Abstract
Maize (Zea mays L.) yield is limited by the poor availability of inorganic phosphate (Pi) in many arable areas worldwide. Phosphorus use efficiency (PUE) is a complex multigene trait, with a single gene contributing only a small percentage to the phenotype. Transcription factors (TFs) are very important as a single TF frequently coordinates the expression of multiple genes in response to environmental signals. Previous studies have indicated that the TFs AtPHR1 and OsPHR2 play important roles in the regulation of plant phosphorus accumulation. However, little is known about the functions of PHR-like genes in maize. In this study, a member of the MYB-CC family encoding a 449-amino acid protein, ZmPHR1, was isolated. The ZmPHR1∷GFP fusion was localized in the nucleus, which indicates that ZmPHR1 is also a TF. Phylogenetic tree analysis revealed that ZmPHR1 belongs to the same subfamily of MYB-CCs as OsPHR1, OsPHR2 and AtPHR1. Transgenic Arabidopsis lines overexpressing ZmPHR1 were used to investigate the pleiotropic effects of this gene under low Pi conditions. Overexpression of ZmPHR1 led to the upregulation of multiple genes that regulate metabolism during Pi-starvation, which in turn resulted in an elevation in Pi content in shoots. Most notably, Arabidopsis overexpressing ZmPHR1 showed better growth under low-Pi conditions. The results presented in this study suggest that PUE could be improved through the manipulation of the TF ZmPHR1 in maize and possibly in other species under Pi-deficient conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phosphate (Pi) deficiency in soil is a worldwide problem, because Pi concentrations are often within the range of 1–10 μM in 30–40 % arable land and cannot meet the demands of plant growth and development (Raghothama 1999; Schachtman et al. 1998; Tan et al. 2012). The application of phosphorus (P) fertilizer in soil is a common practice to maintain plant proper growth and high yield of crops. However, even under adequate levels of P fertilization, only 20 % or less was utilized by plants (Vance 2001). The fertilized P forms insoluble complexes with cations, particularly calcium ions in alkaline soil and aluminum or iron in acidic conditions, and is incorporated into organic matter by microbes (Vance et al. 2003). In addition, leaching and runoff of Pi from soils into water bodies result in eutrophication, which is increasingly becoming a global environmental concern (Sharply and Moyer 2000; Sims et al. 2000). Moreover, excessive application of inorganic P fertilizers to the soil system over decades can exhaust the supply of Pi-rich minerals—a non-renewable resource—which will increase the overall cost of crop production (Vance et al. 2003; Cordell et al. 2009). In order to use genetic and breeding approaches to improve crops with complex traits such as phosphorus use efficiency (PUE), it is necessary to gain comprehensive knowledge of the regulatory mechanisms underlying Pi use, especially under Pi-deficient conditions.
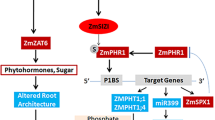
Plants have evolved a variety of adaptive strategies to survive Pi deficiency. In Arabidopsis thaliana, transcriptome analysis indicated that approximately 29 % of the total genes are involved in Pi-starvation responses (Wu et al. 2003). Transcription factors (TFs) such as WRKY75, ZAT6, MYB62, BHLH32, AtPHR1 and PHR1-LIKE1 are the main regulators involved in regulating Pi homeostasis in various plants (Bustos et al. 2010; Chen et al. 2007; Devaiah et al. 2007a, 2007b, 2009; Rubio et al. 2001). AtPHR1, a member of the MYB-CC family, was the first transcription factor demonstrated to mediate the Pi-starvation response at the transcriptional level in Arabidopsis. It regulates the expression of Pi starvation-inducible (PSI) genes by binding as a dimer to the PHR1-specific binding sequence (P1BS) element (GNATATNC)—an imperfect palindromic sequence present in the promoter regions of these genes (Rubio et al. 2001; Franco-Zorrilla et al. 2007; Nilsson et al. 2007; Coelho et al. 2010). Pi content was increased significantly in both wild type and phr1 mutant Arabidopsis plants transformed with AtPHR1, irrespective of Pi conditions (Bari et al. 2006; Nilsson et al. 2007). Recently, OsPHR2, which may function in a manner similar to AtPHR1, was isolated from rice (Oryza sativa L.) using the AtPHR1 protein sequence as a query. Several Pi transporters were upregulated in the shoots of OsPHR2-overexpressing plants, and correlated positively with the accumulation of Pi (Zhou et al. 2008).
Maize—one of the most economically important cultivated crops worldwide—is grown widely in both acidic and calcareous soils, where Pi fertilizers are easily absorbed by soil particles, resulting in the production of relatively insoluble and unavailable forms of Pi (Vance et al. 2003). Many maize genotypes have been screened for PUE; according to both greenhouse and field tests, only a few have turned out to be high phosphorus-utilizing types (Da Silva and Gabelman 1992; Kaeppler et al. 2000). Many important crop traits, including PUE, are complex multi-gene traits, with each gene contributing only a small proportion to the phenotype. For example, HvPHT1-1, which encodes a single Pi transporter, did not increase P assimilation in barley upon introduction into the genome (Rae et al. 2004). The introduction of TFs, which regulate many downstream genes, is a feasible approach to crop modification (Century et al. 2008). However, little is known about the functions of PHR-like TFs in maize compared with those in Arabidopsis and rice.
Here, we describe the isolation of a maize transcription factor, ZmPHR1, and its functional characterization in transgenic Arabidopsis plants. Overexpression of ZmPHR1 in Arabidopsis upregulated a series of phosphorus starvation-inducible genes and transporters, resulting in reduced sensitivity to low Pi stress, improved plant growth and Pi content in shoots in comparison with wild type. These results give hope to breeders aiming to improve PUE in crops by genetic engineering.
