Abstract
Nitrogen management has been intensively studied on several crops and recently associated with variable rate on-the-go application based on crop sensors. Such studies are scarce for sugarcane and as a biofuel crop the energy input matters, seeking high positive energy balance production and low carbon emission on the whole production system. This article presents the procedure and shows the first results obtained using a nitrogen and biomass sensor (N-Sensor™ ALS, Yara International ASA) to indicate the nitrogen application demands of commercial sugarcane fields. Eight commercial fields from one sugar mill in the state of São Paulo, Brazil, varying from 15 to 25 ha in size, were monitored. Conditions varied from sandy to heavy soils and the previous harvesting occurred in May and October 2009, including first, second, and third ratoon stages. Each field was scanned with the sensor three times during the season (at 0.2, 0.4, and 0.6 m stem height), followed by tissue sampling for biomass and nitrogen uptake at ten spots inside the area, guided by the different values shown by the sensor. The results showed a high correlation between sensor values and sugarcane biomass and nitrogen uptake, thereby supporting the potential use of this technology to develop algorithms to manage variable rate application of nitrogen for sugarcane.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sugarcane is the most important crop for sugar and ethanol production in tropical climates and Brazil is the leading producer followed by India (FAO 2010). The cultivated area with sugarcane (Saccharum officinarum L.) in Brazil is rapidly increasing and production doubled in the last 10 years (UNICA 2010), strongly influenced by ethanol production due to the introduction of flex fuel car engines on the local market (ANFAVEA 2010). Currently, sugarcane production is the second-leading local energy source (ethanol and biomass electricity), accounting for 18.1% of whole energy consumption (BEN 2010).
According to a recent investigation (Silva et al. 2011), the sugar-ethanol industry in São Paulo state, which produces 60% of the Brazilian commercial sugarcane, is open to precision agriculture technologies focusing on managerial improvements, higher yields, lower costs, minimized environmental impact and improvements in sugarcane quality. The study also showed that 96% of the sector wants to expand the use of precision agriculture practices.
In the sugarcane production system, especially in humid areas, nitrogen (N) availability in the soil is the most important factor limiting plant growth, development and yield, but N is also the second impact factor on the fuel ethanol lifecycle (Ometto et al. 2009). Although biological N fixation seems to play a role in the N nutrition of sugarcane crops, it seems premature to use inoculants of N2-fixing bacteria since the main contributors to the observed biological N fixation are not well known (Boddey et al. 2003). Nitrogen fertilization of ratoon sugarcane using rates up to 175 kg ha−1 resulted in an increase of productivity and benefits to subsequent ratoons (Prado and Pancelli 2008; Vitti et al. 2007), and enhanced technological attributes of the sugarcane plant (Franco et al. 2010). On the other hand, N fertilization can account for a significant part of total production expenses. Therefore, rational management of such a high cost input can have a great impact on the profitability of crop production. In addition, the potential for environmental impacts has to be considered by the use of better agriculture practices, including more precise use of fertilizers, especially N, since N application rates that exceed the crop requirements may contribute to increased nitrate levels in the soil and increase the risk of nitrate leaching from agricultural lands to surface and ground waters or losses to the atmosphere via denitrification (Spiertz 2010).
Nitrogen fertilization prescriptions have always been a challenge, because of the difficulty to predict the amount of N mineralized from the soil organic matter during the growing season and the high nitrate mobility in the soil profile. In addition, large spatial variability of nitrate content and N supply capability of the soil and N status of plants may exist in the field. The determination of appropriate N rates to be applied to crops is of crucial interest for yield formation. Moreover, fertilizer rate is one of the major tools available to manipulate crops to produce high yields. On the other hand, the amount of N naturally supplied by mineral soils normally is not enough to reach the crop yield potential and, therefore, soil N can be supplemented with fertilizer to sustain high sugarcane yields.
Presently, the application of N fertilizers on sugarcane is done without taking into account soil and crop spatial variability, having fertilizers applied over the field at a uniform rate based on the average needs of the crop. Nevertheless many fields consist of more than one soil type with different N supply and crop yield potentials, requiring distinct fertilizer management for economic and ecological reasonable yields. A consequence of the uniform N management is that some parts of the field may be under or over fertilized (Bredemeier and Schmidhalter 2005).
The fallacies of uniform application rates have led to site specific crop management approaches designed to optimize crop production by managing the crop and soil, taking into account the variability within each field. Map and sensor-based approaches are the basic methods of implementing site specific management of variable-rate application of crop inputs. However, mapping approaches that derive recommendations for variable-rate applications from grid soil and/or plant sampling will not be economical for site specific N fertilization, considering the spatial N soil variability that can exist over very short distances in fields (Solie et al. 1999).
Therefore, sensor based methods using light reflectance measurements have been used to estimate the N status of plants. They use data acquired in real time by the sensor to control site specific field operations while the machine is moving, collecting data about the current plant growth in a high spatial resolution, enabling the detection of N needs in real time, transforming those needs into a fertilizer rate to be applied instantaneously after the sensor measurement. However, a crop specific agronomic algorithm is necessary to convert the sensor readings into a fertilizer rate. In this sense, variable N management based on reflectance sensors is one of the most promising practices of precision agriculture to optimize N use efficiency and decrease the environmental impact of agricultural practices on sugarcane crop production.
The N-Sensor ALS uses light reflectance at 730 and 760 nm to determine N uptake from crops by active remote sensing (Jasper et al. 2009). Vegetation indices calculated as simple ratios from the above wavebands are considered to be superior to classical reflectance ratios that involve one waveband in the visible and one waveband in the near infrared region of the spectrum (Heege et al. 2008; Reusch 2005). In particular, the resulting relationship to N uptake seemed to be largely independent of growth stage and variety (Jasper et al. 2009), and showed less saturation at high N-uptake levels. As there is no information available in the literature for the use of this sensor in sugarcane, its suitability for sugarcane cropping systems needs to be tested and validated (Singh et al. 2006). This article presents the procedure and initial results obtained using the N-Sensor to indicate the N application demands of commercial sugarcane fields.
Materials and methods
Eight commercial fields of sugarcane located around the São Martinho Sugar Mill (21°19′11′′S, 48°07′23′′W), in the state of São Paulo, Brazil, were evaluated during the 2009/10 growing season. Conditions varied from sandy to clay soils, with all crops being mechanically green harvested (with no burn). On four fields, harvesting of the previous crop occurred at the beginning of the season (May, 2009) corresponding to the dry time of the year, and on the other four fields, in late season (October, 2009), corresponding to the wet time of the year. The crops under investigation included first, second, and third ratoon stages. The first four fields were planted with the varieties CTC 9 over sandy soil and RB 855453 over clay soil, monitored on first and second ratoon stages on the sandy soil, and first and third ratoon on the clay soil. All were harvested in the dry season. The last four fields were planted with the varieties CTC 2 on sandy soil and SP 80–3 280 on clay soil, also monitored during the first and second ratoon periods on the sandy soil, and first and third ratoon on the clay soil, and harvested during the wet season (Table 1). Shortly after harvesting all fields were fertilized with a uniform rate of 100 kg ha−1 of N using ammonium nitrate (30% N) as the N source broadcast over the sugarcane rows.
The sugarcane fields were scanned using the N-Sensor™ ALS (Yara International ASA, Duelmen, Germany) that comprises a transmitter with a xenon flashlight, providing multi-spectral light (650–1 100 nm) of high intensity, and a receiver with two photodiodes and interference filters with 730 and 760 nm center wavelength and a half band width of 10 nm (Jasper et al. 2009). The sensor system uses red-edge reflectance data to generate a vegetation index (VI) as show in Eq. 1. The sensor was mounted behind the cabin of a high clearance vehicle (Jacto Uniport NPK Canavieiro, Pompéia, Brazil), providing 1.4 m of free vertical clearance.
The target parameter for the agronomic calibration of the sensor readings is the N-uptake of the above-ground biomass of the crop (Link et al. 2005). As the relationship between sensor readings and crop N uptake might be growth stage specific, each of the eight fields was scanned with the sensor three times in the growing season (at 0.2, 0.4, and 0.6 m average stem height) (Fig. 1).
The sensor was connected to a GPS receiver and the vehicle was driven through the whole field spaced by ten rows of 1.5 m. After the scanning, the sensor data from the field were downloaded to a GIS software (SSToolbox®, SST Development Group, Stillwater, OK, USA) and cleaned, cutting off data points outside of the field boundaries and discrepant points close to the boundaries, where the sensor was scanning surfaces outside the field. The data points were interpolated using 10-m cells and the inverse distance method, establishing five classes by natural breaks to express the variability of the vegetation index read by the sensor inside the field during the scanning.
Two sampling points were located in the middle of representative areas of each class, summarizing ten points per field for every scanning date (Fig. 2). Each sample point consisted of four sugarcane rows of 5-m length (i.e., 30 m2 at 1.5-m row width). At each sample point located in the middle of the 10-m cell, the number of stems was counted, the average height of the stems estimated, and destructive plant samples of the above ground biomass were taken by cutting 1.5-m subplots on three rows, summarizing 4.5-m row length. Biomass samples were weighed in the field, samples were chopped in the laboratory, and sub-samples were dried to estimate above ground dry matter production, then its total N content analyzed (Kjeldahl method) and the N-uptake calculated.
Sensor readings of the respective sample plots were related to the crop parameters, specific calibration functions were derived, and the capacity of the sensor measurements to predict the actual crop biomass and N uptake was investigated.
An exploratory analysis of the data was done running a box plot using SAS®(SAS 2009) to identify outliers, excluding just two sampled points in the entire data set. Sensor data of each field were correlated with biomass and N uptake from the respective sample points, best fit curves were adjusted between data points (exponential model, Eq. 2), and the equation of the curve used to calculate the sensor predicted biomass and N uptake.
where y—real biomass or N-uptake obtained from the laboratory analyses, kg ha−1; a—intercept of the y axis; e—exponential; b—angular coefficient of the fit curve; x—VI from sensor.
From those, simple linear regression models were used to compare sugarcane N uptake and biomass collected data against sensor predicted N uptake and biomass data, using the procREG procedure routine of SAS® at 5% probability.
Results and discussion
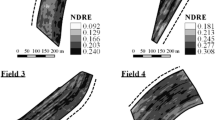
Fields 1–4, harvested at the beginning of the dry season (winter), involved three sensor measurements coincident with the lowest temperatures of the year when crop growth development was low. In-field variability was detected at all scanning dates, i.e., crop growth stages, with increasing average vegetation indices with increasing crop height. The sensor readings per field and date were divided in five classes using natural breaks to better show the variability and locate the sampling points. Thus, the legends of the resulting maps are different for each field and scanning date.
The interpolated maps show the sensor readings at three sugarcane growth stages indicating that the sensor is capable to detect the variability inside the area. Analyzing the maps of one of the fields (Fig. 3), it is possible to see significant variability of the sensor vegetation index in short distances inside the sugarcane field and also the stability of values on low and high zones inside the area among measurement times.
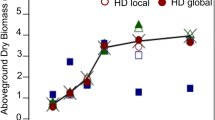
Using the results of the destructive plant sampling of the thirty plots from the three scanning times of each field (0.2, 0.4, and 0.6 m of stem height) against the values predicted by the sensor according to the obtained calibration function, it was possible to investigate the sensor’s capability to predict crop biomass and N uptake, as shown for field 1–4 in Fig. 4.
The sensor was capable of predicting the real sugarcane biomass in the dry season fields, having coefficients of determination varying from R² = 0.76 to R² = 0.94, and also to predict N uptake in the dry season fields with the coefficients of determination varying from R² = 0.79 to R² = 0.94.
The graphs also show that the measurement at 0.2-m stem height was too early, resulting in low values with little differentiation. This can be seen as a consequence of non-uniform sprouting of the stools during the dry and chilly period, showing a low number of stems per meter at this time and resulting in low biomass and N uptake. Aggregated results of the four dry season fields are illustrated in Fig. 5.
The sensor capacity to predict biomass for sugarcane on the dry season of the year shows a coefficient of determination of R² = 0.85 and R² = 0.86 for the N-uptake prediction. It is also evident that the sensor’s capability to predict crop N uptake and biomass is independent of soil properties, variety, or ratoon.
Fields harvested at the beginning of the wet season (summer) had sensor measurements taken during the warmest time of the year, when crop sprouting and growth was maximized. Field 7, corresponding to the first ratoon on a clay soil, had operational problems with the 0.4-m data collection time because of excess rain and traffic problems (missing data set).
The interpolated maps of the fields monitored during the wet season showed the same characteristics as those from the dry season (not shown). Figure 6 presents the results of the destructive plant sampling of the thirty plots from the three scanning times (0.2, 0.4, and 0.6 m of stem height) of each field against the values predicted by the sensor according to the obtained calibration function for the wet season.
The graphics of Fig. 6 show that the sensor was capable of predicting the real sugarcane biomass in the fields during the wet season, having coefficients of determination varying from R² = 0.74 to R² = 0.96, and also to predict N uptake in the fields of the wet season with the coefficients of determination varying from R² = 0.74 to R² = 0.94. Compared to the results of the dry season, the accuracy of the prediction was slightly lower on fields 5 and 6, mainly due to some saturation of the sensor signal at the late measurement date. Also one outlier sample point of the 0.6-m stem height measurement was excluded due to the box plot analyses on each of these fields. Operational problems caused a delay and the sensor measurement was made after the growth stage of 0.6 m average crop height on these two fields. This caused periodic saturation of the sensor signal (Mutanga and Skidmore 2004) where the biomass of the sugarcane was increasing, but the vegetation sensor index was not increasing in the respective scale. Figure 7 shows the aggregated results of the four wet season fields, as showed for the dry season in Fig. 5.
Due to saturation of the sensor signal, especially on fields five and six, at 0.6-m stem height, this evaluation was less accurate than in the earlier growth stages (0.2 and 0.4 m), resulting in lower accuracy (R² = 0.81) of the crop biomass prediction for the wet season than for the dry season (R² = 0.85), resulting in a systematic under estimation of the biomass at such late growth stages. The predicted N uptake consequently showed lower accuracy (R² = 0.82) against the dry season (R² = 0.86). However, the results are consistent and also show that the sensor results have independency of soil properties, variety, or ratoon, supporting the findings found in the dry season.
The relationship between real and sensor predicted crop biomass for the entire data set of all eight fields is shown in Fig. 8. Most of the data points obtained for the 0.6-m stem height in Fig. 8 were under the 1:1 line, indicating an under estimation of the crop biomass, whereas the data points for the measurement at 0.4-m stem height were above the 1:1 line when the biomass was above 4 000 kg ha−1, indicating an over estimation of the crop biomass. These findings suggest that the calibration function for sensor-based estimation of the crop biomass is sensitive to growth stages up to 0.6-m crop height. For a reliable sensor based prediction of crop biomass, the average stem height of the crop should not be above 0.4–0.5 m. Furthermore, the development of separate calibration functions for early and later growth stages within this development phase of the crop should be considered.
N-uptake data are considered to be the most suitable agronomic parameter to guide site-specific N fertilization. The saturation effect at later growth stages is much less distinct, most likely due to auto compensation by the N concentration in the biomass that declines with the development of the crop. For the samples taken at 0.2-m stem height, the N concentration varied from 15 to 20 g kg−1, for the 0.4-m measurement from 11 to 16 g kg−1, and for the 0.6-m measurements from 8 to 14 g kg−1.
By joining the data of all sampling dates across all eight fields (Fig. 9), it was possible to calculate a universally applicable calibration function that allows a sensor based prediction of the N uptake of a sugarcane crop with an coefficient of determination of R² = 0.84, independent of differences in varieties, soil, season period, and ratoon of the sugarcane field being monitored.
The relationship between sensor predicted and measured N uptake was close to the 1:1 line for the entire data set, including measurements taken from 0.2 to 0.6-m stem height. Deviations in growth of the crop and the increase of N uptake were probably influenced at the 0.6-m growth stage where sensor saturation occurred periodically. The root mean square error (RMSE) for this relationship, in order to quantify the accuracy of the sensor based N-uptake estimation against the real N uptake was 9.14 kg ha−1, which is low considering the quantities involved.
Conclusions
The method implemented in this study allowed for the acquisition of a unique data set describing in-field variability of N uptake in commercial sugarcane fields. According to the data collected, the N-Sensor is capable of detecting the variability of sugarcane biomass and N uptake while being unaffected by variety characteristics, soil type, and seasonal growth conditions, thus giving a good estimate of the site-specific N supply by the soil. Results indicate that the current N uptake of a sugarcane crop can be predicted with high accuracy over a wide period of early sugarcane growth (0.2–0.6 m stem height). Therefore, it is concluded that N-Sensor based N-uptake measurement has the potential to provide a reliable basis for the derivation of mathematic algorithms for real time variable-rate application of N fertilizer in commercial sugarcane fields.
References
ANFAVEA (2010). Associação Nacional dos Fabricantes de Veículos Automotores—Brasil. http://www.anfavea.com.br/tabelas.html. Accessed 16 September 2010.
BEN (2010). Balanço Energético Nacional—Brasil. https://www.ben.epe.gov.br/downloads/Relatorio_Final_BEN_2010.pdf. Accessed 16 September 2010.
Boddey, R. M., Urquiaga, S., Alves, B. J. R., & Reis, V. (2003). Endophytic nitrogen fixation in sugarcane: present knowledge and future applications. Plant and Soil, 252, 139–149.
Bredemeier, C., & Schmidhalter, U. (2005). Laser-induced chlorophyll fluorescence sensing to determine biomass and nitrogen uptake of winter wheat under controlled environment and field conditions. In J. V. Stafford (Ed.), Precision agriculture’05. Proceedings of the 5th European conference on precision agriculture (pp. 273–280). Wageningen, The Netherlands, Uppsala, Sweden: Academic Publishers.
FAO (2010). Food and Agriculture Organization, Faostat. http://faostat.fao.org/. Accessed 16 September 2010.
Franco, H. C. J., Trivelin, P. C. O., Faroni, C. E., Vitti, A. C., & Otto, R. (2010). Stalk yield and technological attributes of planted cane as related to nitrogen fertilization (Produção de colmos e atributos tecnológicos da cana planta relacionados com a adubação nitrogenada). Scientia Agricola, 67, 579–590.
Heege, H. J., Reusch, S., & Thiessen, E. (2008). Prospects and results for optical systems for site-specific on-the-go control of nitrogen-top-dressing in Germany. Precision Agriculture, 9, 115–131. doi:10.1007/s11119-008-9055-3.
Jasper, J., Reusch, S., & Link, A. (2009). Active sensing of the N status of wheat using optimized wavelength combination–impact of seed rate, variety and growth stage. In E. J. Van Henten, D. Goense, & C. Lokhorst (Eds.), Precision agriculture’09 Proceedings of the 7th European conference on precision agriculture (pp. 23–30). Wageningen, The Netherlands: Academic Publishers.
Link, A., Jasper, J., & Reusch, S. (2005). Suitability of different crop parameters for the determination of site-specific nitrogen fertilizer demand. In J. V. Stafford (Ed.), Precision agriculture′05. Proceedings of the 5th European conference on precision agriculture (pp. 297–302). Wageningen, The Netherlands. Uppsala, Sweden: Academic Publishers.
Mutanga, O., & Skidmore, A. K. (2004). Narrow band vegetation indices overcome the saturation problem in biomass estimation. International Journal of Remote Sensing, 25, 3999–4014. doi:10.1080/01431160310001654923.
Ometto, R. A., Hauschild, M. Z., & Roma, W. N. L. (2009). Lifecycle assessment of fuel ethanol from sugarcane in Brazil. International Journal of Life Cycle Assessment, 14, 236–247. doi:10.1007/s11367-009-0065-9.
Prado, R. M., & Pancelli, M. A. (2008). Sugarcane ratoon response to nitrogen application in a no burn harvesting system. (Resposta de soqueiras de cana-de-açúcar à aplicação de nitrogénio em sistema de colheita sem queima). Bragantia, 67, 951–959.
Reusch, S. (2005). Optimum waveband selection for determining the nitrogen uptake in winter wheat by active remote sensing. In J. V. Stafford (Ed.), Proceedings of the 5th European conference on precision agriculture (pp. 261–266). Wageningen, The Netherlands: Academic Publishers.
SAS. (2009). SAS Online Doc R 9.2. North Carolina: Cary.
Silva, C. B., de Moraes, M. A. F. D., & Molin, J. P. (2011). Adoption and use of precision agriculture technologies in the sugarcane industry of São Paulo state, Brazil. Precision Agriculture, 12, 67–81. doi:10.1007/s11119-009-9155-8.
Singh, I., Srivastava, A. K., Chandna, P., & Gupta, R. K. (2006). Crop sensors for efficient nitrogen management in sugarcane: Potential and constraints. Sugar Technology, 8, 299–302. doi:10.1007/bf02943572.
Solie, J. B., Raun, W. R., & Stone, M. L. (1999). Submeter spatial variability of selected soil and bermudagrass production variables. Soil Science Society of America Journal, 63, 1724–1733.
Spiertz, J. H. J. (2010). Nitrogen, sustainable agriculture and food security. A review. Agronomy for Sustainable Development, 30, 43–55. doi:10.1051/agro:2008064.
UNICA (2010). União da Indústria de cana-de-açúcar—Brasil. Union of the Brazilian sugarcane industry. http://www.unica.com.br/dadosCotacao/estatistica/. Accessed 16 September 2010.
Vitti, A. C., Trivelin, P. C. O., Gava, G. J. C., Penatti, C. P., Bologna, I. R., Faroni, C. E., et al. (2007). Sugarcane yield related to the residual nitrogen from fertilization and the root system (Produtividade da cana-de-açúcar relacionada ao nitrogênio residual da adubação e do sistema radicular). Pesquisa Agropecuaria Brasileira, 42, 249–256.
Acknowledgments
All this work would not be possible without the collaboration of São Martinho’s Mill team, the support of Máquinas Agrícolas Jacto SA and the Research Fellowship to the first author from the Brazilian Government (CNPq—National Council of Scientific and Technological Development).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Portz, G., Molin, J.P. & Jasper, J. Active crop sensor to detect variability of nitrogen supply and biomass on sugarcane fields. Precision Agric 13, 33–44 (2012). https://doi.org/10.1007/s11119-011-9243-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11119-011-9243-4