Abstract
Phytopathogenic fungi may affect both the cuticular and the stomatal conductance of plant tissue resulting in significant modifications of leaf temperature. Venturia inaequalis colonizes apple leaves below the cuticle (subcuticularly) causing scab disease. The suitability of digital infrared thermography for sensing and quantifying apple scab was assessed by investigating the effects of V. inaequalis on the water balance of apple leaves in relation to the disease stage and the severity of scab. Transpiration was measured by infrared thermo-imaging to evaluate spatial heterogeneity of the leaves in response to localized infections. Fungal development was assessed microscopically. Subcuticular growth of the pathogen caused localized decreases in leaf temperature before symptoms appeared that significantly increased the maximum temperature difference (MTD) of leaves. The MTD increased with scab development and was strongly correlated to the size of infection sites (r²linear = 0.85) and overall disease severity (% diseased leaf area, r²square = 0.71). In later stages of the disease, the MTD decreased because of leaf senescence. Thermographic measurements revealed differences in disease severity resulting from disease stage, resistance of host tissue and differences in the aggressiveness of V. inaequalis isolates. Subcuticular growth of the pathogen was beyond the area of conidia production, therefore, the area of leaf with increased transpiration was larger than the scab lesions; the proportion decreased from >70% in the early stages to <20% for mature lesions. Leaf transpiration was increased by all stages of scab development, therefore, MTD may be used not only for the differentiation between diseased and non-diseased leaves, but also for disease quantification, e.g. in screening systems and monitoring in precision agriculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most important diseases in apple production worldwide is apple scab caused by the ascomycete Venturia inaequalis (Cooke) G. Winter (MacHardy 1995). Chemical control of the pathogen is essential, especially under moderate climatic conditions, as infections and disease progress are favoured by temperatures of 16–20°C and frequent periods of leaf wetness exceeding 6–8 h as described in Mills’ tables (MacHardy and Gadoury 1989).
Apple scab is a unique disease in the sense that the pathogen grows under the cuticle of apple leaves without killing the underlying cells of the epidermal layer and the mesophyll. The disease becomes visible when, with the initiation of sporulation, conidiogenous cells disrupt the cuticle to release melanized conidia into the air. Just like powdery mildew diseases, the fungal structures on the leaf surface are the typical symptoms of apple scab rather than the reaction of the plant to fungal attack. The fungus neither penetrates the mesophyll nor the epidermal cell layer; it takes up nutrients from the subcuticular space between the cuticle and epidermal cells. V. inaequalis affects host plant metabolism by taking up nutrients via corallipherous hyphae and by increasing the water loss through its damaging action on the plant cuticle (Aderhold 1896; Mills 1931; Hignett and Kirkham 1967; Shear and Drake 1971).
Transpiration rate has been shown to be negatively correlated to leaf temperature (Inoue et al. 1990). Leaf temperature results from the incoming radiation, the water status of the plant and the functionality of the epidermal layer (cuticle and stomata) to regulate the transpiration of leaves, as well as from environmental conditions such as air temperature, relative humidity (RH) and wind speed (Jones 1992). Unintentional transpiration is prevented mainly by the cuticle on leaf surfaces which is an effective barrier against water loss (Schönherr 1982), whereas the water status of the shoot tissue determines the temperature of plant leaves through stomatal transpiration. In addition to the abiotic environment, pathogenic organisms may affect both cuticular and stomatal conductance of plant tissue resulting in significant modification of leaf temperature (Ayres and Jones 1975; Smith et al. 1986; Wright et al. 2000; Chaerle et al. 2001; Bassanezi et al. 2002).
As leaf temperature is directly related to the rate of evapotranspiration from the canopy surface, infrared sensing of the canopy temperature may be used to monitor the transpiration rate of plants (Jones et al. 2002; Merlot et al. 2002). Thermal infrared imaging (TIRI) enables the quantitative analysis of spatial and dynamic physiological information on the plant status at the canopy and leaf level without interfering with plants (Jones 2004). In plant sciences, the method has been applied to study the relationship between stomatal conductivity and leaf temperature (e.g. Luquet et al. 2003; Prytz et al. 2003; Wang et al. 2004; Leinonen et al. 2006) to visualize stress in plants or in parts of plants (Pearce and Fuller 2001; Stier et al. 2003; Leinonen and Jones 2004; Möller et al. 2007), the drying of fruits (Fito et al. 2004) and to estimate apple yields (Stajnko et al. 2004). In plant pathology, TIRI has been used to detect disease symptoms before they become visible (Chaerle et al. 1999, 2004; Lindenthal et al. 2005; Allegre et al. 2007; Stoll et al. 2008a) and to identify management zones for disease control (Lenthe et al. 2007; Stenzel et al. 2007).