Materials and Methods
Pi Treatment and Growth Conditions for Maize
The previously isolated P-efficient maize inbred line 478 was used in this experiment. Seeds were surface sterilized with 75 % ethanol for 30 s, rinsed three times with sterile distilled water, and germinated in boxes filled with sterilized perlite. Seven-day-old seedlings were transferred to a continuously aerated nutrient solution with high Pi content (HP, 1 mM KH2PO4) for 7 days and then transferred to a low Pi solution (LP, 10 μM KH2PO4). The basal nutrient solution contained 2 mM Ca(NO3)2, 0.65 mM MgSO4, 25 μM Fe-EDTA, 5 μM MnSO4, 50 μM KCl, 2 μM ZnSO4, 0.5 μM CuSO4, 0.005 μM (NH4)6Mo24 and 25 mΜ H3BO4. In addition, K+ was supplied in the LP solution in the form of 0.99 mM KCl. The seedlings were grown in an artificial climatic chamber at 28 °C/18 °C (day/night) under a 16 h/8 h (light/dark) photoperiod at 100 μmol m−2 s−1 photon flux density and approximately 70 % humidity. Roots, stems, and leaves were harvested at 0, 1, 3, 5 and 7 days after Pi starvation, frozen in liquid nitrogen, and stored at −80 °C for further analysis.
RNA Extraction and Synthesis of First-Strand cDNA
Tissue samples (50–100 mg) were ground into a fine powder in liquid nitrogen. Total RNA was then extracted from various tissues using RNAiso™ Plus (Takara, Dalian, China) according to the manufacturer’s instructions. The quality and quantity of RNA were determined using a BioPhotometer (Eppendorf, Hamburg, Germany) and further confirmed by denaturing agarose gel electrophoresis. Isolated RNA was digested with DNaseI (RNase free, Takara) to remove traces of DNA. First strand cDNA was synthesized using 2.5 μg RNA in a final volume of 20 μl with a reverse transcriptase M-MLV (RNase H−) kit and oligo(T)18 primer (Takara). Reverse transcription was performed at 42 °C for 60 min with a final denaturation at 70 °C for 5 min.
Cloning of ZmPHR1 by Reverse Transcription-PCR
A BLAST search was performed in the TIGR (The Institute of Genomic Research Database-TDB) Maize Database (http://maize.jcvi.org) using the amino acid sequence of AtPHR1 (NP_194590) as a query. A consensus sequence (TC346249) was obtained and used as a template to design the primer pairs (forward: 5′-AAGCAAGGTAATGAAATG-3′ and reverse: 5′-TGAATAGTGCAACCGATA-3′) with Primer Premier 5.0 software (Premier Biosoft International, Palo Alto, CA). Full-length cDNA was amplified from the leaf cDNA of maize inbred line 478. RT-PCR was conducted with 2.5 ng cDNA, 2.5 U Takara Ex Taq polymerase (Takara), 50 μM dNTPs (Takara), 0.2 μM primers, and programmed at 94 °C for 3 min followed by 30 cycles of 94 °C for 1 min, 53 °C for 1 min, and 72 °C for 1 min. The PCR products were recovered with a DNA Fragment Quick Purification/Recovery Kit (DingGuo, Beijing, China), subcloned into a pGEM-T Easy Vector (Promega, Madison, WI) according to the manufacturer’s instructions, and sequenced (Sangon, Shanghai, China).
Bioinformatic Analysis
Analysis of the ZmPHR1 cDNA sequence was conducted with DNAMAN 6.0.40 (http://www.lynnon.com/). Multiple sequence alignment and phylogenetic analysis for ZmPHR1 and related members of the MYB-CC subfamily were conducted with the Clustal X, MEGA 5.0 and GeneDoc programs, respectively (Tamura et al. 2011; Zhang et al. 2011). The gene structure analysis of ZmPHR1, AtPHR1, OsPHR1 and OsPHR2 was carried out by performing a BLAST search of the MaizeGDB genomic database (http://www.maizegdb.org) and NCBI (http://www.ncbi.nlm.nih.gov/), respectively, using the cDNA as a query.
Localization of the ZmPHR1::GFP Fusion Protein
To construct the ZmPHR1::GFP fusion protein, the coding region of ZmPHR1 was amplified from pMD-18T Vector-ZmPHR1 with a forward primer containing a SalI site (gtcgac; 5′-gtcgac ATGAGGAAGTTTAATCTG-3′) and a reverse primer containing a BclI site (tgatca; 5′-CCG tgatca ACTATCTTGCAGTTT-3′). BclI and BamHI are isocaudomers. The PCR products were inserted between the SalI and BamHI sites in the pJIT163-GFP vector (pJIT163 vector, http://www.pgreen.ac.uk) with two CaMV 35S promoters (Fig. 3a). The pJIT163-ZmPHR1::GFP and pJIT163-GFP vectors were transferred into onion epidermal cells using a Biolistic PDS–1000/He particle delivery system (Bio-Rad, Hercules, CA), The GFP signal was detected using a laser confocal scanning microscope (LCSM; Olympus, Tokyo, Japan) at a wavelength of 488 nm.
