Abstract
In order to evaluate genotypic variation, we measured leaf photosynthetic rate (Pn) and its associated factors for the rice diversity research set of germplasm (RDRS) selected from the Genebank in National Institute of Agrobiological Sciences (NIAS). Pn showed large genotypic variation from 11.9 to 32.1 μmol m−2 s−1. The variation in stomatal conductance to CO2 (Gs) explained about 50% of that in Pn, while that in nitrogen concentration (N) in leaves explained about 35%. The genotype group which mainly consists of aus type indica tended to have higher Gs, and the genotype group which corresponds to japonica had a higher nitrogen concentration (N) in leaves. The relationships of Pn with Gs and N were not significantly different among genotype groups, suggesting photosynthetic efficiencies are similar among genotype groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since leaf photosynthetic rate (Pn) is one of the most important traits for rice production, many studies have evaluated its genotypic variation and analyzed its determination factors. For example, Kawamitsu and Agata (1987) measured Pn in 50 rice varieties, and reported a close relationship between Pn and stomatal conductance (Gs). The effect of nitrogen concentration (N) on Pn has also been reported by many investigators (Cook and Evans 1983; Sinclair and Horie 1989). Although breeding efforts decreased depletion of photosynthetic rate during the grain-filling period by increasing N in leaf, potential rate of photosynthesis have not been improved (Kuroda and Kumura 1990; Sasaki and Ishii 1992). In order to increase the potential Pn, Pn and its associated factors must be analyzed more precisely from the viewpoint of genotype and genetics.
Most rice cultivars are Oryza sativa L. and commonly divided into three sub-species, japonica, indica, and javanica. Maruyama and Tajima (1990) measured Gs in 50 cultivars and reported that indica has larger Gs than japonica. Other information about the difference in photosynthetic traits among sub-species is limited. Moreover, other classifications of Oryza sativa have been proposed based on the results of genotype analysis using DNA markers. Kojima et al. (2005) conducted a genome-wide restriction fragment length polymorphism (RFLP) survey for 332 accessions of cultivated rice, and classified them into three genotype groups: corresponding to japonica, aus, and indica except aus. Aus is one of the ecotypes of indica, which is generally characterized by early maturity, short statue, drought tolerant, and grown in summer season. Garris et al. (2005) used 169 single-sequence-repeat (SSR) markers and classified 234 accessions of rice into five genotype groups: aromatic, temperate japonica, tropical japonica, indica and aus. The difference in photosynthetic traits among these genotype groups is vague.
Analysis of quantitative trait loci (QTL) provided another measure for analyzing the relationship between genotype and photosynthetic traits. Teng et al. (2004) reported two putative QTLs for Pn using double haploid population derived from a cross between indica and japonica. Some putative QTLs for carbon isotope discrimination (Δ), which is closely linked with process of Pn (Farquhar et al. 1982; Evans et al. 1986), were also reported (Ishimaru et al. 2001; Takai et al. 2006). These procedures may genetically reveal the determination factors of photosynthetic rate. However, it was indicated that effects of QTLs varied with the phenotype and genotype of parents (Mackill 1999; Ashikari and Matsuoka 2006). In order to increase the efficiency of QTL analysis, selection of genotypes for hybridization is important, and information about genotype and phenotype for diverse varieties may help the selection.
The rice diverse research set of germplasm (RDRS) was selected from about 30,000 accessions at the Genebank in National Institute of Agrobiological Sciences, Japan (NIAS), being expected as sufficiently diverse, yet sufficiently small research set, to enable the analysis of basic differences among rice varieties (Kojima et al. 2005). The non-biased population from the viewpoint of RFLP markers is also one of the features of RDRS. This study aimed to evaluate genotypic variation in Pn and its associated factors in RDRS. The difference among genotype groups was also evaluated.
Materials and method
Field experiments and measurements
The experiment was conducted at the experimental field of Kyoto University in 2004. In total, 64 genotypes of RDRSFootnote 1 and Takanari, which was one of the highest-yielding varieties in the field (Horie et al. 2003), were planted (Appendix 1). RDRS is divided into three genotype groups: corresponding to japonica (J), aus type indica (I1), and indica except aus (I2). Takanari is classified into I2 group (Ebana, personal communication). All genotypes are seeded on 4 May and transplanted on 26 May. A set of 3 × 6 plants of each genotype were transplanted at 0.3 × 0.3 m-spacing. Chemical fertilizer was applied at a rate of 2–14–14 g m−2 (N–P2O5–K2O) as a basal, and 1 g m−2 N was top-dressed every 20 days.
Single leaf photosynthetic rate (Pn) and stomatal conductance to CO2 (Gs) were measured with a portable type apparatus for photosynthesis and transpiration measurements (LI-6400, LICOR). The measurement was conducted 2 weeks before heading (2WBH) and 1 week after heading (1WAH) for all genotypes, except ten genotypes which did not reach heading stage (Appendix 1). Pn and Gs of those ten genotypes were measured in early October and used as the data at 2WBH in this study. More than three fully-expanded-uppermost leaves from different plants were measured from 10:00 to 13:00 under light saturated (PAR: 1,500 μmol m−2) and high-relative humidity (60–80%RH) condition.
Immediately after the measurement of Pn, eight fully-expanded-uppermost leaves, including the leaves measured for Pn, were sampled and the leaf area was determined. After the samples were oven-dried at 80°C for 3 days, carbon isotope compositions against a Pee Dee Belemnite (PDB) standard (δp, 13C/12C) and nitrogen concentration (N) of the samples were measured with a mass spectrometer (Delta-S, Thermoquest, USA) equipped with an elementary analyzer (EA1108, FISON, Italy). N was expressed in the weight per unit leaf area (g m−2). Carbon isotope discrimination (Δ) was calculated by the following equation, assuming that carbon isotope composition of the air against a PDB standard (δa) was −8‰.
These measured values were statistically analyzed by one-way analysis of variance with Duncan’s test at 5% significant level.
Daily weather data during the experiment was obtained from Kyoto Local Meteorological Observatory (Japan Meteorological Agency, 2005) (Fig. 1). Solar radiation was calculated from sunshine hours (Angström 1924) and maximum vapor pressure deficit was calculated from maximum temperature and minimum relative humidity.
Results
Variation of Pn and its associated factors in RDRS
Pn, Gs, Ci/Ca Δ, and N show large variation among genotypes and all traits were normally distributed, suggesting that they are quantitative traits and that the genes controlling the traits were fairly selected by the RFLP survey for RDRS (Fig. 2, also see Appendix 1). The highest value of Pn, 32.1 μmol m−2 s−1, was obtained in Tupa729 (No. 53, J, Landrace, Bangladesh) at 1WAH, and the lowest, 11.9 μmol m−2 s−1, in Neang Phtong (No. 56, I2, Landrace, Cambodia) at 2WBH. Tupa729 also had the highest value of Gs (Table 1).
Gs in the I1 group was significantly higher than that in the other groups at 2WBH (Table 2). The higher Gs in the I1 group at 2WBH associated with a higher Ci/Ca, and higher Δ. However, as N in the I1 group at 2WBH was significantly lower than in the other groups, Pn was not significantly different among the groups.
At 1WAH, Gs was not significantly different among genotype groups, while N in I1 was lower than that in the others. The lower N in I1 associated with lower Pn though not significantly.
Modern varieties in RDRS gave a higher Pn than landrace varieties. The higher values were associated with higher N of the varieties. The other traits, Gs, Δ and Ci/Ca, did not show any significant difference between modern and landrace varieties. A high-yielding cultivar, Takanari, was included in the top-5 genotypes for Pn, Gs, and N (Table 1).
Relationship among Pn and its associated factors
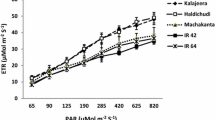
Table 3 shows the correlation coefficients among Pn and its associated factors. Pn was most closely related with Gs and followed by N. Gs explained about 50% of variation in Pn. The correlation between Gs and N was not significant. Ci/Ca had a relatively close positive correlation with Gs and a low-negative correlation with N. These relations did not vary much with the measured time (2WBH and 1WAH) or by the genotype group (Fig. 3).
Pn was negatively correlated with days to heading (DTH). The negative correlation was mostly derived from that between Gs and DTH (Fig. 4). N was not correlated with DTH.
As previous studies indicated, a significant correlation was observed between Δ and Ci/Ca (Table 3 and Fig. 5). Δ also showed a relatively close correlation with Gs.
Relationship between carbon isotope discrimination (Δ) and ratio of intercellular to ambient CO2 concentration (Ci/Ca). Symbols are the same as in Fig. 3. The line represents the relation predicted by Farquhar et al. (1982)
Discussion
Leaf photosynthetic rate (Pn) in this study showed large genotypic variation from 11.4 to 32.1 μmol m−2 s−1. Since previous studies reported similar variations, e.g., 14.0–32.2 μmol m−2 s−1 in 50 varieties (Kawamitsu and Agata 1987) and 10–30 μmol m−2 s−1 in ten varieties (Ohsumi et al. 2007a), the rice diversity research set of germplasm (RDRS) selected by NIAS (Kojima et al. 2005) is thought to almost cover the variation of Pn in rice cultivars. One of the high-yielding cultivars, Takanari, had a higher Pn than standard cultivars (Xu et al. 1997; Ohsumi et al. 2007a), but some landrace genotypes in RDRS had a higher Pn than Takanari. This suggests that Pn can be improved by using genotypic resources in rice cultivars. RDRS may provide useful gene source for the improvement.
Differences among genotype groups were observed in Pn and its associated factors (Table 2). Maruyama and Tajima (1990) reported that indica had a higher Gs than japonica, although such a relation was observed only between I1 and J groups at 2WBH in the present study. Higher carbon isotope discrimination (Δ) in I1 and I2 in this study was also similarly observed in Kondo et al. (2004). The higher Δ may be derived from higher Gs and lower nitrogen concentration (N): higher Gs increases supply of CO2 and lower N decreases demand of CO2, both may increase the rate of intercellular to ambient CO2 concentration (Ci/Ca) and then Δ. These differences among genotype groups imply that genotypes can be genetically classified into phenotype groups, such as extremely higher Gs group, higher N group and higher Pn group. However, the quantitative relations between the factors were not significantly different, e.g. between Pn and stomatal conductance to CO2 (Gs) (Fig. 3), and between Δ and Ci/Ca (Fig. 5). No significant difference in the quantitative relations indicates that photosynthetic efficiencies, such as kinetic constant of ribulose 1,5-bisphosphate (RuBP) carboxylase (Makino et al. 1987), are similar among genotype groups.
The genotypes which had longer days to heading from sowing (DTH) tended to have lower Gs and then lower Pn than those had shorter DTH (Table 3). Although weather conditions may associate such a tendency, the association was not clear in this study. That is, most genotypes had 80–110 DTH and the weather conditions during the duration were relatively stable in terms of air temperature, solar radiation and vapor pressure deficit (Fig. 1). Ohsumi et al. (2007b) used the same materials and reported that the longer-DTH genotypes tended to have shorter stomatal length. The fact may be associated with the tendency in Gs and Pn.
Stomatal conductance to CO2 (Gs) explained about 50% of variation of Pn in this study, while N in leaves did less than 35%. Similar results were reported by Kawamitsu and Agara (1987) and Saitoh et al. (1991). Gs is the index of CO2 supply, and N is the index of the efficiency of photosynthesis. These two factors heighten Pn in different ways. No significant relationship between Gs and N in this study indicates that Gs and N were independently controlled by genes, and able to be improved.
High N in leaves has been one of the important breeding targets since green revolution in 1960s (Rasmusson 1991; Peng et al. 1999). Since higher-N leaves can be distinguished by their dark-green color, recently released cultivars already have higher-N leaves (Cui et al. 2000). In the present study, modern varieties had a higher N than the landrace (Table 2). The improvement of Pn by N is remarkable especially during grain-filling period when leaves of old cultivars start aging process and turns to yellow (Kuroda and Kumura 1990). However, the relation between Pn and N was sparser than that between Pn and Gs as mentioned above. Although significant difference among genotype groups was not observed by regression analysis in the relation between Pn and N, photosynthetic nitrogen use efficiency (PNUE = Pn/N; μmol g−1 s−1) of I1 at 2WBH was significantly higher than those of the others. The result suggests that PNUE can be improved by breeding and one of the major causes of the higher PNUE is the higher Gs. Horie et al. (2003) explained genotypic difference in Pn by the product of Gs and N percentage in leaf dry matter (N%; g 100 g−1). The correlation between Pn and Gs N% in this study was 0.84 at 2WBH and 0.81 at 1WAH, both of which were slightly higher than those between Pn and Gs and significantly higher than those between Pn and N.
Although the above section indicated that breeding improvement in Gs is necessary to improve Pn, the improvement did not obviously progress in the present because the evaluation of Gs required time and labor. Horie et al. (2003) proposed the product of Δ and N% as an index of Pn. Δ may reflect Gs through Ci/Ca. However, Δ N% in this study only explained 57%, 44% and 39% of variation of Pn at 2WBH, 1WAH and in total, respectively (Fig. 6). These insufficient determination factors associate with not strong correlation between Δ and Ci/Ca (Fig. 5). Evans et al. (1986) reported that carbon isotope discrimination deviates from the equation predicted by Farquhar et al. (1982): Δi = a + (b − a) Ci/Ca, where Δi is the expected discrimination, a is the fractionation occurring due to diffusion in air (4.4‰) and b is the net fractionation caused by RuBP carboxylase and phosphoenolpyrvate carboxylase (29‰). The deviation is proportional to Pn and inversely proportional to internal conductance (Evans et al. 1994). Although the deviation in Evans et al. (1994) was observed by concurrent measurement of carbon isotope discrimination and CO2 exchange, Hikosaka et al. (1998) reported that Δ measured with leaf dry matter also deviated from Δi and that Δi − Δ was larger in a higher-photosynthetic-capacity species than that in a lower species. Correlation between Δi − Δ and Pn was also significant at 2WBH in this study but poor (r = 0.30*). Since carbon isotope discrimination occurs not only in photosynthesis but also in respiration, translocation, synthesis of structural materials, and so on, application of the result in Evans et al. (1986) to Δ measured with leaf dry matter may require further study.
This study showed the genotypic variation in Pn and its associated factors. For improvement of Pn, Gs is the key factor. Although it must be considered that Gs is negatively correlated with days to heading, some genotypes in RDRS, such as Keiboba (No. 15, I2, Landrace, China) and Tupa 729 (No. 53, J, Landrace, Bangladesh), may be promising materials for genetic analysis and for breeding.
Notes
When RDRS was distributed to us, RDRS consisted of 64 genotypes. The latest RDRS included five genotypes more (Kojima et al. 2005).
Abbreviations
- 1WAH:
-
1 week after heading
- 2WBH:
-
2 weeks before heading
- Δ:
-
Carbon isotope discrimination
- Ci :
-
Intercellular CO2 concentration
- Ca :
-
Ambient CO2 concentration
- DTH:
-
Days to heading from sowing
- Gs :
-
Stomatal conductance to CO2
- I1:
-
A genotype group corresponding to aus type indica
- I2:
-
A genotype group corresponding to indica except aus
- J:
-
A genotype group corresponding to japonica
- N:
-
Nitrogen weight per unit leaf area
- N%:
-
Nitrogen percentage in leaf dry matter
- PAR:
-
Photosynthetically active radiation
- Pn:
-
Leaf photosynthetic rate
- QTL:
-
Quantitative trait loci
- RFLP:
-
Restriction fragment length polymorphism
- SSR:
-
Single-sequence repeat
- RDRS:
-
Rice diversity research set of germplasm
- RubisCO:
-
Ribulose-1,5-bisphosphate carboxylase/oxygenase
- VPD:
-
Vapor pressure deficit
References
Angström A (1924) Solar and terrestrial radiation. Quart J Roy Met Soc 50:121–126
Ashikari M, Matsuoka M (2006) Identification, isolation and pyramiding of quantitative trait loci for rice breeding. Trends Plant Sci 11:344–350
Cook MG, Evans LT (1983) Nutrient responses of seedlings of wild and cultivated Oryza species. Field Crops Res 6:205–518
Cui J, Kusutani A, Toyota M, Asanuma K (2000) Studies on the varietal difference of harvest index in rice. Relationship between harvest index and morphological characteristics and leaf color. Jpn J Crop Sci 69:365–371 [In Japanese with English abstract]
Evans JR, Sharkey D, Berry JA, Farquhar GD (1986) Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Aust J Plant Physiol 13:281–292
Evans JR, von Caemmerer S, Setchell BA, Hudson GS (1994) The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of Rubisco. Aust J Plant Physiol 21:475–495
Farquhar GD, O’Leary MH, Berry JA (1982) On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust J Plant Physiol 9:121–137
Garris AJ, Tai TH, Coburn J, Kresovich S, McCouch S (2005) Genetic structure and diversity in Oryza sativa L. Genetics 169:1631–1638
Hikosaka K, Hanba YT, Hirose T, Terashima I (1998) Photosynthetic nitrogen-use efficiency in leaves of woody and herbaceous species. Funct Ecol 12:896–905
Horie T, Lubis I, Takai T, Ohsumi A, Kuwasaki K, Katsura K, Nii A (2003) Physiological traits associated with high yield potential in rice. In: Mew TW, Brar DS, Peng S, Dawe D, Hardy B (eds) Rice science: innovations and impact for livelihood. Proceedings of the international rice research conference, Beijing, September 2002. IRRI, Chinese Academy of Engineering and Chinese Academy of Agricultural Sciences, Beijing, pp 117–145
Ishimaru K, Yano M, Aoki N, Ono K, Hirose T, Lin SY, Monna L, Sasaki T, Ohsugi R (2001) Toward the mapping of physiological and agronomic characters on a rice function map: QTL analysis and comparison between QTLs and expressed sequence tags. Theor Appl Genet 102:793–800
Kawamitsu Y, Agata W (1987) Varietal differences in photosynthetic rate, transpiration rate and leaf conductance for leaves of rice plants. Jpn J Crop Sci 53:563–570 [in Japanese with English summary]
Kondo M, Pablico PP, Aragones DV, Agbisit R (2004) Genotypic variations in carbon isotope discrimination, transpiration efficiency, and biomass production in rice as affected by soil water conditions and N. Plant Soil 267:165–177
Kojima Y, Ebana K, Fukuoka S, Nagamine T, Kawase M (2005) Development of an RFLP-based rice diversity research set of germplasm. Breed Sci 55:431–440
Kuroda E, Kumura A (1990) Difference in single leaf photosynthesis between old and new rice varieties. III. Physiological bases of varietal difference in single-leaf photosynthesis between varieties viewed from nitrogen content and the nitrogen–photosynthesis relationship. Jpn J Crop Sci 59:298–302 [in Japanese with English abstract]
Mackill DJ (1999) Genome analysis and breeding. In: Shimamoto K (ed) Molecular biology of rice. Springer-Verlag, Tokyo, pp 17–41
Makino A, Mae T, Ohira K (1987) Variations in the contents and kinetic-properties of ribulose-1,5-bisphosphate carboxylases among rice species. Plant Cell Physiol 28:799–804
Maruyama S, Tajima K (1990) Leaf conductance in japonica and indica rice varieties. I. Size, frequency, and aperture of stomata. Jpn J Crop Sci 59:801–808
Ohsumi A, Hamasaki A, Nakagawa H, Yoshida H, Shiraiwa T, Horie T (2007a) A model explaining genotypic and ontogenetic variation of leaf photosynthetic rate in rice (Oryza sativa) based on leaf nitrogen content and stomatal conductance. Annals Bot 99:265–273
Ohsumi A, Kanemura T, Homma K, Horie T, Shiraiwa T (2007b) Genotypic variation of stomatal conductance in relation to stomatal density and length in rice (Oryza sativa L.). Plant Prod Sci 10:322–328
Peng S, Cassman KG, Virmani SS, Sheehy J, Khush GS (1999) Yield potential trends of tropical rice since the release of IR8 and the challenge of increasing rice yield potential. Crop Sci 39:1552–1559
Rasmusson DC (1991) A plant breeder’s experience with ideotype breeding. Field Crops Res 26:191–200
Saitoh K, Shimoda H, Ishihara K (1991) Characteristics of dry matter production process in high yielding rice varieties. III. Comparisons of leaf photosynthesis. Jpn J Crop Sci 60:65–74 [in Japanese with English abstract]
Sasaki H, Ishii R (1992) Cultivar differences in leaf photosynthesis of rice bred in Japan. Photosyn Res 32:139–146
Sinclair TR, Horie T (1989) Leaf nitrogen, photosynthesis, and crop radiation use efficency: a review. Crop Sci 29:90–98
Takai T, Fukuta Y, Sugimoto A, Shiraiwa T, Horie T (2006) Mapping of QTLs controlling carbon isotope discrimination in the photosynthetic system using recombinant inbred lines derived from a cross between two different rice (Oryza sativa L.) cultivars. Plant Prod Sci 9:271–280
Teng S, Qian Q, Zeng DL, Kunihiro Y, Fujimoto K, Huang DN, Zhu LH (2004) QTL analysis of leaf photosynthetic rate and related physiological traits in rice (Oryza sativa L.). Euphytica 135:1–7
Xu YF, Ookawa T, Ishihara K (1997) Analysis of the photosynthetic characteristics of the high-yielding rice cultivar Takanari. Jpn J Crop Sci 66:616–623 [In Japanese with English abstract]
Acknowledgments
Rice diverse research set of germplasm (RDRS) used in this study was distributed by the Genebank of the National Institute of Agrobiological Science (NIAS), Japan. Special thanks are due to Dr. K. Ebana and Dr. Y. Uga of the NIAS for their comments to this study. We also thank Dr. T. Takai for giving us the information of RDRS.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kanemura, T., Homma, K., Ohsumi, A. et al. Evaluation of genotypic variation in leaf photosynthetic rate and its associated factors by using rice diversity research set of germplasm. Photosynth Res 94, 23–30 (2007). https://doi.org/10.1007/s11120-007-9208-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-007-9208-7