Symptoms of apple scab seem to be associated with disruption of the apple cuticle by the conidiophores of V. inaequalis that result in a localized decrease of leaf temperature (Oerke et al. 2005). However, there is no information on the temporal changes in transpiration as the plant is modified by the pathogenesis of V. inaequalis or on the quantitative relationship between scab severity and plant transpiration. This information is required to evaluate the suitability of TIRI for disease sensing, i.e. the detection and quantification of apple scab. It was hypothesized that (I) minimum colonization of leaf tissue by V. inaequalis increases leaf transpiration even before the appearance of visual symptoms, (II) leaf temperature, especially the maximum temperature difference (MTD, Lindenthal et al. 2005), may be used to differentiate between healthy and diseased leaves and (III) MTD is correlated to disease severity measured as the percentage leaf area affected.
Materials and methods
Plant cultivation
Seedlings of apple (Malus domestica Borkh., cv. Golden Delicious) were grown in pots (diameter 10 cm) filled with standard potting mixture (Klasmann-Deilmann GmbH, Geeste, Germany) at 20 ± 2°C and 16 h daylight (>300 μmol m−2 s−1, Philips SGR 140, Hamburg, Germany) in a glasshouse with 50–70% relative humidity (RH). Plants were irrigated and fertilized (liquid fertilizer Flory 2 special, NPK 16 + 9 + 22) as required. Plants with at least four fully expanded leaves were used for the experiments. Inoculated plants were maintained at 15 ± 1°C, most favourable for rapid scab development, 18 ± 1, and 20 ± 2°C, respectively.
Pathogen isolates and inoculations
Isolates HS1, N5 and SID of V. inaequalis (Cooke) G. Wint., differing in aggressiveness, were cultivated on apple leaves by spraying spore suspensions onto the leaves and incubating plants at 100% RH for 48 h. Leaves with sporulating scab lesions were stored at −18°C for inoculum storage. Conidia of V. inaequalis were washed off the leaves without addition of surfactants, passed through two layers of cheese cloth and adjusted to spore concentrations ranging from 1.25 × 104 to 6 × 105 conidia ml−1 using a Fuchs–Rosenthal hemacytometer. Apple leaves were either spray-inoculated using a commercial hand sprayer or by placing one to six droplets (20 μl) of the spore suspension carefully onto the leaves with a minimum distance between drops of 1 cm before incubation at 100% RH. Two days after inoculation (days pi) the RH was reduced to 50–70%.
Disease assessment
Leaves inoculated with V. inaequalis were assessed daily for the development of scab symptoms. Disease severity was assessed by visually rating the percentage of leaf area showing characteristic symptoms. Leaves were scored on a scale of 0, 1, 3, 5, 10, 20, 30, 40, 50, 60, 70, 80, 90 and 100% of leaf area displaying symptoms.
Apple leaves colonized by V. inaequalis were observed without preparation using a stereomicroscope (MZ16 F, Leica Microsystems Ltd., Heerbrugg, Switzerland) and a Leitz microscope (DMRB Leica, Wetzlar, Germany).
Thermography of apple leaves
For thermographic measurements plants were allowed to adjust to the environmental conditions (20 ± 1°C, 50–65% RH, daylight) for at least 1 h. Thermograms were taken using a Varioscan 3201 ST (Jenoptic Laser, Jena, Germany), a Stirling-cooled infrared scanning camera with a spectral sensitivity from 8 to 12 μm, a geometric resolution of 1.5 mrad (240 × 360 pixels, focal plane array) and a 30° × 20° field of view lens with a minimum focus distance of 0.22 m resulting in a maximum spatial resolution of 0.33 mm/pixel. Thermal resolution was 0.03 K, the accuracy of absolute temperature measurement <±2 K. The camera was controlled using the software IRBIS® control (InfraTec, Dresden, Germany). Images were processed using IRBIS® plus software (Vers. 2.2, InfraTec); leaf emissivity was set to 1 as relative differences in leaf temperature resulting from pathogen development were the main interest of this study. Minimum, maximum and mean leaf temperature, standard deviation and temperature range (maximum temperature difference, MTD) were calculated for leaf areas marked by a polygon, omitting mixed pixels on the leaf edge and for transects; temperature distributions and frequencies were displayed in profiles and histograms. Colour reflectance images of the leaves were taken with commercial digital cameras, such as a Nikon D50 (Nikon, Tokyo, Japan).
Measurements of leaf water status
Leaf water content (LWC) of healthy and diseased areas of apple leaves was assessed gravimetrically. For each treatment, 12 discs (Ø 10 mm) were punched out of the leaves avoiding the central vein and were weighed before and after drying at 106°C for 2 h.