Expression Analysis of ZmPHR1 by Semi-quantitative RT-PCR and Quantitative PCR
Semi-quantitative RT-PCR and quantitative PCR (qPCR) were conducted to analyze the expression of the ZmPHR1 gene in roots, stems and leaves of maize seedlings at various time points (0, 1, 3, 5 and 7 days after LP-treatment). Maize α-tubulin was used as a reference gene to normalize both cDNA concentrations (RT-PCR) and expression data (qPCR). The primers for amplifying α-tubulin were 5′-ACTTCATGCTTTCGTCCTACGCTCCA-3′ (forward) and 5′-CTGGGAGGCTGGTAGTTGATTC-3′ (reverse). ZmPHR1 gene-specific primers A (PA, designed for amplifying complete cDNA sequence) were 5′-AAGCAAGGTAATGAAATG-3′ (forward) and 5′-TGAATAGTGCAACCGATA-3′ (reverse). ZmPHR1 gene-specific primers B (PB, designed for qPCR) were 5′-CACCCTTTATTTCTCAGTCATCCAA-3′ (forward) and 5′-TCATTTTGTGTAGCACTCTCATCAG-3′ (reverse). Semi-quantitative RT-PCR was conducted with 2.5 ng cDNA, 2.5 U Takara Ex Taq polymerase (Takara), 50 μM dNTPs (Takara) and 0.2 μM primers (PA). The PCR program was 94 °C for 3 min followed by 30 cycles of 94 °C for 1 min, 58–60 °C for 1 min, and 72 °C for 1 min. The PCR products were separated on 1 % agarose gels. Next, qPCR was performed on a Bio-Rad platform (CFX96) using an SYBR Green detection chemistry kit (SYBR® Premix Ex Taq™, Takara). The qPCR was performed in a final volume of 20 μL in a mixture containing 10 μL 2× SYBR Premix Ex Taq™, 300 nmol each of specific forward and reverse primers (PB) and 40 ng cDNA. The amplification program was 95 °C for 5 min followed by 40 cycles of 95 °C for 10 s, 60 °C for 30 s, and 72 °C for 1 min. After each PCR reaction, the specificity of the amplification was controlled by melting curve analysis (from 65 to 95 °C). The relative expression levels of the target genes were measured using the 2-△△Ct method of relative quantification (△△Ct = [Ct gene - Ct tubulin]) (Livak and Schmittgen 2001).
Transformation of Arabidopsis and Identification of Transgenic Plants
The fragment containing the 2 × 35S CaMV promoter, ZmPHR1, GFP, and terminator in the vector pJIT163-ZmPHR1::GFP was digested with KpnI and XhoI and inserted into the binary vector pCAMBIA1300 between the KpnI and SalI sites (SalI and XhoI are isocaudomers; Fig. 3a). The constructed binary vector, pZmPHR1::GFP, was transferred into Agrobacterium tumefactions strain GV3101 using the freeze-thaw method.
Next, pZmPHR1::GFP was introduced into Arabidopsis using the floral dip method (Clough and Bent 1998). Arabidopsis thaliana (Ecotype Columbia) was planted in peat mould and vermiculite at a ratio of 3:1, grown at 23 °C, 65 % humidity, under a 16 h light/8 h dark photoperiod at 100 μmol m−2 s−1 photon flux density. The seeds harvested from the transformed plants were surface sterilized with 75 % ethanol for 30 s, followed by 20 % hypochlorite for 20 min. The seeds were germinated and grown in Murashige and Skoog medium containing 0.8 % agar, 1 % sucrose and 50 mg L−1 hygromycin B (Sigma) to select transgenic seedlings. DNA and RNA from the shoots of hygromycin-resistant plants were extracted for PCR, RT-PCR and qPCR with ZmPHR1-specific primers to check for target gene insertion and expression. Homozygous and overexpressing ZmPHR1 transgenic lines were identified from T3 plant lines.
Transgenic and wild-type Arabidopsis plants were used to detect ZmPHR1 green fluorescence protein (GFP) under a living body image-forming system (NightOWL IILB 983, Bad Wildbad, Germany). GFP expressed in root apical meristems was detected through a laser scanning confocal microscope (LSCM) at a wavelength of 488 nm.
Treatment of ZmPHR1 Transgenic and Wild-type Arabidopsis Plants
ZmPHR1 transgenic and wild-type Arabidopsis seeds were planted on sponges moistened with sterile water. Two weeks after germination, the plants were transferred into pots, each containing ten plants and 2.0 L aerated HP nutrient solution (1 mM Pi). The basal nutrient solution composition was the same as that used for maize culture. Each treatment was performed in triplicate. After 2 weeks of HP culture, half of the seedlings were transferred to LP nutrient solution (10 μM Pi) and the other half were maintained in HP nutrient solution. After 7 days of P-starvation treatment, shoots and roots from plants of both genotypes were harvested for qPCR. Two weeks later, five plants from each replicate were dried in an oven prior to biomass weighing and total P measurement, and the other five plants were instantly frozen in liquid nitrogen for inorganic phosphate (Pi) measurement (Nilsson et al. 2007).
Measurement of Total P and Pi
Inorganic Pi contents were measured following previously described methods (Nanamori et al. 2004). Frozen samples were ground using an ice-cold mortar and pestle and placed into a 2.0 mL centrifuge tube. The ground powder was then incubated in 2 % (v/v) glacial acetic acid at 42 °C for 30 min. After centrifugation at 12,000 rpm for 2 min, the Pi content of the supernatant was measured using the Mo (ammonium molybdate)-Sb (antimony potassium tartrate)-Vc (ascorbic acid) method; 0.4 % (w/v) ammonium molybdate melted in 0.5 M H2SO4 was mixed with 0.05 % antimony potassium tartrate (solution A), and 10 % ascorbic acid (solution B; A:B = 6:1) was then added to the mixture. One milliliter of this working solution was added to 1 mL of the sample solution, and the mixture was incubated in a water bath at 37 °C for 1 h. After cooling for 5 min at 4 °C, the absorbance was measured at 820 nm. The Pi concentration was calculated by normalization of fresh weight. The total plant P content was analyzed by the Mo (ammonium molybdate)-Sb (antimony potassium tartrate)-Vc (ascorbic acid) method after the plant material was digested with H2SO4-H2O2 at 300 °C, and normalized by dry weight (Zhou et al. 2008).
