mass cultivation—A general outline. In: Richmond A (ed) Handbook of microalgal mass culture. CRC Press, Inc, Boca Raton, pp 245–263
Setlik I, Sust M, Malek I (1970) Dual purpose open circulation units for large scale culture of algae in temperate zones. 1. Basic design consideration and scheme of pilot plant. Algol Stud 1:111–164
Soeder CJ (1980) The scope of microalgae for food and feed. In: Shelef G, Soeder CJ (eds) Algae biomass. Elsevier/North Holland Biomedical Press, Amsterdam, pp 9–20
Sorokin C (1957) Changes in photosynthetic activity in the course of cell development in Chlorella. Plant Physiol 10:659–666
Stephenson AL, Dennis JS, Howe CJ, Scott SA, Smith AG (2010) Influence of nitrogen-limitation regime on the production by Chlorella vulgaris of lipids for biodiesel feedstocks. Biofuels 1:47–58
Sukenik A, Bennett J, Falkowski PG (1987) Light-saturated photosynthesis—limitation by electron transport or carbon fixation. Biochim Biophys Acta 891:205–215
Terry KL (1986) Photosynthesis in modulated light: quantitative dependence of photosynthetic enhancement on flashing rate. Biotechnol Bioeng 28:988–995
Tredici MR (2004) Mass production of microalgae: photobioreactors. In: Richmond A (ed) Handbook of microalgal culture biotechnology and applied phycology. Blackwell Science, Oxford, pp 273–280
Tredici MR (2010) Photobiology of microalgae mass cultures: understanding the tools for the next green revolution. Biofuels 1:143–162
Vonshak A (1986) Laboratory techniques for the cultivation of microalgae. In: Richmond A (ed) Handbook of microalgal mass culture. Boca Raton, CRC Press, pp 117–145
Vonshak A, Torzillo G, Masojidek J, Boussiba S (2001) Sub-optimal morning temperature induces photoinhibition in dense outdoor cultures of the alga Monodus subterraneus (Eustigmatophyta). Plant Cell Environ 24:1113–1118
Walker DA (2009) Biofuels, facts, fantasy and feasibility. J Appl Phycol 21:509–517
Wassink EC, Kok B, van Oorschot JLP (1953) The efficiency of light-energy conversion in Chlorella cultures compared to higher plants. In: Burlew JS (ed) Algal culture from laboratory to pilot plant, vol 600. Carnegie Institution of Washington Publication, Washington, pp 55–62
Woźniak B, Dera J, Ficek D, Ostrowska M, Majchrowska R (2002) Dependence of the photosynthetic quantum yield in oceans on environmental factors. Oceanologia 44:439–459
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grobbelaar, J.U. Microalgal biomass production: challenges and realities. Photosynth Res 106, 135–144 (2010). https://doi.org/10.1007/s11120-010-9573-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-010-9573-5