Water activity (aW) of leaf tissue was measured using leaf discs (Ø 20 mm) punched out of the apple leaves. Each leaf disc was transferred immediately to the sample drawer of an AquaLab CX-3 (Decagon Devices, IUL Instruments, Königswinter, D) and measured with the chilled-mirror dew point sensor at 23°C (n = 5).
Statistical analysis
Data were analyzed statistically using SAS 9.0 (SAS Institute, Inc., Cary, NC). For normally distributed data a standard analysis of variance (ANOVA) was performed. For significant F values, mean comparisons were performed using Tukey’s HSD test, Fisher’s LSD test or t test statistics (significance level of P = 0.05). Correlations and regressions were calculated using the GLM and REG procedures in SAS. Pearson’s coefficient of determination (r²) was adjusted for the number of explanatory terms.
Results
Effect of apple scab development on leaf temperature
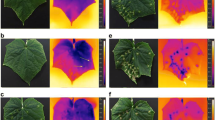
Leaves inoculated with conidia of V. inaequalis show concentric spots of unusual low leaf temperature even before the appearance of visible scab symptoms (Fig. 1, 6 days pi). The affected leaf area as well as its temperature difference from healthy areas increase when typical scab symptoms become visible 8 days pi. Similarly, distinct leaf areas of lower temperature precede the appearance of scab lesions in the later stages of disease development (compare 8 and 11 days pi, respectively, in Fig. 1). The temperature of non-infected apple leaves shows little spatial variation, and the maximum temperature difference (MTD) is typically <1 K (Fig. 2). Fungal infections, especially those of the youngest fully expanded leaves, result in an early increase in MTD, which increases further during later stages of scab development (Fig. 2b). Variation in disease severity related to leaf level (i.e. upper leaves are younger) and with time are revealed by MTD of the respective leaves (Fig. 2b).
Effect of the development of scab colonies caused by V. inaequalis (isolate HS1) infections on spatial heterogeneity in leaf temperature of apple seedlings after 4, 6, 8 and 11 days of incubation. First thermal effects of scab became detectable 6 days pi; similarly, areas of low leaf temperature preceded visible symptoms in later stages of leaf colonization. Left, thermo-images and right, RGB reflectance images
The potential of thermography to detect the first signs of scab symptoms on apple leaves as well as to assess differences in disease severity was compared to a visual assessment of disease in experiments over time with three isolates of V. inaequalis differing in aggressiveness, i.e. the speed of leaf colonization and development of symptoms. Figure 3 shows that at 15°C, a temperature favourable for rapid disease development, isolates HS1 and N5 produce the first visible symptoms 7 days pi; for isolate SID the first scab lesions were detectable after only 8 days of incubation. Thermographically, the first indications of disease were detected 1–2 days earlier. Both methods of disease detection rate isolate HS1 as the most aggressive and isolate SID as the least aggressive, however, the relative differences between disease progress curves described by thermography and visual assessment vary with the isolate (Fig. 3).
According to thermographic measurements, the area of leaf affected by the disease is significantly (p < 0.05) larger than from visual ratings (Fig. 3). The area with decreased leaf temperature exceeds the area of scab lesions by 80% when the first symptoms become visible. This proportion decreases during lesion expansion and was <20% for mature lesions (not shown). Microscopically conidiophores with conidia that had penetrated the cuticle from the subcuticular mycelium are detectable singly or in small groups outside the perimeter of the macroscopic visible scab lesions (Fig. 4a, c). This colonization of the apple leaves exceeds the margin of the macroscopic lesions by about 500 μm, equivalent to about 20% of visible colony diameter at 8 days pi. Infection structures such as runner hyphae, corallipherous hyphae and primordia of conidiophores can be identified at an even greater distance from the visible colony margin (Fig. 4d).
Intensity of leaf colonization by V. inaequalis and symptom development on apple leaves. a Overview under a binocular microscope, b intensively colonized centre of colony with masses of melanized (dark) conidia, c area with subcuticular hyphae and single conidiophores penetrating the apple cuticle not visible with the naked eye and d fungal structures below the intact apple cuticle. Ch corallipherous hyphae, Co conidium, Cp conidiophores, Rh runner hyphae
Heterogeneity of leaf temperature in response to the number and size of scab symptoms
When apple leaves were inoculated with defined droplets of inoculum the concentration of conidia has a significant (p < 0.05) effect on symptom development and thermographic response to scab development (Table 1). As early as 4 days pi, the highest concentration of 250 000 conidia ml−1 results in a significant decrease in leaf temperature in the centre of the developing colony (Fig. 5). Depending on the inoculum concentrations it took up to 2 days more until a change in leaf temperature was measurable. The diameter of the leaf temperature abnormality (= scab colony) as well as the intensity of the temperature decrease increases with the time of incubation until about 8 days pi when all concentrations result in similar changes of leaf transpiration (Fig. 5). At later stages of scab development, higher inoculum concentrations caused an accelerated decay and subsequent drying of the tissue linked to an increase of tissue temperature in the centre of the lesions.