The results of above measurements were subjected to analysis of variance (ANOVA) to determine the significance of the differences in responses to treatment between genotypes. Least significance difference (LSD) post-hoc tests were conducted to determine the differences between the individual treatments (DPS for Windows; Hangzhou RuiFeng Information Technology, Hangzhou, Zhejiang, China; Tang and Zhang 2012).
Expression Analysis of Phosphorous Starvation Response Genes by qPCR
Shoots and roots of the transgenic T3 and wild type plants after low Pi (LP) treatment for 7 days were used for qPCR. The expression of AtIPS1, AtPS2, AtPS3, AtRNS1, Pht1;2, Pht1;4, Pht1;8 and Pht1;9 were analyzed. The specific primers for different genes are listed in Table 1. The above genes were amplified and the relative expression levels of the target genes were measured as described before.
Results
Cloning and Sequence Analysis of ZmPHR1
To isolate the full-length cDNA encoding maize PHR1, the AtPHR1 (NP_194590) amino acid sequence was used as a query to BLAST the TIGR Maize Database (http://maize.jcvi.org). A target fragment 1,703 bp long was obtained. Sequence analysis revealed that it included an open reading frame (ORF) of 1,347 bp, which encoded a predicted protein of 449 amino acids with a calculated molecular weight of 49.59 kDa and theoretical pI value of 6.20 (Fig. 1). Multiple sequence alignment results showed that the predicted amino acids sequence shared 43 %, 64 %, and 45 % identity with the amino acid sequences of AtPHR1, OsPHR1 (AK063486), and OsPHR2 (AK100065), respectively. The results also indicated that the four amino acid sequences were highly conserved in both MYB DNA-binding domain (BD) and CC domain (Fig. 2a). The newly identified gene was designated ZmPHR1 and deposited with GenBank under the accession number JF831533.
MYB-CC domain, phylogenetic and structural analysis of ZmPHR1. a Alignment of MYB-CC domain of ZmPHR1 with AtPHR1, OsPHR1 and OsPHR2. b Phylogenetic tree analysis of ZmPHR1 with other related MYB-CC proteins from Arabidopsis, Oryza sativa and Chlamydomonas reinhardtii. A phylogram of proteins sharing the MYB and predicted CC domains was constructed using the Clustal X program and the neighbor-joining method (Zhang et al. 2011). Bar 0.2 substitutions per site. c Diagram of ZmPHR1, OsPHR1, OsPHR2 and AtPHR1. Exons and introns are indicated as black and white boxes, respectively. Numbers indicate the length of each exon and intron. Start codon (ATG) and stop locations are shown by arrowheads
Phylogenetic analysis was performed based on the neighbor-joining method and bootstrap analysis (n = 1,000) using 19 protein sequences, including the sequences of the four genes described above, CrPSR1 in Chlamydomonas reinhardtii (Wykoff et al. 1999) and another 14 proteins with a conserved MYB and CC Domains in Arabidopsis (Fig. 2b). They were separated into two main groups. ZmPHR1 was clustered in the same group as AtPHR1, OsPHR1 and OsPHR2. ZmPHR1 was sub-grouped with OsPHR1 and OsPHR2, which was consistent with their close evolutionary relationship (Fig. 2b).
The ZmPHR1 gene sequence was obtained by BLAST against the MaizeGDB database. ZmPHR1, which was 3,776 bp in length and contained seven exons and six introns, was located on maize chromosome 1. The gene structure of ZmPHR1 had a similar splicing pattern as AtPHR1, OsPHR1 and OsPHR2 (Fig. 2c).
ZmPHR1 is a TF
In order to determine whether ZmPHR1 encodes a transcription factor like AtPHR1 and OsPHR2, the ZmPHR1∷GFP fusion protein construct (JIT163-ZmPHR1∷GFP) was generated by fusing the ZmPHR1 coding sequence to the 5′ end of the GFP gene (Fig. 3a). This construct was then introduced into onion epidermal cells by particle bombardment. The fluorescence signal was located predominately in the nuclei of ZmPHR1∷GFP transformed cells (Fig. 3b: a, b, c). In contrast, the GFP fluorescence signal was distributed throughout entire control cells that were transformed with the GFP vector (Fig. 3b: d, e, f). The results demonstrated that ZmPHR1 was a nuclear protein.
Subcellular localization of ZmPHR1 in onion epidermal cells. a Diagram of the fusion protein expression vector (JIT163-ZmPHR1∷GFP), which contains an in-frame fusion protein with the ORF of ZmPHR1 and GFP under the control of two CaMV35S promoters (35S-P). CaMV-T CaMV Terminator. b Subcellular localization of ZmPHR1∷GFP fusion protein and GFP protein (control) in the cell. The green fluorescence of ZmPHR1∷GFP was detected in the nucleus (a, b and c) and that of GFP was detected in the cytomembrane and nucleus (d, e and f). The epidermal cells selected for the confocal images (b and e) are the same as the images (a and d) taken with transmitted light. Images c and f are images merged by a and b, d and e, respectively. Bar 100 μm
Expression Patterns of ZmPHR1
To investigate the spatial expression patterns of ZmPHR1 in maize under different Pi conditions, semi-quantitative RT-PCR analysis was performed on different seedling tissues 7 days after Pi treatment. ZmPHR1 showed a constitutive expression pattern in all three tissues, with high expression in leaves and roots and weak expression in stems of plants subjected to either HP or LP treatment (Fig. 4a).
a RT-PCR analysis of ZmPHR1 gene expression. Leaves exhibited the highest levels of ZmPHR1 transcripts among the three tissues. Lanes: R Roots, S stems, L leaves. Maize seedlings were grown hydroponically in high Pi medium (HP, 1 mM Pi) for 7 days and then transferred to medium with high Pi or low Pi (LP, 10 μM Pi), where they were grown for an additional 7 days. Expression of ZmPHR1 was detected by RT-PCR analysis. Tubulin was used as a reference gene. b qPCR analysis of ZmPHR1 gene expression in plants. Seven-day-old seedlings in HP nutrient medium were transferred LP medium, and expression of ZmPHR1 was detected in roots (R), stems (S), and leaves (L) at 0, 1, 3, 5 and 7 days after Pi starvation. The expression of ZmPHR1 expression at 0 day was used to calibrate the samples. Error bars Standard deviation (n = 3)
The temporal expression patterns of ZmPHR1 in plants under Pi-starvation stress were further studied by qPCR. Expression levels of ZmPHR1 were quantified in various tissues at different timepoints (0, 1, 3, 5 and 7 days) during Pi-starvation treatment. In roots and stems, the expression level of ZmPHR1 increased by 1.84-fold and 108.86-fold 3 days after Pi starvation, respectively, followed by a decrease at 5 days. By contrast, ZmPHR1 expression in leaves was reduced dramatically at the beginning of LP-treatment, followed by an increase at 5 days to approximately half of the level observed at 0 day (Fig. 4b).
Transformation of Arabidopsis and Identification of ZmPHR1-Overexpressing Transgenic Plants
Arabidopsis lines overexpressing ZmPHR1 were generated to identify the function of ZmPHR1 in vivo. A binary vector containing the 2 × 35S::ZmPHR1::GFP fusion protein was constructed and introduced into Arabidopsis. Hygromycin-resistant seedlings were selected, and T3 progeny were generated (Fig. 5a). T3 progeny plants from eight independent transgenic lines (T1–T8) were confirmed by PCR (Fig. 5b) and RT-PCR (Fig. 5c). Using a living body image-forming system, green fluorescence was detected, as expected, only in the transgenic Arabidopsis plants (Fig. 5d). Moreover, green fluorescence was detected in the root tips of transgenic plants under an LSCM, which further proved that ZmPHR1 was located in the nucleus and distributed mainly in root apical meristems (Fig. 5e). A 13.06-fold higher ZmPHR1 transcript level was identified by qPCR (Fig. 5f) in T1 plant lines than in their WT counterparts under HP conditions.
Expression of ZmPHR1 in transgenic Arabidopsis. a T3 progeny plants of transgenic Arabidopsis with hygromycin-resistant (left), and wild-type Arabidopsis (right). b Verification of transgenic integration by PCR. Lanes: M 1 kb DNA ladder, CK positive control (recombinant plasmid), WT wild type plant, T1–T8 transformed plant lines. c Expression of transcripts in transgenic plant lines (upper panel) and AtActin (reference gene; lower panel) evidenced by RT-PCR. d Green fluorescence was detected in the transgenic plants (left), but not in control plants (right), using a living body image-forming system (Night OWL II LB 983), indicating the expression of ZmPHR1 protein in transgenic plants. e Green fluorescence was detected in the nucleus of a transgenic root tip with a confocal microscope. Bar 50.0 μm. f Relative quantitative expression of ZmPHR1 in wild-type (WT) and transgenic lines (T) under HP conditions. AtActin was used as a reference gene. Transgenic line was used for calibration. Error bars Standard deviation (n = 3)
Overexpression of ZmPHR1 Improves growth and Pi Content in Shoots under LP Conditions
The phenotype of ZmPHR1-overexpressing lines and WT under different Pi levels was investigated to characterize the function of ZmPHR1. Vegetative growth of both genotypes was inhibited under LP conditions compared to HP conditions (Fig. 6). The shoot sizes and dry matter yield of ZmPHR1-overexpressing lines were much greater (Fig. 6a) and higher (P < 0.05, Table 2), respectively, than those of WT, especially under LP conditions. Although there was no significant difference in root dry matter yield between the two genotypes under either HP or LP conditions, the root-to-shoot ratio, an important indication of plants in response to Pi starvation, of ZmPHR1-overexpressing lines was significantly lower than that of WT under LP conditions (P < 0.05, Table 2). This indicates that the transgenic plants were less sensitive to LP than WT plants, which led to better growth of transgenic vs WT plants.
Total P content of shoots and the distribution of Pi in roots and shoots were also analyzed in hydroponically grown ZmPHR1-overexpressing and wild-type plants (Table 2, Fig. 7). Total P contents of shoots were slightly higher in the ZmPHR1-overexpressing plants than in the WT plants, albeit with no significant difference, irrespective of Pi conditions. However, the level of Pi accumulation in the shoots of ZmPHR1-overexpressing plants was almost twice that in WT under LP conditions with significant difference (P < 0.05; Fig. 7a). No significant difference in Pi accumulation was observed in the roots between both genotypes (Fig. 7a, b). These results suggest that overexpression of ZmPHR1 increased Pi content in shoots under LP conditions.
The distribution of free phosphate (Pi) in shoots and roots of wild-type (white columns) and ZmPHR1-overexpressing (purple columns) Arabidopsis plants. a Grown in hydroponics at LP (10 µM Pi) conditions, the level of Pi accumulation in the shoots of ZmPHR1-overexpressing plants was almost twice that in WT with significant difference. No significant difference in Pi accumulation was observed in the roots between both genotypes. b Grown hydroponics at HP (1mM Pi) conditions, no significant difference in Pi accumulation was observed in the shoots and roots between both genotypes. Letters above the SE values of a particular parameter indicate whether the genotype had a significant influence within each P level (P < 0.05), as determined by analysis of variance (one-way ANOVA) followed by least significance difference (LSD) post-hoc tests. FW Fresh weight. Error bars Standard error (n = 3)
ZmPHR1 Upregulates Pi Starvation-Inducible Genes
The expression patterns of Pi-starvation-inducible genes, including AtIPS1, AtPS2, AtPS3, and AtRNS1, were examined in ZmPHR1-overexpressing Arabidopsis plants (Fig. 8). The upstream promoter regions of these four genes contain one or more P1BS elements, i.e., PHR1-binding sites (Rubio et al. 2001). Under LP conditions, ZmPHR1 regulated the expression of AtIPS1, AtPS2, AtPS3 and AtRNS1, inducing various levels of expression. The expression of all four genes increased significantly in the roots of ZmPHR1-overexpressing plants compared to wild-type plants (Fig. 8a, c, e, g). The expression of the four genes was also upregulated by ZmPHR1 in shoots; however, only the expression of AtPS2 and AtRNS1 obviously increased (Fig. 8c, g). Under HP conditions, AtPS2, AtPS3 and AtRNS1 were also upregulated in the roots of ZmPHR1-overexpressing plants (Fig. 8d, f, h). The expression of AtRNS1 in ZmPHR1-transgenic plants was higher than that in the wild-type, irrespective of Pi treatment (Fig. 8g, h).
Relative expression of AtIPS1 (a, b), AtPS2 (c, d), AtPS3 (e, f) and AtRNS1 (g, h) analyzed by qPCR in shoots and roots of wild-type Arabidopsis (white columns) and ZmPHR1-overexpressing transgenic lines (purple columns). Both genotypes grown in hydroponics at LP (10 µM Pi) and HP (1 mM Pi) conditions, respectively. AtActin was used as a reference gene. ND Not detectable. Error bars Standard error (n = 3). Note different scales was used for calibration analysis of different genes
Overexpression of ZmPHR1 Upregulates Several Pi Transporters
To investigate whether the Pi transport system is regulated by ZmPHR1, the expression levels of four high-affinity Pi transporters (Pht1;2, Pht1;4, Pht1;8 and Pht1;9) were analyzed by qPCR in wild-type and transgenic Arabidopsis plants (Fig. 9). A striking upregulation of Pht1;2, Pht1;8, Pht1;9, was observed (Fig. 9a, e, g) under LP conditions and Pht1;2 and Pht1;9 were also upregulated under HP conditions (Fig. 9b, h) in the roots of ZmPHR1-overexpressing plants compared to wild-type plants. The expression of these three genes was not detectable in the shoots of both genotypes, irrespective of Pi treatment (Fig. 9a, b, e, f, g, h). Moreover, the expression levels of Pht1;2, Pht1;8 and Pht1;9 in roots of ZmPHR1-overexpressing plants were higher under LP conditions than under HP conditions. This result suggested that ZmPHR1 regulates the expression of Pht1;2, Pht1;8 and Pht1;9 in roots, particularly under LP conditions. There was no significant difference in expression of Pht1;4 in the roots and shoots of both genotypes (Fig. 9c,d), implying that ZmPHR1 might not regulate expression of Pht1;4.
Relative expression of Pht1;2 (a, b), Pht1;4 (c, d), Pht1;8 (e, f) and Pht1;9 (g, h) analyzed by qPCR in shoots and roots of wild-type Arabidopsis (white columns) and ZmPHR1-overexpressing transgenic lines (purple columns). Both genotypes grown in hydroponics at LP (10 µM Pi) and HP (1 mM Pi) conditions, respectively. AtActin was used as a reference gene. ND Not detectable. Error bars Standard error (n = 3). Note different scales was used for calibration analysis of different genes
Discussion
Many important traits are under complex polygenic control. The incorporation of a single gene encoding a qualitative trait is often insufficient when attempting to fully activate a target pathway by genetic manipulation. TFs that act as the main regulators of metabolic pathways are excellent candidates for modifying complex traits in crop plants, and TF-based technologies are likely to play a prominent role in the successful production of the next generation of bioengineered crops. PUE has been established as a quantitative characteristic under polygenic control, and plant biomass and Pi concentration are two key indexes for screening PUE genotypes. Our results indicate that a series of genes involved in the Pi-starvation metabolism were up-regulated in Arabidopsis plants overexpressing ZmPHR1, which improved plant growth and increased Pi content in shoots under low-Pi conditions compared to wild-type plants. These results demonstrate the utility of introducing TFs into plants to alter plant metabolism, thus producing plants with superior characteristics.
A series of Pi-starvation response genes function in the Pi rescue system by solubilizing or releasing Pi from organic sources, which increases the capacity of plants to recycle internal Pi (Abel et al. 2002; Bari et al. 2006; Raghothama 1999). Under Pi-starvation conditions, RNases play an important role in the remobilization process (Bariola et al. 1994). AtIPS1 inhibits the cleavage of PHO2 by miR399 and controls P homeostasis (Bari et al. 2006; Lin et al. 2008). AtPS2 is a pyrophosphate-specific phosphatase, and AtPS3 is a glycerol-3-phosphate permease. These genes are responsive to Pi starvation (Devaiah et al. 2009; May et al. 2011). P1BS elements, which are the target sequences of PHR1, exist in the upstream regions of the promoters of AtIPS1, AtRNS1, AtPS2 and AtPS3. Loss of AtPHR1 reduces the expression of AtIPS1, and the overexpression of PHR1 in WT and phr1 mutant Arabidopsis plants leads to the elevated expression of AtIPS1 (Rubio et al. 2001; Nilsson et al. 2007). In rice, OsIPS1 and OsIPS2 are homologs of AtIPS1 and have the same function as AtIPS1. In OsPHR2-overexpressing plants, expression of OsIPS1 and OsIPS2 increased in shoots and roots under Pi-starvation conditions (Hou et al. 2005; Zhou et al. 2008). In this study, AtRNS1 expression in overexpressing ZmPHR1-transgenic Arabidopsis plants was higher than in wild type, irrespective of Pi regime. This was similar to the effect of AtPHR1 on AtRNS1 expression (Nilsson et al. 2007) and indicates that AtRNS1 expression depends strongly on ZmPHR1 levels. The expression of AtIPS1, AtPS2 and AtPS3 were enhanced greatly in roots under LP conditions. These results show that the overexpression of ZmPHR1 greatly increases the expression of AtIPS1, AtRNS1, AtPS2 and AtPS3 in transgenic Arabidopsis plants compared to wild types under LP conditions, especially in roots.
High-affinity phosphate transporters play an important role under LP conditions (1–10 μM Pi) (Raghothama 2000). Members of the high-affinity phosphate transporter family Pht1 are located in the plasma membrane. These transporters acquire Pi from the rhizosphere, as most of them are expressed in root epidermal cells (Muchhal et al. 1996). Pht1;2 (AtPT1), Pht1;4 (AtPT2), Pht1;8 and Pht1;9 are members of the Pht1 family. Genes encoding these transporters contain P1BS elements in the upstream regions of their promoters and are regulated by AtPHR1. Nilsson et al. (2007) found that AtPHR1 regulated only the expression of Pht1;8 and Pht1;9 in the shoots of plants under normal P conditions. The expression of OsPT9, which is related closely to Pht1;8 and Pht1;9, was increased substantially in the shoots and roots of OsPHR2-overexpressing plants under normal P conditions (Zhou et al. 2008). Pht1;4 has been reported to contribute to Pi uptake (Misson et al. 2004). In the current study, ZmPHR1, like AtPHR1 (Nilsson et al. 2007), does not affect the expression of Pht1;4. Under LP conditions, the expression of Pht1;2, Pht1;8, and Pht1;9 in roots of ZmPHR1-overexpressing plants was notably higher than in wild type. Therefore, ZmPHR1 participates in Pi transportation and utilization under Pi-starvation conditions. Together, these results suggest that overexpressing ZmPHR1 upregulates the expression of Pht1;2, Pht1;8 and Pht1;9 in plant roots under LP conditions.
In summary, the overexpression of a single TF gene, ZmPHR1, had pleiotropic effects on numerous metabolic pathways in Arabidopsis plants, as overexpression of this gene stimulated Pi-starvation signaling and triggered the Pi-starvation response. Although it is currently unknown whether the expression of this TF has a direct or indirect effect on metabolism, our results highlight the possibility that enhancing ZmPHR1 expression is a feasible strategy for improving Pi assimilation and use in crop plants, in particular maize under low-Pi conditions. Transgenic maize plants overexpressing ZmPHR1 have been generated in our laboratory and will be the subject of future studies.
References
Abel S, Ticconi CA, Delatorre CA (2002) Phosphate sensing in higher plants. Physiol Plant 115:1–8
Bari R, Pant BD, Stitt M, Scheible WR (2006) PHO2, microRNA399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol 141:988–999
Bariola PA, Howard CJ, Taylor CB, Verburg MT, Jaglan VD, Green PJ (1994) The Arabidopsis ribonuclease gene RNS1 is tightly controlled in response to phosphate limitation. Plant J 6:673–685
Bustos R, Castrillo G, Linhares F, Puga MI, Rubio V, Pérez-Pérez J, Solano R, Leyva A, Paz-Ares J (2010) A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in Arabidopsis. PLoS Genet 6:1–15
Century K, Reuber TL, Ratcliffe OJ (2008) Regulating the regulators: the future prospects for transcription-factor-based agricultural biotechnology products. Plant Physiol 147:20–29
Chen ZH, Nimmo GA, Jenkins GI, Nimmp HG (2007) BHLH32 modulates several biochemical and morphological processes that respond to Pi starvation in Arabidopsis. Biochem J 405:191–198
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Coelho GTCP, Carneiro NP, Karthikeyan AS, Raghothama KG, Schaffert RE, Brandão RL, Paiva LV, Souza IRP, Alves VM, Imolesi A, Carvalho CHS, Carneiro AA (2010) A phosphate transporter promoter from Arabidopsis thaliana AtPHT1;4 gene drives preferential gene expression in transgenic maize roots under phosphorus starvation. Plant Mol Biol Rep 28:717–723
Cordell D, Drangert JO, White S (2009) The story of phosphorus: global food security and food for thought. Glob Environ Chang 19:292–305
Da Silva ÁE, Gabelman W (1992) Screening maize inbred lines for tolerance to low-P stress condition. Plant Soil 146:181–187
Devaiah BN, Karthikeyan AS, Raghothama KG (2007a) WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis. Plant Physiol 143:1789–1801
Devaiah BN, Nagarajan VK, Raghothama KG (2007b) Phosphate homeostasis and root development in Arabidopsis is synchronized by the zinc finger transcription factor ZAT6. Plant Physiol 145:147–159
Devaiah BN, Madhuvanthi R, Karthikeyan AS, Raghothama KG (2009) Phosphate starvation responses and gibberellic acid biosynthesis are regulated by the MYB62 transcription factor in Arabidopsis. Mol Plant 2:43–58
Franco-Zorrilla JM, Valli A, Todesco M, Mateos I, Puga MI, Rubio-Somoza I, Leyva A, Weigel D, Garcia JA, Paz-Ares J (2007) Target mimicry provides a new mechanism for regulation of microRNA activity. Nat Genet 39:1033–1037
Hou XL, Wu P, Jiao FC, Jia QJ, Chen HM, Yu J, Song XW, Yi KK (2005) Regulation of the expression of OsIPS1 and OsIPS2 in rice via systemic and local Pi signalling and hormones. Plant Cell Environ 28:353–364
Kaeppler SM, Parke JL, Mueller SM, Senior L, Stuber C, Tracy WF (2000) Variation among maize inbred lines and detection of quantitative trait loci for growth at low phosphorus and responsiveness to Arbuscular mycorrhizal fungi. Crop Sci 40:358–363
Lin SI, Chiang SF, Lin WY, Chen JW, Tseng CY, Wu PC, Chiou TJ (2008) Regulatory network of microRNA399 and PHO2 by systemic signaling. Plant Physiol 147:732–746
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)). Methods 25:402–408
May A, Berger S, Hertel T, Köck M (2011) The Arabidopsis thaliana phosphate starvation responsive gene AtPPsPase1 encodes a novel type of inorganic pyrophosphatase. Biochim Biophys Acta 1810:178–185
Misson J, Thibaud MC, Bechtold N, Raghothama K, Nussaume L (2004) Transcriptional regulation and functional properties of Arabidopsis Pht1;4, a high affinity transporter contributing greatly to phosphate uptake in phosphate deprived plants. Plant Mol Biol 55:727–741
Muchhal US, Pardo JM, Raghotama KG (1996) Phosphate transporters from the higher plant Arabidopsis thaliana. Proc Natl Acad Sci USA 93:10519–10523
Nanamori M, Shinano T, Wasaki J, Yamamura T, Rao IM, Osaki M (2004) Low phosphorus tolerance mechanisms: phosphorus recycling and photosynthate partitioning in the tropical forage grass, Brachiaria hybrid cultivar Mulato compared with rice. Plant Cell Physiol 45:460–469
Nilsson L, Müller R, Nielsen TH (2007) Increased expression of the MYB-related transcription factor, PHR1, leads to enhanced phosphate uptake in Arabidopsis thaliana. Plant Cell Environ 30:1499–1512
Rae AL, Jarmey JM, Mudge SR, Smith FW (2004) Overexpression of a high-affinity transporter in transgenic barley plants does not enhance phosphate uptake rates. Funct Plant Biol 31:141–148
Raghothama KG (1999) Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol 50:665–693
Raghothama KG (2000) Phosphate transport and signaling. Curr Opin Plant Biol 3:182–187
Rubio V, Linhares F, Solano R, Martin AC, Iglesias J, Leyva A, Paz-Ares J (2001) A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev 15:2122–2133
Schachtman DP, Reid RJ, Ayling SM (1998) Phosphorus uptake by plants: from soil to cell. Plant Physiol 116:447–453
Sharply A, Moyer B (2000) Phosphorus forms in manure and compost and their release during simulated rainfall. J Environ Qual 29:1462–1469
Sims JT, Edwards AC, Schoumans OF, Simard RR (2000) Integrating soil phosphorus testing into environmentally based agricultural management practices. J Environ Qual 29:60–71
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2439–2442
Tan ZJ, Hu YL, Lin ZP (2012) Expression of NtPT5 is correlated with the degree of colonization in tobacco roots inoculated with Glomus etunicatum. Plant Mol Bio Rep 30:885–893
Tang QY, Zhang CX (2012) Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Science 00:1–7
Vance CP (2001) Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant Physiol 127:390–397
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157:423–447
Wu P, Ma LG, Hou XL, Wang MY, Wu YR, Liu FY, Deng XW (2003) Phosphate starvation triggers distinct alterations of genome expression in Arabidopsis roots and leaves. Plant Physiol 132:1260–1271
Wykoff DD, Grossman AR, Weeks DP, Usuda H, Shimogawara K (1999) Psr1, a nuclear localized protein that regulates phosphorus metabolism in Chlamydomonas. Proc Natl Acad Sci USA 96:15336–15341
Zhang XG, Yin DM, Ma CZ, Fu TD (2011) Phylogenetic analysis of S-locus genes reveals the complicated evolution relationship of S haplotypes in Brassica. Plant Mol Biol Rep 29:481–488
Zhou J, Jiao FC, Wu ZC, Li YY, Wang XM, He XW, Zhong WQ, Wu P (2008) OsPHR2 is involved in phosphate-starvation signaling and excessive phosphate accumulation in shoots of plants. Plant Physiol 146:1673–1686
Acknowledgments
We thank Dr. Yiping Tong, Dr. