Abstract
A transient in chlorophyll fluorescence after cessation of actinic light illumination, which has been ascribed to electron donation from stromal reductants to plastoquinone (PQ) by the NAD(P)H-dehydrogenase (NDH) complex, was investigated in Arabidopsis thaliana. The transient was absent in air in a mutant lacking the NDH complex (ndhM). However, in ndhM, the transient was detected in CO2-free air containing 2% O2. To investigate the reason, ndhM was crossed with a pgr5 mutant impaired in ferredoxin (Fd)-dependent electron donation from NADPH to PQ, which is known to be redundant for NDH-dependent PQ reduction in the cyclic electron flow around photosystem I (PSI). In ndhM pgr5, the transient was absent even in CO2-free air with 2% O2, demonstrating that the post-illumination transient can also be induced by the Fd- (or PGR5)-dependent PQ reduction. On the other hand, the transient increase in chlorophyll fluorescence was found to be enhanced in normal air in a mutant impaired in plastid fructose-1,6-bisphosphate aldolase (FBA) activity. The mutant, termed fba3-1, offers unique opportunities to examine the relative contribution of the two paths, i.e., the NDH- and Fd- (or PGR5)-dependent paths, on the PSI cyclic electron flow. Crossing fba3-1 with either ndhM or pgr5 and assessing the transient suggested that the main route for the PSI cyclic electron flow shifts from the NDH-dependent path to the Fd-dependent path in response to sink limitation of linear electron flow.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Light energy conversion of photosynthesis primarily occurs through linear electron transport, in which electrons are transported from photosystem II (PSII) to photosystem I (PSI) via the intersystem carriers and cytochrome b 6 f complex (Cyt b 6 f). Besides this flow, cyclic electron transport operates around PSI. PSI cyclic electron flow is thought to act (1) to supply, via generation of the proton motive force, additional ATP to the stromal metabolism and (2) to induce, via acidification of the intra-thylakoid space, non-photochemical quenching or down-regulation of PSII (reviewed by Bendall and Manasse 1995; Rumeau et al. 2007; Shikanai 2007).
Two paths for PSI cyclic electron flow have been proposed (Fig. 1a) (Bendall and Manasse 1995; Rumeau et al. 2007; Shikanai 2007). One is dependent on the membrane-bound NAD(P)H-dehydrogenase (NDH) complex, being homologous to the proton-pumping NADH-ubiquinone oxidoreductase complex (Complex I or Type I NADH dehydrogenase) in the mitochondrial respiratory chain (Ohyama et al. 1986; Shinozaki et al. 1986). The other path is ferredoxin (Fd)-dependent, in which putative Fd–PQ reductase (FQR) and PGRL1/PGR5 complex are involved (Munekage et al. 2002; DalCorso et al. 2008). The NDH- and Fd-dependent cyclic electron flows are redundant (Munekage et al. 2004) and share the pathway with linear electron flow from plastoquinone (PQ) to PSI via the Cyt b 6 f (Bendall and Manasse 1995). The occurrence of PSI cyclic electron flow, especially under non-stressed conditions, has been under debate. However, a double mutant impaired in both the NDH- and Fd-dependent paths showed severely retarded growth even under low light (Munekage et al. 2004).
Transient in post-illumination chlorophyll fluorescence. a Schematic model of the two paths of cyclic electron flow around photosystem I. Cyt b 6 f cytochrome b 6 f complex, Fd ferredoxin, FNR Fd–NAD(P)H oxidoreductase, PQ plastoquinone, PSI photosystem I. b Induction kinetics and post-illumination transient in chlorophyll fluorescence. Typical induction kinetics of chlorophyll fluorescence in a dark-adapted leaf of Arabidopsis wild type under illumination with actinic light (AL, 50 μmol photons m−2 s−1) are shown. The measurements were made using measuring light (ML) of approx. 0.2 μmol photons m−2 s−1 under normal air conditions
Supporting evidence for the involvement of the NDH complex in the PSI cyclic electron flow has been that normal plants display a transient increase and decrease in chlorophyll fluorescence after light is switched off (Fig. 1b, boxed area) and that the transient is absent in tobacco transformants with inactivated plastid-encoded ndh genes (Burrows et al. 1998; Kofer et al. 1998; Sazanov et al. 1998; Shikanai et al. 1998). The link between the fluorescence signal and the NDH complex has been conclusively proven by discoveries of Arabidopsis mutants lacking subunits of the NDH complex using the transient signal as a key to screen them from the mutated (M2) population (Shikanai 2007) or from the T-DNA insertion mutants of candidate genes for the NDH subunits being identified by in silico approach (Takabayashi et al. 2009). The transient has been interpreted as being due to PQ reduction via the NDH complex by stromal reductants (mainly NADPH) which had been accumulated during previous illumination.
The details of the mechanisms of how the PGRL1/PGR5 complex plays its role in the Fd-dependent cyclic electron flow have not been elucidated. However, the Arabidopsis mutants pgr5 and pgrl1ab, the latter a double mutant lacking two homologous genes for PGRL1 protein, were impaired in electron donation from NADPH to PQ via Fd in vitro, just as required in the Fd-dependent flow (Munekage et al. 2004; DalCorso et al. 2008). Avenson et al. (2005) reported, using an electrochromic shift decay measurement, an approx. 13% decrease of steady-state proton flux into the lumen (υH +) at a given rate of linear electron flow in pgr5 as compared to the wild type. This is likely to be a realistic contribution of the PSI cyclic electron flow, since the same degree of proton influx (approx. 14%) is likely to be lost due to linear electron flow alone for CO2 assimilation in the Benson–Calvin cycle (reviewed by Allen 2003). On the other hand, the pgr5 mutant was shown to be capable of performing the Fd-dependent cycle (Nandha et al. 2007), but this was shown through redox changes of P700 (the chlorophyll dimmer in PSI) under infra-red light and thus in the absence of strict competition between linear and cyclic electron flow. Therefore, the absence of linear electron flow may be responsible for pgr5 inducing the Fd-dependent cycle. Importance of competition on the regulation of the PSI cyclic electron flow has been proposed (Breyton et al. 2006).
Whereas the physiological significance of the cyclic electron transport around PSI is well established, the division of roles between the two paths or compensatory mechanisms between them are still far from being understood. As has been pointed out, the main reason for this is that direct measurements of activity of the cyclic electron flow are problematic (reviewed by Johnson 2005; Baker et al. 2007). This is particularly true for NDH-dependent flow. Whereas an impairment of the Fd- (or PGR5)-dependent flow resulted in distinct disturbance of the steady-state redox conditions of the reaction center of PSI (Munekage et al. 2002; DalCorso et al. 2008), a defect in the NDH-dependent flow did not have any influence on short-term regulation of photosynthesis (Burrows et al. 1998; Horváth et al. 2000; Joët et al. 2001; Rumeau et al. 2005; Kamruzzaman Munshi et al. 2005; Muraoka et al. 2006). It has been even proposed that the concentration of the NDH complex is so low (0.2% of total thylakoid protein in pea) that involvement of the NDH complex in an efficient PSI cyclic flow is unlikely under physiological conditions (Sazanov et al. 1998; Joliot and Joliot 2005). A new approach is needed to detect, if possible, the individual activities of PSI cyclic electron flow.
During our search for the Arabidopsis mutants, derived from ethyl methanesulfonate (EMS) mutagenesis, impaired in the PSI cyclic electron flow, we encountered a mutant with an altered post-illumination transient of chlorophyll fluorescence, a mutation in which was later identified in the gene encoding plastid-targeted putative fructose-1,6-bisphosphate aldolase (FBA). In the mutant, fba3-1, the transient increase in post-illumination chlorophyll fluorescence was markedly accelerated. Here, we attempted qualitative analysis of the regulation of PSI cyclic electron flow using fba3-1 and other relevant mutants, based on the post-illumination fluorescence transient. We showed that the phenomenon is caused not only by NDH-dependent PQ reduction but also by Fd-dependent PQ reduction. However, no other pathways are involved, allowing the analysis of in vivo regulation of the two types of electron recycling around PSI. The results obtained suggest that the NDH- and Fd-dependent cyclic electron flow operate mainly under mild and severe limitation of linear electron flow, respectively. We will discuss the fluorescence transient in the light of the regulation of PSI cyclic electron flow.
Materials and methods
Plant materials
Arabidopsis thaliana wild type (Columbia gl1 background), pgr5 (protongradientregulation) (Munekage et al. 2002), npq4-1 (nonphotochemicalquenching) (Li et al.2000) and fba3-1 were used. The mutants were generated by EMS mutagenesis and are in the Columbia gl1 background. A T-DNA insertion null mutant of ndhM (SALK_087707, At4g37920) (Alonso et al. 2003; Rumeau et al.2005) and T-DNA insertional mutated allele of fba3-1 (designated fba3-2, SALK_073444) were also used. These insertion mutants were in the Columbia (Col-0) background. The plants were grown in soil under growth chamber conditions (60 μmol photons m−2 s−1, 50% RH, 16 h-light/8 h-dark cycle, 23°C). Representative leaves from 3 to 4 week-old-plants were selected for analysis.
Identification of the insertion mutants
A homozygous T-DNA insertion was verified by PCR analysis using the T-DNA-specific primer (Lba1) 5′-GGTTCACGTAGTGGGCCATCG-3′ and gene-specific primers: for fba3-2, 5′-CACTTGTTGGATCCAACAATG-3′ (forward) and 5′-TGCTCGGTTTTAGGAGGATAC-3′ (reverse); for ndhM, 5′-ATGGTTCTGTAACCGGACAAC-3′ (forward) and 5′-GATTTGAGCAACCATAGAAGG-3′ (reverse). The position of the T-DNA insertion was determined by sequencing the PCR products, on the basis of information available from of the Salk Institute database (http://signal.salk.edu/cgi-bin/tdnaexpress).
RT-PCR analysis of the FBA3 transcript
Total RNA was extracted from wild-type seedlings and mutants using the RNeasy Plant Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. RT was performed using a RT-PCR kit, RNA PCR kit version 3.0 (Takara Bio, Otsu, Japan), and an oligo(dT)12 primer. RT-PCRs were performed using the FBA3-specific primers 5′-CACTTGTTGGATCCAACAATG-3′ (forward) and 5′-TGCTCGGTTTTAGGAGGATAC-3′ (reverse). ACTIN8 transcripts were analyzed using the primers 5′-GAGAGATTCAGGTGCCCAG-3′ (forward) and 5′-AGAGCGAGAGCGGGTTTTCA-3′ (reverse). The PCR-amplified samples were electrophoresed using 1.2% (wt/vol) agarose gel and detected with 0.1 mg l−1 ethidium bromide.
Immunoblot analysis
Immunoblotting was carried out using polyclonal antisera to NDH-L, PGR5, and Cyt f as described previously (Munekage et al. 2002; Shimizu et al. 2008). Leaves were homogenized in a medium containing 330 mM sorbitol, 20 mM tricine/NaOH (pH 7.6), 5 mM EGTA, 5 mM EDTA, 10 mM NaCO3, 0.1% (w/v) BSA, and 330 mg l−1 ascorbate. After centrifugation for 5 min at 2,000×g, the pellet was resuspended in 300 mM sorbitol, 20 mM HEPES/KOH (pH 7.6), 5 mM MgCl2, and 2.5 mM EDTA. Intact chloroplasts were purified by passing through 40%. Intact chloroplasts (10 μg chlorophyll ml−1) were osmotically ruptured in a medium containing 50 mM HEPES/NaOH (pH 7.6), 7 mM MgCl2, 1 mM MnCl2, 2 mM EDTA, 30 mM KCl, and 0.25 mM KH2PO4. After centrifugation for 10 min at 3,000×g, proteins from the thylakoid membrane fraction (0.25 μg Chl) were fractioned through 12.5 or 15% (in a Tris–tricine buffer system for PGR5) SDS-PAGE and transferred to a polyvinylidene fluoride membrane.
Chlorophyll fluorescence analysis
Modulated chlorophyll fluorescence was measured using a PAM 101 fluorometer (H. Walz, Germany). For determining minimal (F o) fluorescence, leaves were kept in darkness for at least 1 h prior to use. F o was induced by red-modulated measuring light with a photon flux density (PFD) of approx. 0.2 μmol photons m−2 s−1, unless otherwise noted. The quantum yield of PSII (ΦPSII) was determined as (F m′ − F s)/F m′, where F m′ and F s are the maximum fluorescence and steady-state fluorescence in the light, respectively (Genty et al. 1989). The relative rate of electron flow (ETR) was estimated by the product of ΦPSII and PFD. Infra-red light (>720 nm) was obtained by passing light from the halogen light source through a long pass filter.
In vitro assay of relative rate of electron flow
The relative rate of linear electron flow ETR was estimated under varying light intensities using isolated thylakoids with methyl viologen (20 μM) as an artificial electron acceptor.
Aldolase activity assay
The activity of cleavage reaction of fructose-1,6-bisphosphate (Fru-1,6-P2) into dihydroxyacetone-phosphate (DHAP) and glyceraldehyde-3-phosphate (GAP) was assayed (Ogawa 2005). The activity of reduction of DHAP by NADH catalyzed by glycerol-3-phosphate dehydrogenase (GPDH) was determined spectrophotometrically by monitoring the decrease in absorbance at 340 nm that was caused by the NADH oxidation. The assay mixture contained 25 mM Tris–HCl buffer (pH 7.0 or 8.0), 2.5 mM reduced glutathione, 0.35 mM NADH, 3.45 units per ml of GPDH, 0.5 mM Fru-1,6-P2, and the enzyme solution, i.e., the stromal fraction obtained from intact chloroplasts isolated as above with slight modification. The reaction was run at 25°C for 2 min, and the activity was calculated from the initial linear rate. One unit of aldolase activity was defined as the quantity that catalyzed the cleavage of 1 μmol of Fru-1,6-P2 (equivalent to 1 μmol of NADH oxidized) per min.
Map-based cloning
The fba3-1 mutation was mapped with molecular markers based on a cleaved amplified polymorphic sequence (Konieczny and Ausubel 1993). The forward and reverse primers (and restriction enzyme) used were 5′-GATGTTCTTCATCCGTAATGG-3′ and 5′-TGATTTGTTATAGGGCCAAGC-3′ (EcoRV) for T9A14, and 5′-ACATAGCACACAGAGATATCG-3′ and 5′-CTTTCACTGCCTCTTTGATTC-3′ for T5J17 with no site for the restriction enzyme but yielding PCR products with different sizes between ecotype Columbia (269 bp) and Landsberg (250 bp). Genomic DNA was isolated from F2 plants derived from a cross between fba3-1 and the wild type (Landsberg erecta). The genomic sequences of the candidate genes were amplified by PCR using ExTaq DNA polymerase (Takara, Kyoto, Japan). The resulting PCR products were directly sequenced using a dye terminator cycle sequencing kit and an ABI Prism 3100 Sequencer (Applied Biosystems, Foster, CA).
For complementation of the fba3-1 mutation, the 3.1-kb wild-type genomic sequence was amplified from a BAC clone F19H22 using primers 5′-TGGGAAGATTGTCGACGAAATGATTG-3′ (forward) and 5′-AAGAGTATCGAATTCCTATTCGGAG-3′ (reverse), cloned separately in pBIN19, and introduced into fba3-1 via Agrobacterium tumefaciens c58.
Results
Transient in chlorophyll fluorescence after cessation of actinic light illumination
Figure 1b shows typical result of the measurements of the post-illumination chlorophyll fluorescence to monitor electron donation from stromal reductants to the intersystem electron carrier PQ. In the wild type, a rapid increase in chlorophyll fluorescence is induced upon illumination with actinic light, followed by a gradual decrease to the steady-state level. The fluorescence drops just after turning off the actinic light, and then increases transiently for about 20–30 s (boxed area in Fig. 1b, enlarged in Fig. 2a, i). In contrast, the transient was absent in the T-DNA insertion null mutant of ndhM (Fig. 2b) (Rumeau et al. 2005). Western blotting analysis reconfirmed almost complete disruption of the NDH complex (Fig. 4): although the analyses have been done using antibody against another subunit of the complex (NDH-L), the complex is unstable without its subunit (Rumeau et al. 2005). The results verify that the transient is attributable to PQ reduction by the NDH complex (Burrows et al. 1998; Kofer et al. 1998; Sazanov et al. 1998; Shikanai et al. 1998; Rumeau et al. 2005). As expected, the transient was detected in pgr5 lacking Fd-dependent electron flow (Figs. 2c, 4). The amount of NDH complex in pgr5 was also comparable to that in the wild type (Fig. 4).
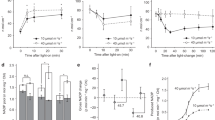
Analyses of post-illumination chlorophyll fluorescence transient. a–i Typical transient in the wild type (WT) (a), ndhM (b), pgr5 (c), fba3-1 (d), fba3-2 (e, black), fba3-1 transformed by wild-type genomic FBA3 (fba3-1 + FBA3) (e, gray), fba3-1 npq4-1 (f), fba3-1 ndhM (g), and fba3-1 pgr5 (h). The transients were compared at a higher time resolution in (i) (WT, black; fba3-1, red; fba3-1 ndhM, blue; fba3-1 pgr5, green). The experimental procedures are as in Fig. 1. j Dependence of magnitude of the transient on the measuring light intensity. The magnitude of the transient obtained at the indicated intensities of measuring light are shown for leaves of WT (circles), fba3-1 (triangles), ndhM (diamonds), and pgr5 (squares). The magnitude was estimated as (F peak − F o′)/F o′, where F peak and F o′ are the fluorescence peak level during the transient and the minimum level after actinic illumination (approx. 50 μmol photons m−2 s−1), respectively. The data points represent the means ± SD of at least three experiments. k Effects of prolonged illumination on the transient. The transient was detected after 45 min illumination of actinic light (approx. 50 μmol photons m−2 s−1). l Effects of infra-red (IR) light on the transient. IR light with a PFD of approx. 60 μmol photons m−2 s−1 was applied at around the peak fluorescence level during the transient. The dotted line shows the minimum F o level. AL: approx. 50 μmol photons m−2 s−1. Except in the case of (j), the measurements were made using measuring light of approx. 0.2 μmol photons m−2 s−1 and the fluorescence levels were normalized to the F o level. All of the measurements were performed under normal air conditions
It is known that a relatively strong measuring light for exciting chlorophyll fluorescence is required to elicit the post-illumination fluorescence transient. In our experiments, the intensity of the measuring light for exciting chlorophyll fluorescence was stronger than that desired for strict measurements of chlorophyll fluorescence. Normally, the weaker the intensity, the more suitable it is for the measurement of chlorophyll fluorescence: weak measuring light (e.g., 0.1 μmol photons m−2 s−1) is preferred to elicit the correct minimum chlorophyll fluorescence (F o) that represents full oxidation of the secondary quinone electron acceptor QA in PSII. On the other hand, the F o is affected by the redox equilibrium among QA, QB (secondary quinone acceptor in PSII) and the PQ pool: redox equilibrium results in a 10% reduction of QA under 50% reduction of the PQ pool (Groom et al. 1993; Corneille et al. 1998). Hence, a strong measuring light was intended to detect QA reduction due to PQ reduction, at the expense of the possible influence of the measuring light itself on the redox equilibrium.
Due to the above reason, there is a possibility that the different transient shown in this study merely reflects a different dependency on the measuring light intensity of the transient among the plants used. To examine this possibility, we tested the dependency. As shown in Fig. 2j, there was a dependency of the magnitude of the transient on the measuring light intensity in the wild type, and the magnitude became larger with increasing intensity of the measuring light. However, it was found that (1) in the wild type and pgr5, the transient was detectable at all the intensities including the lowest intensity tested (0.03 μmol photons m−2 s−1); and (2) in ndhM, the transient was not detected at any of the intensities including the strongest intensity examined (0.72 μmol photons m−2 s−1). These results suggest that the differences in transient are not due to different dependencies of the transient on the measuring light intensity.
In addition, to elicit the transient, we routinely illuminated a leaf with actinic light for 5 min. This illumination time is normally too short to reach steady-state photosynthesis for Arabidopsis. Therefore, the transient may be merely a consequence or influence of photosynthetic induction, during which chlorophyll fluorescence emission shows a well-characterized (O-I-D-P-S[-M-T]) pattern. In order to show that the transient can be apart from such photosynthetic induction, we tested the transient after prolonged illumination for 45 min. Within this range, steady-state photosynthesis was sufficiently attained in Arabidopsis (data not shown). As shown in Fig. 2k, the transient was not affected by the prolonged illumination in the wild type and pgr5. In ndhM, the transient was also absent. These results suggest that the transient is not necessarily related to photosynthetic induction events.
Fd-dependent transient in post-illumination chlorophyll fluorescence
In ndhM, PQ reduction by the Fd- (or PGR5)-dependent electron flow should be possible. However, importantly, no such contribution was indicated by the post-illumination transient in ndhM (Fig. 2b). To investigate the possible involvement of the Fd-dependent flow into the post-illumination PQ reduction, the same experiment was done in 2% O2 in the absence of CO2 (Fig. 3). In the wild type, the sink limitation of electron flow resulted in a marked increase in the transient (Fig. 3a). This was also the case for ndhM (Fig. 3b). This result indicates that the Fd-dependent flow is capable of transporting electrons from the stromal reductants to the intersystem carrier PQ. It is worth noting that the amount of PGR5 protein was not affected by the mutational defect in ndhM (Fig. 4). In pgr5, the transient was detected (Fig. 3c), but not enhanced, unlike that in the wild type and ndhM (Fig. 3a, b). This implies lower sensitivity of the NDH-dependent flow to strong sink limitation in comparison with the Fd-dependent flow.
Post-illumination chlorophyll fluorescence transient. The measurements were carried out in CO2-free air containing 2% O2 in the wild type (WT, a), ndhM (b), pgr5 (c), and ndhM pgr5 (d). Experimental procedures are as in Fig. 1
Immunoblot analysis of the NDH complex and PGR5 protein. Proteins were extracted from the thylakoid membrane fraction of the chloroplasts isolated from leaves of the wild type (WT), ndhM, pgr5, and fba3-1. Each lane was loaded with protein samples corresponding to 0.25 μg chlorophyll and antibodies to a NDH subunit (NDH-L), PGR5 and a subunit (Cyt f) of the cytochrome b 6 f complex were used. Cyt f was analyzed as a loading control
In a double mutant ndhM pgr5 generated by crossing each single knockout and screening the resulting F2 generation for homozygous double mutants, the post-illumination fluorescence transient was not detected, even in CO2-free air with 2% O2 (Fig. 3d). This result indicates that the transient is specific to PQ reduction by the NDH- and Fd- (or PGR5)-dependent electron flow. In the following, we assume that this notion is applicable to other mutants in the fba3 background in normal air, because the redox state of the electron transport chain in the light was always lower in those mutants under this condition than in the double mutant ndhM pgr5 in CO2-free air with 2% O2 (data not shown).
Acceleration of post-illumination reduction of PQ pool in fba3 mutant
In this study, by screening Arabidopsis EMS-M2 seedlings, we isolated a mutant with a new phenotype in the post-illumination chlorophyll fluorescence transient, which is characterized by acceleration of post-illumination fluorescence increase (Fig. 2d).
By crossing the mutant (Columbia gl1 background) with the polymorphic wild type (Landsberg erecta), we identified the gene responsible for this phenotype by map-based cloning (Fig. 5a). The mutation was found by genotyping 480 F2 plants of the mapping population to the south region of chromosome 4 between the molecular markers T9A14 and T5J17. Genes potentially encoding the predicted chloroplast targeting signals (Predotar (http://urgi.versailles.inra.fr/predotar/predotar.html) and TargetP (http://www.cbs.dtu.dk/services/TargetP/)) were then sequenced. As a result, a point mutation from G to A was found in the seventh exon of the gene for putative fructose-bisphosphate aldolase (At4g38970, designated FBA3), leading to an amino acid replacement from Gly382 to Glu (Fig. 5a). This mutant allele, fba3-1, was suggested to be recessive by the segregation ratio at the F2 generation of the phenotype in the post-illumination chlorophyll fluorescence (about 25%, data not shown). To verify that the mutation is responsible for the accelerated increase of post-illumination chlorophyll fluorescence (Fig. 2d), genetic complementation tests were carried out: the wild-type genomic sequence containing At4g38970 was introduced into the fba3-1 mutant. This transformation suppressed the mutant phenotype with respect to the fluorescence transient (Fig. 2e), supporting the assumption that the phenotype was due to the mutation in At4g38970 (FBA3).
Characterization of fba3 mutants. a Schematic diagram showing the position of fba3-1 mutation and the site of T-DNA insertion in fba3-2 (SALK_073444). In the fba3-1 mutant, a single nucleotide substitution was found in the seventh exon of At4g38970 (see text). In the fba3-2 mutant, the insertion was located in the third intron at position +948 bp relative to the start codon. The orientation of the left border (LB) is indicated, and the opposite end of the insertion was not characterized. Boxes and lines represent exons and introns, respectively. b Genomic PCR analysis demonstrating homozygous T-DNA insertion in fba3-2 mutant. Using a T-DNA primer and a gene-specific primer (panel 2), a 390-bp PCR product was amplified from mutant DNA but not from wild-type DNA. On the other hand, using gene-specific primers spanning the insertion site (panel 1), a 200-bp PCR product was amplified from wild-type DNA but not from mutant DNA. WT wild type. c RT-PCR analysis demonstrating the lack of FBA3 transcript in fba3-2. An amplified fragment (348 bp) corresponding to FBA3 was observed in the wild type (WT) but was not detectable in the fba3-2 mutant, demonstrating that FBA3 expression was disrupted in the mutant. ACTIN8 transcripts (650 bp) were analyzed as a loading control
fba3-2, a T-DNA insertional mutated allele of fba3-1, was obtained from publicly available T-DNA insertion collections (Fig. 5a, b). This plant, in which full gene disruption was confirmed by RT-PCR (Fig. 5c), displayed the same acceleration of post-illumination fluorescence increase as fba3-1 (Fig. 2e). From these results, while further confirming the identity of the gene, we regarded fba3-1 as a null mutant, although we did not determine protein abundance using FBA3-specific antibody.
The dependency of the transient on the measuring light intensity as described above was examined in fba3-1 (Fig. 2j). In fba3-1, the transient was detected at all the measuring light intensities including the lowest intensity tested as in the wild type and pgr5. The magnitude in fba3-1 was larger than those in the wild type and pgr5 at all the measuring light intensities tested. These results suggest that the phenotype in the transient in fba3-1 is not due to differences in the dependency of the transient on the measuring light intensity. In addition, we confirmed that the transient was not affected by prolonged illumination in fba3-1 (Fig. 2k). This result suggests that differences in photosynthetic induction, if present, are not responsible for the different transients in fba3-1.
Further, to confirm that the transient in fba3-1 is also due to PQ reduction, the effect of infra-red (IR) light was tested. As previously shown (Burrows et al. 1998; Shikanai et al. 1998) and also seen in the wild type in Fig. 2l, IR light applied on top of the measuring light at around the peak of the transient caused a large decline in fluorescence to the minimum F o level in fba3-1. As IR light preferentially excites PSI, this result can be interpreted to indicate that the IR light promoted drains of electrons from the PQ pool to PSI, which supports the above notion.
The transient is monitored under conditions where non-photochemical quenching (NPQ) of chlorophyll fluorescence relaxes rapidly. In many cases, photosynthesis mutants have different degrees of NPQ. In such cases, the sensitivity of fluorescence to PQ reduction may vary and affect the transient. There is thus a possibility that the transient in fba3-1 was caused by an altered NPQ. To examine this possibility, we used an npq4-1 mutant that lacks the PsbS chlorophyll-binding protein and is impaired in energy dependent quenching (qE), a major component of NPQ (Li et al. 2000). The fba3-1 npq4-1 double mutant was generated by crossing each single knockout. In fba3-1 npq4-1, acceleration of the post-illumination rise was detected (Fig. 2f), indicating that the transient in fba3-1 was not due to such an unexpected alternation in NPQ, but due to increased PQ reduction by the stromal reductants.
The amounts of NDH complex and PGR5 protein in fba3-1 were comparable to those in the wild type (Fig. 4). These results indicate that the NDH and PGR5 content are not related to the acceleration of the post-illumination fluorescence rise (Fig. 1d).
Inhibition of the activity of aldolase and linear electron transport
The aldolase activity measured in chloroplast suspensions was much higher at pH 8.0 than at pH 7.0 (Fig. 6a). This is due to the alkaline pH optimum of the enzyme (Murphy and Walker 1981). At pH 8.0, the activity was approx. 20% lower in the fba3-1 mutant than in the wild type (Fig. 6a). The plastidic FBA is encoded by three homologous genes: At2g01140 (FBA1), At2g21330 (FBA2), and At4g38970 (FBA3, this study) (BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi)). Sequence similarities (and identities) of FBA1 and FBA2 to FBA3 are 85% (and 74%) and 94% (and 88%), respectively. Therefore, the remaining aldolase activity in the fba3-1 (approx. 80%, Fig. 6a) is considered to be ascribed to that of two homologous proteins.
Analysis of fba3-1. a Activity of plastidic aldolase. The activity was measured in the wild type (WT) and fba3-1 at pH 7.0 (white bar) and 8.0 (dark bar) in consideration of the pH optimum of the enzyme. b Relative rate of electron transport (ETR) in leaves of the wild type (WT, circles), fba3-1 (triangles), fba3-2 (diamonds), and fba3-1 transformed by wild-type genomic FBA3 (fba3-1 + FBA3, squares) as a function of light intensity. Inset: ETR in the thylakoids isolated from the wild type leaves (WT, circles) and fba3-1 leaves (triangles). Data points represent the means ± SD of at least five experiments
Plastid aldolase catalyzes the two reactions in the regenerative phase of ribulose-1,5-bisphosphate (RuBP) in the Calvin cycle: condensation of GAP and DHAP to Fru-1,6-P2, and erythrose-4-phosphate and DHAP to sedoheptulose-1,7-bisphosphate. The relative rate of electron flow (ETR) measured in intact leaves was approximately 40% lower in fba3-1 than in the wild type (Fig. 6b). This was also the case in fba3-2. The ETR was restored in the transformant lines fba3-1 + FBA3. Therefore, the partial inhibition of total aldolase activity in fba3-1 is considered to lower the overall rate of electron transport in the thylakoid.
The estimation of ETR depends on the assumption of equal leaf absorption properties (normally about 80%) and equal light energy distribution between PSII and PSI (typically 50%). However, the mutational defect in fba3-1 may cause unanticipated pleiotropic effects on photosynthetic functions, making the comparisons of ETR as above ambiguous. To address this problem, ETR measured in the thylakoids was compared between the wild type and fba3-1. In the presence of methyl viologen as an electron acceptor, no substantial difference in ETR in the thylakoids was found between the two plants (Fig. 6b, inset). This result suggests that the lower ETR in fba3-1 leaves than in wild-type leaves (Fig. 6b) is not due to unexpected pleiotropic effects but to the inhibition of aldolase activity (Fig. 6a). In this experiment using the thylakoids, ETR became progressively smaller at higher light intensities over 400 μmol photons m−2 s−1 in the two plants (data not shown). This is probably because a proportion of PSII lost quantum efficiency. It would also be worth noting that leaf chlorophyll content and chl a/b ratio were similar between the wild type (616.8 ± 45.4 μg chl a + b per g fresh weight, chl a/b = 3.17, n = 3) and fba3-1 (585.8 ± 1.8 μg g−1 f.w., chl a/b = 3.20, n = 3), suggesting no significant pleiotropic effects on the photosynthetic apparatus of the mutational defect in fba3-1.
Changing contribution of NDH- and Fd-dependent flow to the post-illumination transient in fba3-1
The fba3-1 ndhM double mutant was generated by crossing. fba3-1 ndhM showed, although it was very slight, a post-illumination fluorescence transient (Fig. 2g, asterisk). In the double mutant fba3-1 pgr5 generated, the transient was obvious (Fig. 2h). Figure 2i shows the post-illumination fluorescence transient on a more enlarged timescale. As compared to the transient in the wild type, the fluorescence signal increased and declined faster in fba3-1. Within the same time range (approx. 20 s), the transients in the double mutants, fba3-1 ndhM and fba3-1 pgr5, were almost completed. Although we did not analyze the triple mutant ndhM pgr5 fba3-1, these results provide confirmation that the transient in fba3-1 originated from both the NDH- and Fd-dependent electron flows.
Discussion
Link between the post-illumination transient and cyclic electron flow around PSI in the steady state
The transient of post-illumination fluorescence was specific to the NDH- and Fd-dependent electron flows (Fig. 3d). In addition, the appearance, magnitude, and time range of the transient appeared to respond to mutational deletions that alter the activity of the PSI cyclic electron flow (Figs. 2, 3). These results strongly prompted us to further analyze regulation of the activity of the PSI cyclic electron flow from the post-illumination fluorescence transient.
The greatest problem here is the slow kinetics of the post-illumination fluorescence transient. A much more rapid turnover of the PSI cyclic electron flow has been reported based on the rate of redox changes of P700 (Laisk and Oja 1994; Bukhov et al. 2002; Chow and Hope 2004; Joliot and Joliot 2006) or decay of the electrochromic shift (ECS) (Avenson et al. 2005). ECS decay or P700 re-reduction is normally monitored at the time the light is switched off and provides the rate constant of the linear and cyclic electron flows operating during steady-state photosynthesis. Although the value significantly changes according to the experimental conditions, it reached several dozen or a hundred and several tens in s−1. In such a time frame, chlorophyll fluorescence simply declines as shown in Fig. 1b. The post-illumination transient we focused on, the rate constant of which appears to be approximately 1 s−1, is detected in the ensuing dark period. Therefore, we were obliged to consider that the slow kinetics are not directly related to the steady-state rate of linear and PSI cyclic electron flows.
On the other hand, relatively slow phases of P700 re-reduction kinetics with a rate constant below approximately 1–2 s−1, which are roughly consistent in time scale with our slow kinetics for the post-illumination transient, have also been analyzed previously. Bukhov et al. (2002) suggested the presence of two types of pathway for PQ reduction by stromal reductants. Chow and Hope (2004) suggested PGR5- (or Fd)-dependent and NDH-dependent electron donation from stromal reductants NADPH and ascorbate. Therefore, we tentatively assume that, during a restricted short period (e.g., ~30 s) immediately after illumination, PGR5 and NDH complex are continuously capable of mediating electron donation from stromal reductants to PQ at rates reflecting the activities of PSI cyclic electron flow in the previous light period.
Another problem may be the source of stromal reductants. As an origin of the reducing power for post-illumination PQ reduction, Mano et al. (1995) previously suggested the involvement of Calvin cycle intermediates such as DHAP. They observed acceleration of the transient by adding DHAP to a chloroplast suspension during the transient and proposed reverse reactions (in the Calvin cycle in the dark immediately after switching off the actinic light) from DHAP to 3-phosphoglycerate (3-PGA), via GAP and 1,3-bisphosphoglycerate (1,3-PGA), catalyzed by triosephosphate isomerase, GAP dehydrogenase, and phosphoglycerate kinase. In our study, a mutant impaired in the Calvin cycle enzyme FBA, a substrate of which is DHAP, showed accelerated increase in the transient, supporting the conclusion drawn by Mano et al. (1995). It is possible to assume that the post-illumination fluorescence transient is finally attributable to accumulation of DHAP in the stroma in the light. The accumulated DHAP is considered to be metabolized via the reverse reactions in the Calvin cycle, releasing NADPH in the stroma in the dark after illumination.
Regulation of the NDH- and Fd-dependent PSI cyclic electron flow
After illumination of actinic light at 50 μmol photons m−2 s−1 in normal air conditions, the transient by the NDH-dependent flow was detected, as shown in pgr5 (Fig. 2c), but that by the Fd- (or PGR5)-dependent flow was not, as shown in ndhM (Fig. 2b). These results suggest that the NDH-dependent flow functions under low light where no limitation of linear electron flow is expected. On the other hand, the transient by the Fd- (or PGR5)-dependent flow was largely induced in 2% O2 as shown in ndhM (Fig. 3b). In such conditions, the NDH-dependent transient seemed not to be prevailing anymore, as shown in pgr5 (Fig. 3c). It is, therefore, suggested that the Fd- (or PGR5)-dependent flow requires a strong sink limitation to be present and primarily operates under the severe limitation of linear electron flow.
This idea is supported by a comparison of the transient in the two double mutants fba3-1 ndhM (Fig. 2g) and fba3-1 pgr5 (Fig. 2h). As no pathways other than the NDH- and Fd-dependent electron flow were suggested to be involved in the transient (Fig. 3), the transient in fba3-1 ndhM (Fig. 2g) is attributable to the Fd- (or PGR5)-dependent flow and that in fba3-1 pgr5 to the NDH-dependent flow. In the presence of the slight limitation of linear electron flow in the genetic background of the fba3-1 mutant, the transient due to the NDH-dependent flow prevailed, as in fba3-1 pgr5 (Fig. 2h). Under the conditions, the Fd- or PGR5-dependent flow could be first induced, as in fba3-1 ndhM (Fig. 2g). This flow is considered to be enhanced with increasing sink limitation of the linear electron flow as in 2% O2 (Fig. 3b). These results suggest that the NDH-dependent flow is more preferentially accelerated than the Fd- (or PGR5)-dependent flow under the mild limitation of linear electron flow in fba3-1.
The reason for the Fd- (or PGR5)-dependent flow being induced under more severe acceptor limitations is not known. However, if the Fd-dependent electron flow operates exclusively in the series NAD(P)H → Fd-NAD(P)H oxidoreductase → Fd → PQ (but not directly PSI → Fd → PQ), over-reduction of the NAD(P)H system would be a prerequisite for the operation. The trivial signal in fba3-1 ndhM (Fig. 2g) likely indicates that the acceptor limitation was not severe enough to cause an increase in Fd-dependent flow.
As to the compensatory response between the two types of PSI cyclic electron flow, it has been shown in Arabidopsis that the NDH-dependent flow plays a minor role and the Fd-dependent flow a major role, on the basis of several phenotypes (e.g., growth and non-photochemical chlorophyll fluorescence quenching) of the pgr5 mutant as well as of various NDH mutants (Munekage et al. 2004). In contrast, analyses of delayed luminescence rise suggest that the NDH-dependent path predominates in Arabidopsis, while in tobacco the Fd-dependent path predominates (Havaux et al. 2005). It is, therefore, possible to propose that the relative contribution of the two paths on the overall rate of PSI cyclic electron flow is not fixed, but changes in response to the extent of the acceptor limitation of linear electron flow.
It is worth noting that, using ruptured chloroplasts from pgr5, the Fd-dependent flow was shown to be regulated by the redox situation in the stroma (Okegawa et al. 2008), and that, in the tobacco ndhB disruptant, stromal reductants were more highly reduced than in the wild type in supra-saturating light (Endo et al. 1999). These studies suggest that both the NDH- and Fd- (or PGR5)-dependent flow have the opportunity to be activated when linear electron flow is limited by a lack of electron acceptors. This is consistent with (and has been well established from) studies quantifying the rate of PSI cyclic electron flow (Golding and Johnson 2003; Miyake et al. 2005; Avenson et al. 2005; Laisk et al. 2006). It should be noted that the overall rate of PSI cyclic electron flow is regulated via redox poising of carriers of the electron transport chain (Breyton et al. 2006).
It was previously reported that the transient in post-illumination fluorescence was enlarged by photo-oxidative treatment (Martín et al. 2004) and high-temperature treatment (Sazanov et al. 1998; Wang et al. 2006). Of these studies, high-temperature treatment resulted in enhancement of the post-illumination fluorescence rise in the ndh deletion tobacco mutants (Sazanov et al. 1998; Wang et al. 2006). It is, therefore, suggested that the Fd-dependent flow is stimulated under these conditions. It should be noted that no involvement of the NDH complex in changed fluorescence yield at high temperatures was demonstrated (Yamane et al. 2000).
fba3 mutant as a tool to investigate in vivo regulation of photosynthesis
Potato was previously transformed with antisense gene constructs to reduce the amount of aldolase (Haake et al. 1998, 1999). Using the antisense potato, it was shown that aldolase is not generally accumulated in excess in chloroplasts for CO2 assimilation, in contrast to other enzymes that are highly regulated and are in considerable excess like Rubisco (Stitt and Schulze 1994), fructose-1,6-bisphosphatase (Koßmann et al. 1994), and phosphoribulokinase (Paul et al. 2000). As a result, decreased expression of aldolase induced strong suppression of photosynthesis in both low and high light. The extent of the inhibition was comparable to that in tobacco expressing antisense construct to a key enzyme of the Calvin cycle Rubisco (Stitt and Schulze 1994). A plant with inactivated aldolase activity is likely to be suitable for analyzing photosynthetic control.
An underlying mechanism responsible for the photosynthesis inhibition by the antisense decreases of aldolase was in the shortfall of RuBP, which was accompanied by a decrease in 3-PGA and thus low consumption of ATP and NADPH in the Calvin cycle (Haake et al. 1998, 1999). Therefore, similar situations might promote the accumulation of reductants in the stroma and enhance PSI cyclic electron flow in fba3-1.
Based on this idea, ETR in the wild type was inhibited 40% (i.e., to the same level as in fba3-1 mutant) by glyceraldehyde (1 mM), a distinct inhibitor of Calvin cycle activity (Stokes and Walker 1972). However, the acceleration of post-illumination fluorescence rise as in fba3-1 (Fig. 2d) could not be reproduced (data not shown). Therefore, not only inhibition of the Calvin cycle but also accumulation of DHAP (Mano et al. 1995) may be needed to reproduce the post-illumination fluorescence transient.
Why is fba3 special in relation to the post-illumination fluorescence transient? We have attempted to analyze the relationship between the post-illumination fluorescence transient and DHAP levels in chloroplasts. However, the transient was never reproduced in intact chloroplasts, no matter how carefully we tried to isolate intact chloroplasts from Arabidopsis. The transient is indeed dealt with as a rather subtle signal, since its detection is sensitive to the conditions under which the measurements are carried out. The transient readily disappears on altering factors that influence photosynthesis, for example, actinic light intensity, CO2 and O2 concentrations, temperature, and mutations. However, we recently found that the transient in fba3-1 can be detected under a wide range of actinic light intensities (even under saturating light of 2,000 μmol photons m−2 s−1) and that the transient is affected by changes in photosynthate partitioning, i.e., the distribution of sugars deriving from CO2 assimilation between the export of triose phosphate from chloroplasts and the accumulation of transitory starch in chloroplasts (Gotoh et al. in preparation). On the other hand, using mutants defective in starch synthesis (adg1-1) and triose-phosphate export (ape2 or tpt-1), Häusler et al. (2009) reported a link between downstream events in photosynthesis and gene expression for inducing adaptive responses to severe stress conditions. As discussed by the authors, such a long-term and widespread regulation has been difficult to investigate because of the lack of an appropriate mutant. It is expected that fba3-1 will provide a new platform for the in vivo analysis of photosynthesis and bridge the gap between investigations of the short- and long-term regulation of photosynthesis. The mechanisms of the transient and its relevance to photosynthetic control require further experimentation.
Conclusion
The data obtained in the fba3 mutant reinforce the view that in normal plants, when the rate of electron flow in the thylakoid exceeds that of electron consumption in the Calvin cycle, excess electrons return to the intersystem carriers by PSI cyclic electron flow. In fba3-1 ndhM, the transient in post-illumination fluorescence was largely, but not completely, suppressed, indicating that the acceleration of the PSI cyclic electron flow in fba3-1 was mainly due to enhancement of the NDH-dependent flow. The low signal in fba3-1 ndhM was likely due to induction of Fd- (or PGR5)-dependent flow. In fba3-1 pgr5, the transient was only slightly suppressed, indicating the minor contribution of Fd-dependent flow to the accelerated PSI cyclic electron flow in fba3-1. Taken together with the results obtained under severe acceptor limitations in CO2-free air with 2% O2, it was concluded that the relative contribution of the two paths shifts from the NDH-dependent path to the Fd-dependent path in response to an increase in acceptor limitation of linear electron flow in Arabidopsis.
References
Allen JF (2003) Cyclic, pseudocyclic and noncyclic photophosphorylation: new links in the chain. Trends Plant Sci 8:15–19
Alonso JM, Stepanova AN, Leisse TJ et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301:653–657
Avenson TJ, Cruz JA, Kanazawa A, Kramer DM (2005) Regulating the proton budget of higher plant photosynthesis. Proc Natl Acad Sci USA 102:9709–9713
Baker NR, Harbinson J, Kramer DM (2007) Determining the limitations and regulation of photosynthetic energy transduction in leaves. Plant Cell Environ 30:1107–1125
Bendall DS, Manasse RS (1995) Cyclic photophosphorylation and electron transport. Biochim Biophys Acta 1229:23–38
Breyton C, Nandha B, Johnson GN, Joliot P, Finazzi G (2006) Redox modulation of cyclic electron flow around photosystem I in C3 plants. Biochemistry 45:13465–13475
Bukhov N, Egorova E, Carpentier R (2002) Electron flow to photosystem I from stromal reductants in vivo: the size of the pool of stromal reductants controls the rate of electron donation to both rapidly and slowly reducing photosystem I units. Planta 215:812–820
Burrows PA, Sazanov LA, Svab Z, Maliga P, Nixon PJ (1998) Identification of a functional respiratory complex in chloroplasts through analysis of tobacco mutants containing disrupted plastid ndh genes. EMBO J 17:868–876
Chow WS, Hope AB (2004) Electron fluxes through photosystem I in cucumber leaf discs probed by far-red light. Photosynth Res 81:77–89
Corneille S, Cournac L, Guedeney G, Havaux M, Peltier G (1998) Reduction of the plastoquinone pool by exogenous NADH and NADPH in higher plant chloroplasts. Characterization of a NAD(P)H-plastoquinone oxidoreductase activity. Biochim Biophys Acta 1363:59–69
DalCorso G, Pesaresi P, Masiero S, Aseeva E, Schünemann D, Finazzi G, Joliot P, Barbato R, Leister D (2008) A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell 132:273–285
Endo T, Shikanai T, Takabayashi A, Asada K, Sato F (1999) The role of chloroplastic NAD(P)H dehydrogenase in photoprotection. FEBS Lett 457:5–8
Genty B, Briantais JM, Baker N (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Golding AJ, Johnson GN (2003) Down-regulation of linear and activation of cyclic electron transport during drought. Planta 218:107–114
Groom QJ, Kramer DM, Crofts AR, Ort DR (1993) The non-photochemical reduction of plastoquinone in leaves. Photosynth Res 36:205–215
Haake V, Zrenner R, Sonnewald U, Stitt M (1998) A moderate decrease of plastid aldolase activity inhibits photosynthesis, alters the levels of sugars and starch, and inhibits growth of potato plants. Plant J 14:147–157
Haake V, Geiger M, Walch-Liu P, Christof E, Zrenner R, Stitt M (1999) Changes in aldolase activity in wild-type potato plants are important for acclimation to growth irradiance and carbon dioxide concentration, because plastid aldolase exerts control over the ambient rate of photosynthesis across a range of growth conditions. Plant J 17:479–489
Häusler RE, Geimer S, Kunz HH, Schmitz J, Dörmann P, Bell K, Hetfeld S, Guballa A, Flügge UI (2009) Chlororespiration and grana hyperstacking: how an Arabidopsis double mutant can survive despite defects in starch biosynthesis and daily carbon export from chloroplasts. Plant Physiol 149:515–533
Havaux M, Rumeau D, Ducruet JM (2005) Probing the FQR and NDH activities involved in cyclic electron transport around Photosystem I by the ‘afterglow’ luminescence. Biochim Biophys Acta 1709:203–213
Horváth EM, Peter SO, Joët T, Rumeau D, Cournac L, Horváth GV, Kavanagh TA, Schäfer C, Peltier G, Medgyesy P (2000) Targeted inactivation of the plastid ndhB gene in tobacco results in an enhanced sensitivity of photosynthesis to moderate stomatal closure. Plant Physiol 123:1337–1349
Joët T, Cournac L, Horvath EM, Medgyesy P, Peltier G (2001) Increased sensitivity of photosynthesis to Antimycin A induced by inactivation of the chloroplast ndhB gene. Evidence for a participation of the NADH-dehydrogenase complex to cyclic electron flow around photosystem I. Plant Physiol 125:1919–1929
Johnson GN (2005) Cyclic electron transport in C3 plants: fact or artifact? J Exp Bot 56:407–416
Joliot P, Joliot A (2005) Quantification of cyclic and linear flows in plants. Proc Natl Acad Sci USA 102:4913–4918
Joliot P, Joliot A (2006) Cyclic electron flow in C3 plants. Biochim Biophys Acta 1757:362–368
Kamruzzaman Munshi M, Kobayashi Y, Shikanai T (2005) Identification of a novel protein, CRR7, required for the stabilization of the chloroplast NAD(P)H dehydrogenase complex in Arabidopsis. Plant J 44:1036–1044
Kofer W, Koop HU, Wanner G, Steinmüller K (1998) Mutagenesis of the genes encoding subunits A, C, H, I, J and K of the plastid NAD(P)H-plastoquinone-oxidoreductase in tobacco by polyethylene glycol-mediated plastome transformation. Mol Gen Genet 258:166–173
Konieczny A, Ausubel FM (1993) A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J 4:403–410
Koßmann J, Sonnewald U, Willmitzer L (1994) Reduction of the chloroplastic fructose-1,6-bisphosphatase in transgenic potato plants impairs photosynthesis and plant growth. Plant J 6:637–650
Laisk A, Oja V (1994) Range of photosynthetic control of postillumination P700+ reduction rate in sunflower leaves. Photosynth Res 39:39–50
Laisk A, Eichelmann H, Oja V, Rasulov B, Rämma H (2006) Photosystem II cycle and alternative electron flow in leaves. Plant Cell Physiol 47:972–983
Li XP, Björkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK (2000) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403:391–395
Mano J, Miyake C, Schreiber U, Asada K (1995) Photoactivation of the electron flow from NADPH to plastoquinone in spinach chloroplasts. Plant Cell Physiol 36:1589–1598
Martín M, Casano LM, Zapata JM, Guéra A, de Campo EM, Schmitz-Linneweber C, Maier RM, Sabater B (2004) Role of thylakoid Ndh complex and peroxidase in the protection against photo-oxidative stress: fluorescence and enzyme activities in wild-type and ndhF-deficient tobacco. Physiol Plant 122:443–452
Miyake C, Miyata M, Shinzaki Y, Tomizawa K (2005) CO2 response of cyclic electron flow around PSI (CEF-PSI) in tobacco leaves—relative electron fluxes through PSI and PSII determine the magnitude of non-photochemical quenching (NPQ) of Chl fluorescence. Plant Cell Physiol 46:629–637
Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T (2002) PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 110:361–371
Munekage Y, Hashimoto M, Miyake C, Tomizawa K, Endo T, Tasaka M, Shikanai T (2004) Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 429:579–582
Muraoka R, Okuda K, Kobayashi Y, Shikanai T (2006) A eukaryotic factor required for accumulation of the chloroplast NAD(P)H dehydrogenase complex in Arabidopsis. Plant Physiol 142:1683–1689
Murphy DJ, Walker DA (1981) Aldolase from wheat leaves—its properties and subcellular distribution. FEBS Lett 134:163–166
Nandha B, Finazzi G, Joliot P, Hald S, Johnson GN (2007) The role of PGR5 in the redox poising of photosynthetic electron transport. Biochim Biophys Acta 1767:1252–1259
Ogawa K (2005) Glutathione-associated regulation of plant growth and stress responses. Antioxid Redox Signal 7:973–981
Ohyama K, Fukuzawa H, Kohchi T, Shirai H, Sano T, Sano S, Umesono K, Shiki Y, Takeuchi M, Chang Z, Aota S, Inokuchi H, Ozeki H (1986) Chloroplast gene organization deduced from complete sequence of liverwort Marchantia polymorpha chloroplast DNA. Nature 322:572–574
Okegawa Y, Kagawa Y, Kobayashi Y, Shikanai T (2008) Characterization of factors affecting the activity of photosystem I cyclic electron transport in chloroplasts. Plant Cell Physiol 49:825–834
Paul MJ, Driscoll SP, Andralojc PJ, Knight JS, Gray JC, Lawlor DW (2000) Decrease of phosphoribulokinase activity by antisense RNA in transgenic tobacco: definition of the light environment under which phosphoribulokinase is not in large excess. Planta 211:112–119
Rumeau D, Bécuwe-Linka N, Beyly A, Louwagie M, Garin J, Peltier G (2005) New subunits NDH-M, -N, and -O, encoded by nuclear genes, are essential for plastid Ndh complex functioning in higher plants. Plant J 17:219–232
Rumeau D, Peltier G, Cournac L (2007) Chlororespiration and cyclic electron flow around PSI during photosynthesis and plant stress response. Plant Cell Environ 30:1041–1051
Sazanov LA, Burrows PA, Nixon PJ (1998) The chloroplast Ndh complex mediates the dark reduction of the plastoquinone pool in response to heat stress in tobacco leaves. FEBS Lett 429:115–118
Shikanai T (2007) Cyclic electron transport around photosystem I: genetic approaches. Annu Rev Plant Biol 58:199–217
Shikanai T, Endo T, Hashimoto T, Yamada Y, Asada K, Yokota A (1998) Directed disruption of the tobacco ndhB gene impairs cyclic electron flow around photosystem I. Proc Natl Acad Sci USA 95:9705–9709
Shimizu H, Peng L, Myouga F, Motohashi R, Shinozaki K, Shikanai T (2008) CRR23/NdhL is a subunit of the chloroplast NAD(P)H dehydrogenase complex in Arabidopsis. Plant Cell Physiol 49:835–842
Shinozaki K, Ohme M, Tanaka M et al (1986) The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J 5:2043–2049
Stitt M, Schulze ED (1994) Does Rubisco control the rate of photosynthesis and plant growth? An exercise in molecular ecophysiology. Plant Cell Environ 17:465–487
Stokes DM, Walker DA (1972) Photosynthesis by isolated chloroplasts. Inhibition by dl-glyceraldehyde of carbon dioxide assimilation. Biochem J 128:1147–1157
Takabayashi A, Ishikawa N, Obayashi T, Ishida S, Obokata J, Endo T, Sato F (2009) Three novel subunits of Arabidopsis chloroplastic NAD(P)H dehydrogenase identified by bioinformatic and reverse genetic approaches. Plant J 57:207–219
Wang P, Duan W, Takabayashi A, Endo T, Shikanai T, Ye JY, Mi H (2006) Chloroplastic NAD(P)H dehydrogenase in tobacco leaves functions in alleviation of oxidative damage caused by temperature stress. Plant Physiol 141:465–474
Yamane Y, Shikanai T, Kashino Y, Koike H, Satoh K (2000) Reduction of QA in the dark: another cause of fluorescence Fo increases by high temperatures in higher plants. Photosynth Res 63:23–34
Acknowledgments
We thank Dr. Toshiharu Shikanai (Kyoto University) for his gift of mutant plants and helpful discussions. This work was supported by a Grant-in-aid for Exploratory Research (20658036) from JSPS to MT and for Scientific Research on Priority Areas (16085206) from MEXT to Toshiharu Shikanai.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gotoh, E., Matsumoto, M., Ogawa, K. et al. A qualitative analysis of the regulation of cyclic electron flow around photosystem I from the post-illumination chlorophyll fluorescence transient in Arabidopsis: a new platform for the in vivo investigation of the chloroplast redox state. Photosynth Res 103, 111–123 (2010). https://doi.org/10.1007/s11120-009-9525-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-009-9525-0