Changes in temperature of scab lesions on apple leaves in relation to inoculum concentration of V. inaequalis (12 500, 50 000 and 250 000 conidia ml−1). Temperature profiles of scab lesions from the centre of lesions to healthy tissue after 4, 5, 6, 8, 11 and 14 days of incubation (repeated measures of infection sites from the same leaf)
Experiments with three droplets of 6 × 104 conidia ml−1 on the right side and 6 × 105 conidia ml−1 on the left side of apple leaves confirm that the intensity of the temperature decline and the extent of affected leaf area increases with inoculum concentration. Temperature profiles of the affected leaf tissue taken at 5 days pi clearly reflect the differences in size of scab lesion and in the intensity of tissue colonization by the pathogen (Fig. 6). The MTD is strongly correlated to the diameter of scab lesions (y = 0.213x − 0.464; r² = 0.85). When leaf sides were inoculated with 1 (left side) and 5 (right side) droplets, respectively, of the same concentration, the larger number of infection sites results in a higher MTD of the right leaf side throughout the experiment (p ≤ 0.05; Fig. 7a). Histograms of the leaf sides indicate a Gaussian distribution for the temperature of healthy leaves (r² = 0.971) and Gaussian distributions (r² = 0.967 and r² = 0.958 for 1 and 5 lesions, respectively) with a negative skew for diseased leaf sides at 4 days pi (Fig. 7b). The larger number of infection sites per leaf side is associated with a significantly (p ≤ 0.05) broader temperature range, whereas the mean temperature is only slightly less than that of the leaf side with one infection site because some tissue also showed reduced transpiration associated with a rise in temperature (Fig. 7b). In later stages of the disease, the heterogeneity in MTD within the affected leaf side slowly decreased, associated with the overall senescence of apple leaves.
Effect of inoculum concentration of V. inaequalis on the temperature of apple leaves after 5 days of incubation (left leaf side 6 × 105 conidia ml−1, right side 6 × 104 conidia ml−1). a Reflectance image, b thermogram with transects, c thermogram visualising isotherms and d temperature profile of transects through lesions resulting from high (L01, L02, L03) and low inoculum concentration (L04, L05, L06), respectively
Effect of the number of V. inaequalis (isolate HS1) infection sites on maximum temperature difference of apple leaves during scab development (a, n = 9), and distribution of leaf temperature 4 days post inoculation for a healthy leaf side and leaf sides with 1 and 5 infection site(s), respectively (b). Plants were kept at 18°C after inoculation; thermographic measurements at 22°C
Effect of scab development on the water status of apple leaves
When symptoms became visible, diseased leaf areas had a significantly higher water content (68.2% vs. 65.5%) and a larger water activity aw (0.973 ± 0.001 vs. 0.954 ± 0.003) than non-diseased leaves. The difference in LWC decreased in later disease stages.
Relationship between disease severity and maximum temperature difference
The results presented above demonstrate a relationship between scab severity and the extent to which leaf temperature was affected. The correlation between MTD and disease severity—i.e. % visibly diseased leaf area—is described best by the function y = 0.936 + 0.0523x − 0.0004x² (Table 2) with a maximum for a disease severity of about 67% diseased leaf area; the coefficient of determination for a linear function was considerably weaker. A considerable amount of variation was due to the time of measurement as thermograms were recorded over several days. Analysis of the various thermograms day by day gives r² values that range from 0.69 to 0.72 (quadratic function) and 0.53 to 0.61 (linear function), respectively. Calculations based on the standard deviation (SD) of leaf temperature instead of MTD give similar results, whereas absolute temperature values, e.g. mean leaf temperature result in considerably smaller coefficients of determination (Table 2). A similar set of data showed that the SD of leaf temperature was closely correlated to MTD (y = 0.027x² +0.133x; r² = 0.88).
Discussion
Digital infrared thermography proved to be very suitable for the detection and quantification of V. inaequalis development on apple leaves. Modification of the plant’s water status could be detected 1–3 days earlier than visible symptoms of fungal colonization. These results agree with earlier ones from cucumber leaves infected by the oomycete Pseudoperonospora cubensis (Lindenthal et al. 2005; Oerke et al. 2006). The development of V. inaequalis caused a localized temperature drop associated with an increase in leaf water content.
Maximum temperature difference of leaves was affected more by fungal colonization than absolute changes in the mean, minimum, maximum and standard deviation of leaf temperature. Primary changes in leaf temperature due to differences in leaf water status were spatially confined at the early stages of the disease, and, in contrast to other parameters, MTD assesses these rare pixels. The standard deviation (SD), although strongly correlated to MTD, was less sensitive, especially at low levels of disease, when the variation in temperature of diseased leaves was similar to that of non-infected leaves. As MTD of non-infected leaves is usually <1 K (Lindenthal et al. 2005; Oerke et al. 2006) and MTD values of diseased leaves exceed the corresponding SD values by a factor of >3, this parameter should be more appropriate in screening assays to discriminate between healthy and diseased tissue or plants.
Thermography was able to assess differences in scab intensity resulting from the increase in scab resistance of older leaves as well as from isolate-specific differences in aggressiveness. In all cases, however, the leaf area with a reduced temperature was larger than that covered with scab lesions. The extent of subcuticular colonization of leaves by V. inaequalis significantly exceeded the dimensions of the visible symptoms. Runner hyphae beneath the cuticle form secondary stromata (i.e. compact masses of fungal hyphae) which produce conidiophores penetrating the cuticle to liberate conidia for further dispersal of the pathogen (Nusbaum and Keitt 1938; Stadelmann and Schwinn 1982; Corlett et al. 1976; Gessler and Stumm 1984). The centres of scab lesions are the sites of initial spore production and become visible to the naked eye because of the production of numerous melanized conidia. Visible symptoms, however, are surrounded by an area where the pathogen has also penetrated the cuticle and produces conidia at a lower density (Fig. 4). The proportion of this area to the total area affected depends on the stage of the disease and the growth rate of the pathogen in the host, which is determined by temperature. The proportion commonly decreases with time and should be small for mature colonies. Hyphal growth of the pathogen between the cuticle and epidermal layer is likely to affect the water status of leaf tissue over an even larger area. It seems likely that leaf areas where the corallipherous hyphae are still covered by intact plant cuticle contribute to the localized decrease in leaf temperature. The leaf area with decreased temperature, therefore, is likely to represent scab severity of leaves better than the leaf area showing ‘visible’ scab symptoms.
The severity of host colonization increased with inoculum concentration and resulted in a very early decrease in surface temperature at higher concentrations. Rapid and intense damage of leaf tissue, however, led to necrotic tissue in the centre of colonies associated with a spatially limited upsurge in temperature. An equalization of the temperature of V. inaequalis-infected areas to the temperature of uninfected leaf parts may also occur during late stages of the disease in senescent leaves associated with an overall decrease in transpiration rate (Oerke et al. 2005). However, in early stages of the disease that are relevant for disease detection in the field, apple scab resulted in a decrease in leaf temperature; this unidirectional effect is a prerequisite for the use of thermal effects to quantify disease. In other host-pathogen systems, e.g. downy mildew of cucumbers (Lindenthal et al. 2005), TMV infection of tobacco and Cercospora leaf spot of sugar beet (Chaerle et al. 2004), the stage of the disease has a pronounced effect on whether leaf temperature decreases or increases. Increases and decreases in temperature may alternate, spatially or temporally when tissue colonization and disease symptoms are associated with localized cell death. Contrasting thermal effects have also been reported to result from the water status of the host plant as shown for downy mildew of grapevine (Stoll et al. 2008b).
The proportion of leaf area with an elevated transpiration rate increased with the number of scab colonies. Interestingly, MTD of leaves not only increased with the size of lesions but also with their number per leaf indicating that the probability of large lesions rises with the frequency of scab lesions. The increase in MTD with colony size may be explained by the high thermal conductivity of water (Kümmerlen et al. 1999). With small lesions, which have a very limited effect on transpiration or increase in leaf water content, the cooling effect dissipates quickly because of high lateral thermal flow, and the effect on tissue temperature is restricted in size and magnitude. With large lesions, the cooling effect is greater and the largest lesions even displayed a temperature gradient from the margin to the centres of colonies.
The strong correlation between leaf area affected by V. inaequalis and MTD of apple leaves confirmed earlier results on downy mildew of cucumber (Oerke et al. 2006). The regression curve was described better by a quadratic equation, as disease levels of more than about 70% of leaf area resulted in severe damage of the leaf tissue associated with premature leaf senescence and the collapse of leaf hydraulics. These changes finally result in a more or less homogenous loss of tissue vitality and a decrease in MTD. The linear increase of MTD with scab severity up to 70% diseased leaf area indicates that thermography is suitable for quantifying scab severity, e.g. in screening systems for disease resistance. Although the SD of leaf temperature gave similar coefficients of determination, MTD showed lower day-to-day variability and greater sensitivity at low disease severity.
In contrast to Pseudomonas syringae pv. syringae (Di Giorgio et al. 1996) and Phytophthora sojae (McDonald and Cahill 1999), which rapidly reduce host transpiration, V. inaequalis is not known to produce toxic metabolites that may affect stomatal conductivity directly. The pathogen, however, induces a transpiration sink to divert plant solutes (Hignett and Kirkham 1967) to the subcuticular space for its own growth. The diffusion of nutrients to the pathogen is associated with an increase in leaf water content of the mesophyll below the infection site. This effect was detectable thermographically before the perforation of the cuticle by emerging conidiophores and conidia of V. inaequalis (typically on adaxial leaf sides) increased cuticular conductivity of this astomatous surface. The increase in transpiration of scabby leaves has been reported several times to result from damage to the cuticle (Aderhold 1896; Mills 1931; Hignett and Kirkham 1967; Shear and Drake 1971). The pre-symptomatic increase in transpiration of the leaf areas with subcuticular colonization is evidence that there is also a local increase in stomatal transpiration of the abaxial leaf side due to an increase in leaf water potential induced by fungal activity—at least in earlier disease stages, when perforation of the cuticle is not detectable microscopically.
Thermography may be used for sensing plant diseases that affect plant transpiration; it has the potential for early detection and is as well suited to quantify disease when severity does not exceed 60–70% of the leaf area. Although it lacks diagnostic properties—various abiotic and biotic factors have an effect on transpiration—thermography may be used in inoculation experiments in plant breeding to screen many genotypes for disease resistance, in which case the capability of disease diagnosis is not needed. The method gives reproducible results and has the potential for automation. Remote sensing may be used also in early warning systems; Delalieux et al. (2007, 2009) used non-imaging hyperspectroscopy for the differentiation of scab-infected and healthy apple leaves and reported that the 1460, 1940 and 2400 nm wavelengths (the first and third associated to water content) are suitable for discrimination at early disease stages, whereas the visible range, especially around 650–700 nm, became more important at later stages. This sensing system, however, was neither tested for its sensitivity (for low disease severities) nor for its potential to quantify the severity of scab disease.
Conclusions
Thermography seems to be suitable for the detection and quantification of V. inaequalis infections, but the technique still has to be evaluated under field conditions. The temperature range (MTD) of leaves or other plant parts (of a canopy) is robust to side-effects from abiotic factors, however, it also seems to be very sensitive to pathogen-induced changes in plant transpiration in the field. Diseases affecting plant transpiration may be detected at early stages of epidemics, i.e. when primary foci occur in the field that can be controlled site-specifically. Thermal infrared imaging has great potential as a sensor technique for precision agriculture in the near future because of its high degree of sensitivity. Since thermography largely lacks diagnostic capabilities, the technique has to be combined with other sensors, e.g. hyperspectral imaging, in cases when disease identification is needed.
References
Aderhold, R. (1896). Die Fusicladien unserer Obstbäume, 1. Teil. Diels Landwirtschaftliches Jahrbuch, 25, 875–914.
Allegre, M., Daire, X., Heloir, M. C., Trouvelot, S., Mercier, L., Adrian, M., et al. (2007). Stomatal deregulation in Plasmopara viticola-infected grapevine leaves. New Phytologist, 173, 832–840.
Ayres, P. G., & Jones, P. (1975). Increased transpiration and the accumulation of root absorbed 86Rb in barley leaves infected by Rhynchosporium secalis (leaf blotch). Physiological Plant Pathology, 7, 49–58.
Bassanezi, R. B., Amorim, L., Bergamin, F. A., & Berger, R. D. (2002). Gas exchange and emission of chlorophyll fluorescence during the monocycle of rust, angular leaf spot and anthracnose on bean leaves as a function of their trophic characteristics. Journal of Phytopathology, 150, 37–47.
Chaerle, L., Van Caeneghem, W., Messens, E., Lambers, H., van Montagu, M., & van der Straeten, D. (1999). Presymptomatic visualization of plant–virus interactions by thermography. Nature Biotechnology, 17, 813–816.
Chaerle, L., de Boever, F., van Montagu, M., & van der Straeten, D. (2001). Thermographic visualization of cell death in tobacco and Arabidopsis. Plant, Cell and Environment, 24, 15–25.
Chaerle, L., Hagenbeek, D., de Bruyne, E., Valcke, R., & van der Straeten, D. (2004). Thermal and chlorophyll-fluorescence imaging distinguish plant–pathogen interactions at an early stage. Plant Cell Physiology, 45, 887–896.
Corlett, M., Chong, J., & Kokko, E. G. (1976). The ultrastructure of the Spilocea state of Venturia inaequalis in vivo. Canadian Journal of Microbiology, 22, 1144–1152.
Delalieux, S., van Aardt, J., Keulemans, W., Schrevens, E., & Coppin, P. (2007). Detection of biotic stress (Venturia inaequalis) in apple trees using hyperspectral data: Non-parametric statistical approaches and physiological implications. European Journal of Agronomy, 27, 130–143.
Delalieux, S., Somers, B., Verstraeten, W. W., van Aardt, J. A. N., Keulemans, W., & Coppin, P. (2009). Hyperspectral indices to diagnose leaf biotic stress of apple plants, considering leaf phenology. International Journal of Remote Sensing, 30, 1887–1912.
Di Giorgio, D., Camoni, L., Mott, K. A., Takemoto, J. Y., & Ballio, A. (1996). Syringopeptins, Pseudomonas syringae pv. syringae phytotoxins, resemble syringomycin in closing stomata. Plant Pathology, 45, 564–571.
Fito, P. J., Ortola, M. D., de los Reyes, D., Fito, P., & de los Reyes, E. (2004). Control of citrus surface drying by image analysis of infrared thermography. Journal of Food Engineering, 61, 287–290.
Gessler, C., & Stumm, D. (1984). Infection and stroma formation by Venturia inaequalis on apple leaves with different degrees of susceptibility to scab. Phytopathologische Zeitschrift, 110, 119–126.
Hignett, R. C., & Kirkham, D. S. (1967). The role of extracellular melanoproteins in Venturia inaequalis in host susceptibility. Journal of General Microbiology, 48, 269–275.
Inoue, Y., Kimball, B. A., Jackson, R. D., Pinter, P. J., & Reginato, R. J. (1990). Remote estimation of leaf transpiration rate and stomatal resistance based on infrared thermometry. Agricultural and Forest Meteorology, 51, 21–33.
Jones, H. G. (1992). Plant and microclimate (2nd ed.). Cambridge, UK: Cambridge University Press.
Jones, H. G. (2004). Application of thermal imaging and infrared sensing in plant physiology and ecophysiology. Advances in Botanical Research, 41, 107–163.
Jones, H. G., Stoll, M., Santoa, T., de Sousa, C., Chaves, M. M., & Grant, O. M. (2002). Use of infrared thermography for monitoring stomatal closure in the field: Application to grapevine. Journal of Experimental Botany, 53, 2249–2260.
Kümmerlen, B., Dauwe, S., Schmundt, D., & Schurr, U. (1999). Thermography to measure water relations of plant leaves. In B. Jähne (Ed.), Handbook of computer vision and applications (Vol. 3, pp. 763–781). London: Academic Press.
Leinonen, I., & Jones, H. G. (2004). Combining thermal and visible imagery for estimating canopy temperature and identifying plant stress. Journal of Experimental Botany, 55, 1423–1431.
Leinonen, I., Grant, O. M., Tagliavia, C. P. P., Chaves, M. M., & Jones, H. G. (2006). Estimating stomatal conductance with thermal imagery. Plant, Cell and Environment, 29, 1508–1518.
Lenthe, J.-H., Oerke, E.-C., & Dehne, H.-W. (2007). Digital infrared thermography for monitoring canopy health of wheat. Precision Agriculture, 8, 15–26.
Lindenthal, M., Steiner, U., Dehne, H.-W., & Oerke, E.-C. (2005). Effect of downy mildew development on transpiration of cucumber leaves visualized by digital infrared thermography. Phytopathology, 95, 233–240.
Luquet, D., Begue, A., Vidal, A., Clouvel, P., Dauzat, J., et al. (2003). Using multidirectional thermography to characterize water status of cotton. Remote Sensing of Environment, 84, 411–421.
MacHardy, W. E. (1995). Apple scab: Biology, epidemiology, and management. St. Paul, MN: APS Press.
MacHardy, W. E., & Gadoury, D. M. (1989). A revision of Mills’s criteria for predicting apple scab infection periods. Phytopathology, 79, 304–310.
McDonald, K. L., & Cahill, D. M. (1999). Evidence for a transmissible factor that causes rapid stomatal closure in soybean at sites adjacent to and remote from hypersensitive cell death induced by Phytophthora sojae. Physiological and Molecular Plant Pathology, 55, 197–203.
Merlot, S., Mustilli, A. C., Genty, B., North, H., Lefebre, V., et al. (2002). Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant Journal, 30, 601–609.
Mills, W. D. (1931). A method of detecting and demonstrating early leaf infections of apple scab. Phytopathology, 21, 338–339.
Möller, M., Alchanatis, V., Cohen, Y., Tsipris, J., Naor, A., et al. (2007). Use of thermal and visible imagery for estimating crop water status of irrigated grapevine. Journal of Experimental Botany, 58, 827–838.
Nusbaum, C. J., & Keitt, G. W. (1938). A cytological study of host-parasite relations of Venturia inaequalis on apple leaves. Journal of Agricultural Research (Washington), 56, 595–618.
Oerke, E.-C., Lindenthal, M., Fröhling, P., & Steiner, U. (2005). Digital infrared thermography for the assessment of leaf pathogens. In J. V. Stafford (Ed.), Precision agriculture ‘05 (pp. 91–98). Wageningen: Wageningen University Press.
Oerke, E.-C., Steiner, U., Dehne, H.-W., & Lindenthal, M. (2006). Thermal imaging of cucumber leaves affected by downy mildew and environmental conditions. Journal of Experimental Botany, 57, 2121–2132.
Pearce, R. S., & Fuller, M. P. (2001). Freezing of barley studied by infrared video thermography. Plant Physiology, 125, 227–240.
Prytz, G., Futsaether, C. M., & Johnsson, A. (2003). Thermography studies of the spatial and temporal variability in stomatal conductance of Avena leaves during stable and oscillatory transpiration. New Phytologist, 158, 258–259.
Schönherr, J. (1982). Resistance of plant surfaces to water loss: Transport properties of cutin, suberin and associated lipids. In Encyclopedia of plant physiology (Vol. 12B, pp. 153-179). Berlin: Springer-Verlag.
Shear, G. M., & Drake, C. R. (1971). Calcium accumulation in apple fruit infected with Venturia inaequalis (Cooke) Wint. Physiological Plant Pathology, 1, 313.
Smith, R. C. G., Heritage, A. D., Stapper, M., & Barrs, H. D. (1986). Effect of stripe rust (Puccinia striiformis West.) and irrigation on the yield and foliage temperature of wheat. Field Crops Research, 14, 39–51.
Stadelmann, F. X., & Schwinn, F. J. (1982). Contribution to the biology of Venturia inaequalis. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz, 89, 96–109.
Stajnko, D., Lakota, M., & Hoevar, M. (2004). Estimation of number and diameter of apple fruits in an orchard during the growing season by thermal imaging. Computers and Electronics in Agriculture, 42, 31–42.
Stenzel, I., Steiner, U., Dehne, H.-W., & Oerke, E.-C. (2007). Occurrence of fungal leaf pathogens in sugar beet fields monitored with digital infrared thermography. In J. V. Stafford (Ed.), Precision agriculture ‘07 (pp. 529–535). Wageningen: Wageningen Academic Publishers.
Stier, J. C., Filiault, D. L., Wisniewski, M., & Palta, J. P. (2003). Visualization of freezing progression in turfgrasses using infrared video thermography. Crop Science, 43, 415–420.
Stoll, M., Schultz, H. R., Baecker, G., & Berkelmann-Loehnertz, B. (2008a). Early pathogen detection under different water status and the assessment of spray application in vineyards through the use of thermal imagery. Precision Agriculture, 9, 407–417.
Stoll, M., Schultz, H. R., & Berkelmann-Loehnertz, B. (2008b). Exploring the sensitivity of thermal imaging for Plasmopara viticola pathogen detection in grapevines under different water status. Functional Plant Biology, 35, 281–288.
Wang, Y., Holroyd, G., Hetherington, A. M., & Ng, C. K. Y. (2004). Seeing ‘cool’ and ‘hot’-infrared thermography as a tool for non-invasive, high-throughput screening of Arabidopsis guard cell signaling mutants. Journal of Experimental Botany, 55, 1187–1193.
Wright, K. N., Duncan, G. H., Pradel, K. S., Carr, F., Wood, S., et al. (2000). Analysis of the N gene hypersensitive response induced by a fluorescently tagged tobacco mosaic virus. Plant Physiology, 123, 1375–1385.
Acknowledgments
We would like to thank Phil E. Russell for critical reading of a former version of the manuscript. We are grateful to Alexander Prange, Hochschule Niederrhein, Mönchengladbach (D) for providing the AquaLab CX-3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oerke, EC., Fröhling, P. & Steiner, U. Thermographic assessment of scab disease on apple leaves. Precision Agric 12, 699–715 (2011). https://doi.org/10.1007/s11119-010-9212-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11119-010-9212-3