Abstract
Photosynthetic state transitions are a well-known phenomenon of short-term adaptation of the photosynthetic membrane to changes in spectral quality of light in low light environments. The principles of the monitoring and quantification of the process in higher plants are revised here. The use of the low-temperature excitation fluorescence spectroscopy for analysis of the photosystem I antenna cross-section dynamics is described. This cross section was found to increase by 20–25% exclusively due to the migration and attachment of LHCIIb complex in State 2. Analysis of the fine structure of the additional PSI cross-section spectrum revealed the 510 nm band, characteristic of Lutein 2 of LHCIIb and present only when the complex is in a trimeric state. The excitation fluorescence spectrum of the phospho-LHCII resembles the spectrum of aggregated and hence quenched LHCII. This novel observation could explain the fact that at no point in the course of the state transition high fluorescence and long lifetime components of detached trimeric LHCII have ever been observed. In the plants lacking Lhcb1 and 2 proteins and unable to perform state transitions, compensatory sustained adjustments of the photosystem I and II antennae have been revealed. Whilst the major part of the photosystem II antenna is built largely of CP26 trimers, possessing less chlorophyll b and more of the red-shifted chlorophyll a, photosystem I in these plants contains more than 20% of extra LHCI antenna enriched in chlorophyll b. Hence, both photosystems in the plants lacking state transitions have less spectrally distinct antennae, which enable to avoid energy imbalance due to the changes in the light quality. These alterations reveal remarkable plasticity of the higher plant photosynthetic antenna design providing the basis for a flexible adaptation to the light environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
State transitions: what are they and why do they occur?
The photosynthetic state transitions are a process of changing the balance between the excitation energy flows into the photosystem I and photosystem II reaction centres. This phenomenon first discovered in algae nearly 40 year ago (Bonaventura and Myers 1969; Murata 1969a, b) is triggered by changes in the spectral quality of light environment. It is a short-term adaptation strategy, since it occurs on a timescale of minutes (with total completion within 10–20 min) and does not involve any known alterations in protein gene expression. This adaptation is very pronounced in cyanobacteria and green algae, which normally inhabit low light environments. State transitions are also present in higher plants, where the molecular mechanism was very intensively investigated during the 1970s.
The fundamental cause of the need for state transitions is the fact that the photosynthetic reaction centres of photosystem I and II have different chlorophyll excited state energies, 700 and 680 nm, respectively (Fig. 1a). This difference dictates a different spectral design of the respective antenna systems manifesting in the photosystem II antenna having more blue-shifted absorption relative to the one of the photosystem I (Fig. 1a). In higher plants, the blue shift of the photosystem II antenna is determined largely by enrichment in chlorophyll b (most abundant in the antenna-dominating LHCIIb complex) and rather moderately red-shifted chlorophyll a forms, most of which absorb in 670–676 nm region (van Grondelle and Novoderezhkin 2006). The photosystem I antenna has a smaller amount of chlorophyll b and possesses a number of red-shifted chlorophyll a forms with absorption expanding to 690–710 nm region (Fig. 1a).
a Absorption spectra of isolated PSII-containing membranes (BBY) and PSI complexes prepared by the sucrose gradient separation as described by Ruban et al. 2006. Difference between PSI and PSII absorption spectra is displayed by hollow symbols. Black arrows indicate absorption wavelengths of the reaction centres of both photosystems. b Pulse-modulated chlorophyll fluorescence measurement of the state transitions in Arabidopsis leaf. F o and F m are the fluorescence levels corresponding to open and closed PSII reaction centres, respectively. r and fr are red and far red continuous lights. FsI and FsI′ are the fluorescence levels at State I with and without far red light on, respectively. FsII and FsII′ are the fluorescence levels at State II with and without far red light on, respectively. qT is a reduction in F m level as a result of the transition into State II
The frequent light quality changes can cause a very strong alteration in the ratio between energy inputs into photosystems, which work in series supporting the linear electron transfer flow from water (PSII) to ferredoxin (PSI). This variation in the energy flow can cause the growth inefficiency of the photosynthetic processes in the thylakoid membrane. The consequences can be particularly detrimental for the plants living in light starved environments typical of shaded ecological niches. Therefore, state transitions are an essential strategy for photosynthetic organisms helping them to utilise every photon they manage to capture in order to survive and proliferate in low light environments.
The measurement and quantification of state transitions
The development of approaches to measure chlorophyll fluorescence enabled the discovery of state transitions (Bonaventura and Myers 1969; Murata 1969a, b). Later the development of pulse amplitude modulated fluorimetry (PAM) at the end of 1970s by Schreiber (1986) played a great role in studies of the short-term adaptation mechanisms of the photosynthetic membrane. Since pulse modulated fluorescence approach can easily separate the photochemical and nonphotochemical quenching events in the thylakoid membrane it seems to be a most valid method to analyse state transitions and gain the consistent and quantitative information about them.
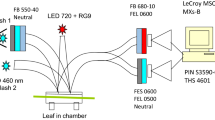
Figure 1b displays the typical fluorescence transients measured using PAM fluorimeter on Arabidopsis leaves. It is essential in these measurements to use low light intensity for selective excitation of photosystem I or II in order to keep the majority of reaction centres in the open state. Before applying spectrally defined light, a saturation pulse is used to measure the level of maximum fluorescence, F m, in order to assess the state of photosystem II reaction centres. The spectral quality of excited actinic light (r—red, and fr—far red) was chosen using the spectra displayed on the Fig. 1a. Photosystem II can be preferentially excited by the light defined by the broad interference filter at 650 nm with 20 nm bandpath. Combination with a tungsten halogen light source enables a light intensity of several tens of micromoles per square meter per second to be achieved. For the PSI light a long path filter allowing light with wavelengths above 690 nm or an interference filter at around 700–710 nm are required.
Since plants used for these experiments are normally dark-adapted, the first illumination cycle is applied in order to induce transients related to the activation of the electron transport. Both PSII and PSI lights are turned on. After the induction phase, far red light was turned off, causing a marked increase in the fluorescence level far above F o level (FsI′). This rise indicates that the electron removal from the photosystem II has been slowed down leading to the additional reduction of Q A. If the filter combination is right and the balance between the red and far red light intensities is optimised, one should expect the Fs level to increase after switching off the far red light more than 50% of the amplitude of F o. Removal of the far red light also triggers the onset of a gradual decrease in the Fs level, lasting for about 15 min (FsII′). This is the manifestation of the transition from State 1 to State 2, tuning the balance of excitation energy towards the PSI consequently re-oxidising the plastoquinone pool and causing a decrease in fluorescence. Turning the far red light on in this state causes a small drop of FsII′–FsII level. The smaller this drop the more completely state transitions are effectively compensating for the spectral differences between photosystems to that extent that in State 2 the photosystem II redox state becomes almost insensitive to the spectral quality of excited light. Saturation pulse reveals a decrease in F m level, associated with the transition into State 2. During the next 15 min of illumination with both PSI and PSII lights a slow increase in the FsII fluorescence level to FsI can be observed. The use of a saturation pulse in the end of this period reveals complete restoration of the F m fluorescence level (Fig. 1b). Now, turning off the far red light again causes the rise in the Fs level suggesting the restoration of the spectral difference between the photosystems typical of State 1. One can run another cycle of illumination and reveal that these transients are perfectly reversible, so that there is no accumulation of any sustained Fm quenching, typical in the measurements of the ΔpH-triggered nonphotochemical fluorescence quenching, NPQ.
Several important parameters of the fluorescence transients related to the state transitions can be calculated.
First is a degree of energy imbalance induced by the removal of the far-red light:
This is somewhat an arbitrary parameter, which depends on the combination of filters and ratio between red and far-red light intensities. It is useful in tuning the set-up for the measurements as a standard for optimum lights choice. The imbalance parameter can also be used to assess the effect of antenna composition and structure on the spectral differences between photosystems, which affect the efficiency of the linear electron transport rate.
Second state transition characteristic is the level of F m fluorescence drop in State 2. It is called qT and calculated as:
qT is regarded as a fluorescence decline, not a true quenching, since it reflects the decrease in the LHCII antenna size (see the next paragraph). This is normally variable from 0 to 0.25.
Third useful parameter for the calculation of state transitions can be referred to as qS:
qS simply indicates how effectively state transitions can cope with the changing quality of light in optimisation of the electron transport. The parameter varies from 0 to 1, where 1 indicates 100% efficiency in the rebalancing of the electron transport rate after the changes in the spectral quality of light.
Fourth characteristic of state transitions is the rate of Fs fluorescence changes. It can simply be estimated as 1/t 1/2 , where t 1/2 is the time required to change Fs by 50%. In addition, Fs can be fitted with, for example, an exponential function in order to obtain the value of the rate constant.
The mechanism
During the first decade since their discovery, state transitions were believed to reflect spillover-type alterations between the photosystems, related to the cation-induced movements causing stacking/unstacking of grana in higher plants (Myers 1971; Murata 1969a, b; Arntzen and Dito 1976; Barber 1980). Indeed the fluorescence decrease during the grana unstacking process seems to imitate well the transition-related fluorescence changes. However, the problem of this explanation of the mechanism was that nobody could observe any detectable grana unstacking as well as fluctuations in the cation levels under physiological conditions. Moreover, whilst the state transitions did not have any significant effect on the photosystem II intrinsic efficiency, spillover caused a large decrease in F v/F m (Butler 1978).
These fundamental discrepancies have been resolved by the emergence of a new model explaining the state transitions—the LHCII phosphorylation mechanism (Allen et al. 1981; Bennett 1983; Horton 1983). Two major discoveries have been crucial for a development of this model. First is a discovery of phosphorylation of the chloroplast membrane proteins (Bennett 1977) and second is the finding of the phosphokinase activation by the redox state of the plastoquinone pool (Horton and Black 1980). According to the model, the imbalance in the energy input into both photosystems is reflected by the redox state of the plastoquinone pool, which acts as a governor operating two enzymes—kinase and phosphatase—which phosphorylate and dephosphorylate some of the LHCIIb polypeptides, respectively. The phosphorylation alters the affinity of a subpopulation of LHCII to photosystem II causing its detachment and migration towards photosystem I in order to serve as an additional antenna for this photosystem (Fig. 2). As a result, PS II cross section decreases, whilst the one of the PSI increases. In addition, the increase in PSI cross section occurs at the expense of chlorophyll b and short-wavelength forms of chlorophyll a, which make photosystem I antenna less spectrally distinguishable from that of the photosystem II facilitating further the balanced redistribution of the excitation energy in the thylakoid membrane. Oxidised plastoquinone acting as an activator of phosphatase causes removal of the phosphate group from LHCII and subsequent migration of the complex towards photosystem II.
The LHCII phosphorylation model of the state transitions. Imbalance in the light energy input into the antennae of photosystems leading to the preferential excitation of PSII causes the reduction of the plastoquinone pool (PQH2). Reduced PQ activates kinase, which phosphorylates polypeptides of LHCII. The phosphorylated LHCII detaches from PSII, migrates towards PSI and incorporates into its antenna system. As a result of this incorporation PSI gains more excitation energy which leads to the increase in the reaction centre (RC1) turnover with the subsequent oxidation of the plastoquinone pool. When PSI gains relatively more excitation light than PSI it oxidises PQ further. PQ+ activates phosphatase—an enzyme which dephosphorylates LHCII attached to the PSII. Dephosphorylated LHCII detaches from PSI, and migrates and incorporates into PSII
The LHCII phosphorylation model was tested by numerous experiments in the 1980s. The effect of redox state of plastoquinone pool on the kinase has been demonstrated by groups of Allen and Horton (for the review see Horton et al. 1981). The role of the spectral quality of light in the phosphorylation of thylakoid proteins has been shown on both thylakoids and leaves (Horton and Black 1980; Chow et al. 1981; Owens and Ohad 1982; Telfer et al. 1983; Bennett 1983). Chlorophyll fluorescence measurements have confirmed reversible phosphorylation-related alteration in the energy redistribution between photosystems (Bennett et al. 1980; Haworth et al. 1982; Horton and Black 1983).
However, the question of the contribution of spillover to the phosphorylation-related fluorescence transients continued to cause some controversy. Whilst Kyle et al. (1982) found that the spillover takes place during the in vitro LHCII phosphorylation, Horton and Black argued for the LHCII dissociation from PSII as a sole cause of the fluorescence decrease accompanying phosphorylation (Horton and Black 1982, 1983). Spillover was found to depend upon magnesium concentration in the incubation medium. Bennett (1984) has frequently argued that in vivo the cation concentration should be high enough to prevent spillover and therefore preferred the α-transfer idea (migration of LHCII only) to the spillover explanation of the consequences of LHCII phosphorylation. However, it has been suggested that spillover could be a second energy-balancing event, following α-transfer, resulting from prolonged phosphorylation and exacerbated by high temperature (Jennings et al. 1986; Sundby and Andersson 1985; Weis 1985; Ruban 1991; Ruban and Trach 1991). In addition, there are indications that the spectral quality of the growth light environment can trigger spillover in leaves during state transitions (Dau and Hansen 1988).
Various microscopy and biochemistry experiments proved the migration of phosphorylated LHCII into the stroma lamellae, which carry PSI (Chow et al. 1981; Andersson et al. 1982; Kyle et al. 1983). It was proposed that only a subpopulation of LHCII, a mobile phosphorylated complex, migrated towards PSI. This complex was proposed to be enriched in the lighter polypeptide of 25 kDa (Lhcb2 in the modern nomenclature) (Larsson and Andersson 1985). Migrating phospho-LHCII was suggested to be in oligomeric state (Andersson et al. 1982). Recently the analysis of fractions containing grana and stroma PSI obtained from phosphorylated thylakoid membranes revealed that in the State 2 phospho-LHCII interacts with the photosystem I localised in grana margins (Tikkanen et al. 2008). It was proposed that this region of thylakoid membrane is very important in the regulation of balance between cyclic and linear electron transport. These findings are coherent with the earlier observations of heat-induced migration of LHCII towards the grana PSI (Ruban and Trach 1991).
Questions regarding the functional consequences of LHCII migration towards PSI as a result of phosphorylation have been addressed too. The phosphorylation-related decrease in PSII and increase in PSI electron transport have been observed (Farchaus et al. 1982; Forti and Vianelli 1988). However, experiments on the measurements of oxygen quantum evolution efficiency have failed to detect any phosphorylation-related alterations in the O2 yield. (Sinclair and Cousineau 1981). In addition to this discrepancy, measurements of P700 photooxidation kinetics revealed only a small increase in the PSI cross section following LHCII phosphorylation (Haworth and Melis 1983). Later work on P700 by Allen and Melis also failed to detect any increase in the PSI antenna size (Allen and Melis 1988). However, similar experiments performed by other groups on PSI-containing isolated membranes have shown about 20% increase in the rate of P700 oxidation (Telfer et al. 1984). Low temperature fluorescence induction measurements have also indicated significant redistribution of excitation energy towards PSI after LHCII phosphorylation (Black and Horton 1984; Larsson et al. 1986).
Photosystem I absorption cross-section changes during the state transitions
There are only a few papers in which direct observations of functional interaction between the phospho-LHCII and PSI have been reported. Kyle et al. using the 77 K excitation fluorescence spectroscopy of chloroplasts and subchloroplast PSI-containing fractions have found that PSI receives additional energy from chlorophyll b after LHCII phosphorylation (Kyle et al. 1984). This finding suggested that LHCII was functionally coupled to PSI in State 2. Later, Larsson et al. confirmed the presence of chlorophyll b and some chlorophyll a forms in the Soret band excitation fluorescence spectra of thylakoids and PSI particles after phosphorylation. Moreover, these authors have estimated that phosphorylation caused a 15% increase in PSI antenna size (Larsson et al. 1986).
Difficulties of the Soret band structure interpretation and uncertainties arising from the normalisation of excitation spectra have led to the application of the PSI excitation fluorescence spectroscopy in the red chlorophyll absorption region (Ruban 1991). The method utilises the large separation between PSI and PSII 77 K fluorescence bands (Fig. 3a) and can be used for PSI preparations as well as intact chloroplasts and potentially even leaves. Photosystem I F735 band is emitted by the long wavelength chlorophyll a forms absorbing around 705–710 nm and known as C705. The fluorescence excitation spectrum in the red region for chloroplasts is shown in Fig. 3b. It represents a sum of amplitudes of reversed transmission, (1 − T), of i spectral components multiplied by their corresponding energy transfer coefficients to C705 chlorophyll/s, k i :
a 77 K fluorescence spectra of Photosystem II membranes (PSII) and Photosystem I complex (PSI). Spectra are normalised to 1. b A diagram explaining the origin of the PSI F735 excitation fluorescence spectrum. Pigment energy levels are displayed as horizontal lines. C705 is the self-absorption of F735 band, where the fluorescence is being detected. The amplitudes in the excitation fluorescence spectra of each individual pigment form are proportional to the product of (1 − T), a reversed transmission, and the energy transfer efficiency towards C705, k i . c Relationship between OD and (1 − T) displaying its nonlinearity. The dashed line highlights this nonlinear relationship. Inset on the left displays two almost overlapping spectra, OD and excitation fluorescence for isolated LHCII, normalised at 650 nm for low chlorophyll concentration (OD = 0.1). Inset on the right displays OD (solid line) and excitation fluorescence (dashed line) spectra (normalised at 650 nm) for the complex at OD = 0.45
where f is a proportionality coefficient. The C705 band can be clearly seen in the fluorescence excitation spectrum (Fig. 3b). This is because k i are all smaller than 1, reflecting some losses in energy during its transfer from chlorophyll a and b to C705 and therefore causing a decrease in amplitudes across the whole spectrum relative to C705. Thus, excitation fluorescence spectra of different states can be normalised to C705—an exclusively PSI terminal emitter region—in order to obtain information on the PSI cross-section changes. The excitation fluorescence spectra also need to be corrected for the contribution of the PSII vibronic satellite band, which can contribute up to 20% of intensity at 733 nm (Fig. 3a).
Another point needing to be taken into consideration is the fact that the excitation fluorescence spectrum being a (1 − T) type is not linearly related to the optical density or OD spectrum even when all k i = 1. This is because it is proportional to the amount of absorbed energy and therefore does not contain a logarithmic operator as OD. Figure 3c displays the relationship between (1 − T) intensity and OD (solid curve). Only for OD < 0.1, i.e. for low chlorophyll concentrations one can see almost linear relationship between those parameters. For OD > 0.2 (1 − T) spectrum becomes less and less proportional to the OD and hence concentration of spectral pigment forms. This tendency will lead to the reduction in the amplitudes of the major spectral components relative to those of the minor ones causing broadening of the whole excitation fluorescence spectrum. Two insets on Fig. 3c illustrate this point. OD and fluorescence excitation spectrum for isolated LHCII at the chlorophyll concentration giving maximum OD = 0.1 are almost indistinguishable. On the contrary, the same pair of spectra for the maximum OD = 0.45 are clearly different from each other. The excitation fluorescence spectrum looks broader with relative reduction in the chlorophyll a region and slight increase in the vibrational satellite area.
Taking into consideration the above mentioned peculiarities of the excitation fluorescence profiles, one has to take great care ensuring that the spectra are:
-
(a)
corrected by the contribution of PSII vibronic satellite;
-
(b)
measured always at low chlorophyll concentration, identical for all samples.
For the samples measured at different and somewhat higher concentrations, one has to measure (1 − T) spectra and use them for a logarithmic conversion of the excitation fluorescence spectra. The procedure would include a transformation of the excitation fluorescence spectrum into the T-type and then calculation of log(T o/T), which would give a form of OD spectrum and hence make its shape independent from the sample concentration. However, the caution has to be exercised in the extraction of the meaningful information on k i after this procedure.
Figure 4 shows 77 K fluorescence spectra of chloroplasts at low chlorophyll concentration (2 µM) prepared from the leaves used in the experiment described in Fig. 1b. Spectra are normalised to 680 nm region. The difference State 2-minus-State 1 spectrum closely resembles that of purified PSI (Fig. 3a). A typical PSI band at 690 nm can be clearly identified in the difference spectrum (arrow). Figure 5a shows the F735 fluorescence excitation spectra for States 1 and 2 measured in the whole visible region and normalised at 705 nm. The difference State 2-minus-State 1 spectrum is shown below in Fig. 5b. It clearly resembles the (1 − T) spectrum of isolated LHCIIb complex. Structural features at 435, 660, 670 and 676 nm (chlorophyll a), and 472 and 650 nm (chlorophyll b) can be clearly seen here. The ratio between chlorophyll a and b in the red and Soret regions suggests that the PSI cross section is enhanced in State 2 due to the coupling of LHCIIb complex. No long wavelength chlorophyll a forms typical for PSII core complexes can be detected in this experiment, suggesting the absence of spillover. Previously those forms have been shown to appear only after in vitro phosphorylation of thylakoids in the presence of dithionite or triggered by high incubation temperature (Ruban 1991, Ruban and Trach 1991). The relative cross-section increase expressed as a ratio between the difference spectrum and the spectrum for the State 1 was found to be around 24%, which is consistent with the previous reports on the PSI cross-section changes mentioned above. Taking into account that PSI carries 168 chlorophylls (Amunts et al. 2007) 24% of that number would correspond to 40 molecules in additional cross section in the State 2. This roughly matches the number of chlorophyll molecules in LHCIIb trimer or three LHCIIb monomers (42 chlorophylls a + b).
a 77 K F735 excitation fluorescence spectra of Arabidopsis chloroplasts in States 1 and 2 (for experimental details see Ruban et al. 2006). b 77 K difference excitation spectrum State 2-minus-State 1 (S2 − S1) and reversed transmission spectrum of isolated trimeric LHCII (for the isolation procedure see Ruban et al. 2006)
What is the structural and functional state of phospho-LHCIIb interacting with PSI ?
The calculated spectrum of the PSI additional cross section (S2 − S1) is particularly rich in information on various pigments and their states in the phosphor-LHCII interacting with PSI. The question, deserving of particular attention, is what is the oligomeric state of the interacting complex, a trimer or a monomer? It has been previously suggested that the LHCII phosphorylation causes a conformational transition within LHCII leading to its monomerisation, detachment from PSII and migration towards PSI in the monomeric state (Nilsson et al. 1997). Migration of a smaller, monomeric complex with an altered conformation allowing recognition of PSI is an elegant and attractive scenario explaining the promptness, precision and effective reversibility of the state transitions (Allen and Forsberg 2001). However, recently, electron microscopic evidence has emerged suggesting that in State 2 PSI interacts with a particle closely resembling an LHCII trimer (Kouril et al. 2005).
The controversy about the oligomerisation state of migrating phospho-LHCII can be addressed by analysing the Soret part of the calculated additional cross-section spectrum of PSI in State 2. Figure 6 displays the state transition-related spectrum and its second derivative as well as excitation fluorescence spectra for the LHCIIb in trimeric and monomeric states. The second derivative spectrum shows maxima corresponding to chlorophylls and xanthophylls. The latter have 485 nm (neoxanthin) and 495 nm (lutein 1) components (Ruban et al. 2000, 2001). In addition, a band at 510 nm corresponding to lutein 2 absorption is clearly seen. This band is present exclusively in LHCII in the trimeric, not monomeric state as evident from the comparison of their fluorescence excitation spectra (Fig. 6). The cause for the red shift of the lutein 2 can be explained using the structure of LHCII. Figure 7 shows the fragment of LHCII trimer showing lutein 2 of the one of the monomers positioned within the van-der-Waals contact with the chlorophyll a603 from one of the other monomers within the trimer. Nearly all five oxygen atoms of the chlorine ring of the chlorophyll a are facing the end ring of lutein 2. This creates highly polar environment for the xanthophyll affecting its excited state energy and leading to the red shift in absorption. Monomerisation removes this interaction, leading to disappearance of the 510 nm absorption band. Therefore, the excitation spectral analysis of the state transitions on isolated chloroplasts made it possible to not only identify and quantify LHCII interaction with PSI during the state transitions but also helped to define its state of oligomerisation as a trimer.
The Soret band 77 K F735 excitation fluorescence spectral difference, State 2-minus-State 1 (S2 − S1), and its second derivative (2d derivative). This solid and dashed lines represent excitation fluorescence spectra of isolated LHCII trimers and monomers, respectively (detection wavelength was 680 nm). Vertical line highlights the 510 nm band characteristic for the LHCII trimer and absent in the complex in the monomeric state
Further analysis of the phospho-LHCII cross-section spectrum has revealed additional interesting information. Its comparison to the excitation fluorescence spectrum of isolated LHCIIb trimers shows that the spectra are not entirely identical (Fig. 8). The (S2 − S1) spectrum indicates the presence of 681 nm band and relative decrease in the carotenoid region at around 485–495 nm as emerged in the difference spectrum, (S2 − S1)-minus-LHCII. This spectrum notably resembles the difference aggregated-minus-trimeric LHCII (Ruban et al. 1997). It is well-known that LHCII trimers are in a low fluorescing state when in the conformational form present in aggregates (Ruban and Horton 1992). Therefore, it is likely that the phospho-LHCII exists in a similar quenched conformation during state transitions. This possibility could now explain why strong fluorescence of the detached phospho-LHCII has never been observed during the process. Fluorescence quenching in the phospho-LHCII would not significantly reduce its antenna function for PSI since this photosystem is more powerful trap of excitation energy than PSII and is likely to compete successfully with the excitation quencher in the interacting LHCII.
77 K F735 excitation fluorescence spectral difference, State 2-minus-State 1 (S2 − S1), and the fluorescence excitation spectrum of LHCII trimers (LHCII) (detection in the vibronic satellite band at 740 nm). Below is the difference between them, (S2 − S1)-minus-LHCII. Vertical line at 681 nm indicates the long wavelength chlorophyll a transition typical for the spectrum of aggregated/quenched LHCII
Chromatic adaptations in plants lacking LHCII polypeptides
The importance of keeping a balance of excitation energy input into both photosystems was highlighted by experiments on Lhcb2 antisense plants (asLhcb2) lacking the two major LHCII polypeptides, Lhcb1 and 2, which phospho-LHCII is build of Anderson et al. (2003). Andersson et al. (2003) have shown that these plants have only moderately reduced PSII antenna and possess normal granae. The state transitions were completely inhibited, as one would anticipate. What was surprising is the fact that the imbalance parameter, IB, was found to be drastically reduced in comparison to the wild type plants (IBas = 0.1 vs. IBwt = 0.65) (Fig. 9a). This clearly indicated some alterations in photosystems antennae composition/structure. Later experiments have revealed that significant compensatory adjustments took place in the photosystem II antenna of asLhc2 (Ruban et al. 2003). LHCII trimers built of Lhcb5 and Lhcb3 proteins have been discovered serving as a dominating component of PSII antenna. Close comparison of the spectral properties of these trimers and the timers isolated from the wild type reveal significant decrease in the chlorophyll b absorption and a shift towards longer wavelengths of the spectrum of chlorophyll a population (Fig. 9b). These differences make PSII antenna effective in capturing less red and more far red photons and therefore make it similar to PSI antenna. Another series of experiments discovered a significant increase in PSI antenna size caused by an increased amount of LHCI complexes (Ruban et al. 2006) (Fig. 9c, see the inset of the polypeptide profiles). This was a first observation that LHCI antenna can be considerably upregulated. The excitation fluorescence spectroscopy of isolated PSI has confirmed the increase in C705 cross section by approximately 20%. However, this figure is probably much higher taking into account the removal of a large proportion of peripheral LHCI from PSI during its purification in the mutant (Ruban et al. 2006). PSI antenna gaining more chlorophyll b and blue-shifted chlorophyll a with additional LHCI becomes more blue-shifted in the antisense plants—the response completely opposite to that of the PSII antenna, as described above. Analysing changes in both PSI and PSII one could easily conclude that in asLhcb2 plants the antennae of photosystems are less spectrally distinct than in the wild type plants. This makes the photosynthetic membrane less sensitive to the changes in spectral quality of light as was clearly indicated by the dramatically decreased imbalance parameter in the state transition measurements shown in the Fig. 9a. Absence of ability to perform the state transitions seems to be compensated by the decrease in the spectral differences between PSI and II antennae in asLhcb2 plants.
a State transition measurement (as in Fig. 1b) of Lhcb2 antisense Arabidopsis leaf. IB, is the imbalance parameter. b 77 K absorption spectra of isolated LHCII trimers from the wild type (solid line) and Lhcb2 antisense (dashed line) Arabidopsis leaves. c 77 K reversed transmission (solid lines) and fluorescence excitation (dashed lines) spectra of isolated PSI from the wild type (wt) and Lhcb2 antisense (as) plants. Inset is the polypeptide profiles of the isolated PSI complexes of the wild type (wt) and Lhcb2 antisense plants. LHCI polypeptides are highlighted by the bracket
Figure 10 illustrates the antenna adjustments in the asLhcb2 plants. The state transitions are absent and compensated by the decreased PSII antenna, which is less spectrally different from the PSI antenna (highlighted by less different shades of grey). Photosystem I antenna is increased with more LHCI units attached and with somewhat lower affinity to PSI than the wild type LHCI.
The model illustrating compensatory adjustments in both photosystem I and II antennae as a response to the lack of Lhcb1 and 2 polypeptides of the major LHCII complex. Lack of the state transitions is compensated by the reduced PSII and increased PSI LHC antenna size. In addition, in the asLhcb2 plants, PSII antenna absorption is red-shifted in comparison to that of the wild type plants. PSI antenna absorption, on the other hand, shifted more to the blue, since it is more enriched in chlorophyll b-containing LHCI complexes
Conclusions
Although the phenomenon of the state transitions was discovered nearly four decades ago, some crucial mechanistic details of the process are still not completely understood, in particular, interaction of phospho-LHCII with PSI. Excitation fluorescence technique seems to be a powerful tool to study this interaction, since it can not only reveal the fact of this interaction, but also quantify it. In addition, this approach enables the study of the oligomerisation state of interacting LHCII as well as the excited state properties or the pigments involved. A few important conclusions have already resulted from the application of this approach:
-
(1)
phospho-LHCIIb does interact effectively with PSI;
-
(2)
attached LHCII is in a trimeric state;
-
(3)
phospho-LHCII is likely to be in a quenched state;
-
(4)
absence of the major LHCIIb polypeptides in antisense plants leads to a complete abolition of state transitions;
-
(5)
both photosystems in plants lacking Lhcb1 and 2 possess less spectrally distinct antennae—an indication of compensatory adjustments helping to cope with inability to perform state transitions.
Future perspectives
State transitions remain one of the most interesting topics in photosynthesis research. Invention of new experimental approaches is likely to ignite a new wave of discoveries in this field.
Structural methods
Development of various microscopies, suitable for studies of the membrane structure and dynamics in vivo can be very promising in elucidating the mechanism of the phospho-LHCII delivery to the PSI, i.e. migration character, rates, role of the membrane composition and macrostructure, character of interaction of the phospho-LHCII with PSI.
Genetic manipulation
Availability of various protein and pigment (xanthophyll) antenna mutants provides a rich field for experimentation, which could help to determine the energy state/states (oligomeric vs. monomeric, harvesting vs. dissipative) of migrating phospho-LHCII and roles of the immobile PSII antenna complexes and LHCI in the state transitions.
Physiology
Important physiological questions regarding the state transitions are waiting to be addressed. Among them are possible effect of LHCII antenna size and composition; effect of energy-dependent quenching, NPQ; effect of the xanthophyll cycle activity; effect of PsbS; effect of the change in the photosystem stoichiometry and PSII connectivity; role of the state transitions in the plant survival under the light starvation regimes in natural environments.
These and other new approaches should engender a greater understanding of the role of the state transitions in the context of the fundamental adaptative responses of the photosynthetic membrane and indeed the life of the plant as a whole.
Abbreviations
- LHCIIb:
-
The major light harvesting complex of photosystem II
- PSI and II:
-
Photosystems I and II
- CP26:
-
A minor antenna complex of photosystem II
- PAM:
-
Pulse amplitude modulated
- NPQ:
-
Nonphotochemical fluorescence quenching
- OD:
-
Optical density
- T:
-
Transmission
- F o and F m :
-
Fluorescence levels when all PSII reaction centres are open or closed, respectively
- F v :
-
Variable fluorescence (F v = F m − F o)
References
Allen JF, Forsberg J (2001) Molecular recognition in thylakoid structure and function. Trends Plant Sci 6:317–326
Allen JF, Melis A (1988) The rate of P700 photooxidation under continuous illumination is independent of state-1–state-2-transitions in green algae Scenedesmus obliquus. Biochim Biophys Acta 933:95–106
Allen JF, Bennett J, Steinback KE et al (1981) Chloroplast protein phosphorylation couples plastoquinone redox state to distribution of excitation energy between photosystems. Nature 291:1–5
Amunts A, Drory O, Nelson N (2007) The structure of a plant photosystem I supercomplex at 3.4 Å resolution. Nature 447:58–63
Anderson J, Wentworth M, Walters RG, Howard C, Ruban AV, Horton P, Jansson S (2003) Absence of Lhcb1 and Lhcb2 proteins of the light-harvesting complex of photosystem II-effects on photosynthesis, grana stacking and fitness. Plant J 35:350–361
Andersson B, Åkerlund H-E, Jergil B, Larsson C (1982) Differential phosphorylation of the light-harvesting chlorophyll-protein complex in appressed and non-appressed regions of the thylakoid membrane. FEBS Lett 149:181–185
Arntzen JC, Dito CL (1976) Effects of cations upon chloroplast membrane subunit interactions and excitation energy distribution. Biochim Biophys Acta 449:259–274
Barber J (1980) An explanation for the relationship between salt-induced thylakoid stacking and the chlorophyll fluorescence changes associated with changes in spillover of energy from photosystem II to photosystem I. FEBS Lett 118:1–10
Bennett J (1977) Phosphorylation of chloroplast membrane proteins. Nature 269:344–346
Bennett J (1983) Regulation of photosynthesis by reversible phosphorylation of the light-harvesting chlorophyll a/b protein. Biochem J 212:1–13
Bennett J (1984) Chloroplast protein phosphorylation and the regulation of photosynthesis. Physiol Plant 60:583–590
Bennett J, Steinback KE, Arntzen CJ (1980) Chloroplast phosphoproteins: regulation of excitation energy transfer by phosphorylation of thylakoid membranes. Proc Natl Acad Sci USA 77:5253–5257
Black MT, Horton P (1984) An investigation into the mechanistic aspects of excitation redistribution following thylakoid membrane protein phosphorylation. Biochim Biophys Acta 767:568–573
Bonaventura C, Myers J (1969) Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim Biophys Acta 189:366–383
Butler WL (1978) Energy distribution in the photochemical apparatus of photosynthesis. Annu Rev Plant Physiol 29:345–378
Chow WS, Telfer A, Chapman DJ et al (1981) State 1–State 2 transitions in leaves and its association with ATP induced chlorophyll fluorescence quenching. Biochim Biophys Acta 638:60–68
Dau H, Hansen U-P (1988) The involvement of spillover changes in State 1–State 2 transitions in intact leaves at low light intensities. Biochim Biophys Acta 934:156–159
Farchaus JW, Widger WR, Cramer WA, Dilley RA (1982) Kinase-induced changes in electron transport rates of spinach chloroplasts. Arch Biochem Biophys 217:362–367
Forti G, Vianelli A (1988) Influence of thylakoid protein phosphorylation on photosystem I photochemistry. FEBS Lett 231:95–97 638:60–68
Haworth P, Melis A (1983) Phosphorylation of chloroplast thylakoid membrane proteins does not increase the absorption cross-section of photosystem I. FEBS Lett 160:277–280
Haworth P, Kyle DJ, Horton P, Arntzen CJ (1982) Chloroplast membrane protein phosphorylation. Photochem Photobiol 36:743–748
Horton P (1983) Control of chloroplast electron transport by phosphorylation of thylakoid proteins. FEBS Lett 152:47–52
Horton P, Black MT (1980) Activation of adenosine 5′-triphosphate induced quenching of chlorophyll fluorescence by reduced plastoquinone. The basis of State I–State II transitions in chloroplasts. FEBS Lett 119:141–144
Horton P, Black MT (1982) On the nature of the fluorescence decrease due to phosphorylation of chloroplast membrane proteins. Biochim Biophys Acta 680:22–27
Horton P, Black MT (1983) A comparison between cation and protein phosphorylation effects on the fluorescence induction curve in chloroplasts treated with 3-(3, 4-dichlorophenyl)-1, 1-dimethylurea. Biochim Biophys Acta 722:214–218
Horton P, Allen JF, Black MT, Bennett J (1981) Regulation of phosphorylation of chloroplast membrane proteins by the redox state of plastoquinone. FEBS Lett 125:193–196
Jennings RC, Islam K, Zucchelli G (1986) spinach-thylakoid phosphorylation: studies on the kinetics of changes in photosystem antenna size, spill-over and phosphorylation of light-harvesting chlorophyll a/b protein. Biochim Biophys Acta 850:483–489
Kouril R, Zygadlo A, Arteni AA, de Wit CD, Dekker JP, Jensen P-E, Scheller HV, Boekema EJ (2005) Structural characterization of a complex of photosystem I and light-harvesting complex II of Arabidopsis thaliana. Biochemistry 44:10935–10940
Kyle DJ, Haworth P, Arntzen CJ (1982) Thylakoid membrane protein phosphorylation leads to a decrease in connectivity between photosystem II reaction centers. Biochim Biophys Acta 680:336–342
Kyle DJ, Staehelin LA, Arntzen CJ (1983) Lateral mobility of the light-harvesting complex in chloroplast membranes controls excitation energy distribution in higher plants. Arch Biochem Biophys 222:527–541
Kyle DJ, Kuang T-Y, Watson JL, Arntzen CJ (1984) Movement of a sub-population of the light harvesting complex (LHCII) from grana to stroma lamellae as a consequence of its phosphorylation. Biochim Biophys Acta 765:89–96
Larsson UK, Andersson B (1985) Different degrees of phosphorylation and lateral mobility of two polypeptides belonging to the light-harvesting complex of PSII. Biochim Biophys Acta 809:396–402
Larsson UK, Ogren E, Oquist G, Andersson B (1986) Electron transport and fluorescence studies on the functional interaction between phospho-LHCII and Photosystem I in isolated stroma lamellae vesicles. Photobiochem Photobiophys 13:29–39
Murata N (1969a) Control of excitation transfer in photosynthesis. I. Light-induced change of chlorophyll a fluorescence in Porphyridium cruentum. Biochim Biophys Acta 172:242–251
Murata N (1969b) control of excitation transfer in photosynthesis. II. Magnesium ion-dependent distribution of excitation energy between two pigment systems in spinach chloroplasts. Biochim Biophys Acta 189:171–181
Myers J (1971) Enhancement studies in photosynthesis. Annu Rev Plant Physiol 22:289–312
Nilsson A, Stys D, Drakenberg T, Spanford MD, Forsén S, Allen JF (1997) Phosphorylation controls the three-dimensional structure of plant light harvesting complex II. J Biol Chem 272:18350–18357
Owens GC, Ohad I (1982) Phosphorylation of Chlamidomonas reinhardii chloroplast membrane proteins in vivo and in vitro. J Cell Biol 93:712–718
Ruban AV (1991) The use of excitation fluorescence spectroscopy in the study of short-term chloroplast membrane reorganisation. Biochim Biophys Acta 1058:411–415
Ruban AV, Horton P (1992) Mechanism of ΔpH-dependent dissipation of absorbed excitation energy by photosynthetic membranes. I Spectroscopic analysis of isolated light harvesting complexes. Biochim Biophys Acta 1102:30–38
Ruban AV, Trach VV (1991) Heat-induced reversible changes in photosystem 1 absorption cross-section of pea chloroplasts and sub-chloroplast preparations. Photosynth Res 29:157–169
Ruban AV, Calkoen F, Kwa SLS, van Grondelle R, Horton P, Dekker JP (1997) Characterisation of the aggregated state of the light harvesting complex of photosystem II by linear and circular dichroism spectroscopy. Biochim Biophys Acta 1321:61–70
Ruban AV, Pascal A, Robert B (2000) Xanthophylls of the major photosynthetic light-harvesting complex of plants: identification, conformation and dynamics. FEBS Lett 477:181–185
Ruban AV, Pascal AA, Robert B, Horton P (2001) Configuration and dynamics of carotenoids in light-harvesting antennae of the thylakoid membrane. J Biol Chem 276:24862–24870
Ruban AV, Wentworth M, Yakushevska AE, Keegstra W, Lee PJ, Dekker JP, Jansson S, Boekema E, Horton P (2003) Plants lacking the main light harvesting complex retain photosystem II macro-organisation. Nature 421:648–652
Ruban AV, Solovieva S, Lee PJ, Ilioaia C, Wentworth M, Ganeteg U, Klimmek F, Chow WC, Anderson JM, Jansson S, Horton P (2006) Plasticity in the composition of the light harvesting antenna of higher plants preserves structural integrity and biological function. J Biol Chem 281:14981–14990
Schreiber U (1986) Detection of rapid induction kinetics with a new type of high-frequency modulated chlorophyll fluorometer. Photosynth Res 9:261–272
Sinclair J, Cousineau C (1981) The effect of adenosine-5′-triphosphate on oxygen-evolution, fluorescence emission and Emerson enhancement effect. FEBS Lett 136:213–215
Sundby C, Andersson B (1985) Temperature-induced reversible migration along the thylakoid membrane of photosystem II regulates its association with LHC-II. FEBS Lett 191:24–28
Telfer A, Allen JF, Barber J, Bennett J (1983) Thylakoid protein phosphorylation during State 1–State 2 transitions in osmotically shocked pea chloroplasts. Biochim Biophys Acta 722:176–181
Telfer A, Bottin H, Barber J, Mathis P (1984) The effect of magnesium and phosphorylation of light-harvesting chlorophyll a/b protein on the yield of P700 photooxidation in pea chloroplasts. Biochim Biophys Acta 764:324–330
Tikkanen M, Nurmi M, Suorsa M, Danielsson R, Mamedov F, Styring S, Aro E-M (2008) Phosphorylation-dependent regulation of excitation energy distribution between the two photosystems in higher plants. Biochim Biophys Acta. doi:10.1016/j.bbabio.2008.02.001
van Grondelle R, Novoderezhkin VI (2006) Energy transfer in photosynthesis: experimental insights and quantitative models. Phys Chem Chem Phys 8:798–807
Weis E (1985) Light- and temperature-induced changes in the redistribution of excitation energy between photosystem I and photosystem II in spinach leaves. Biochim Biophys Acta 807:118–126
Acknowledgements
We would like to acknowledge BBSRC and The Royal Society for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruban, A.V., Johnson, M.P. Dynamics of higher plant photosystem cross-section associated with state transitions. Photosynth Res 99, 173–183 (2009). https://doi.org/10.1007/s11120-008-9387-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-008-9387-x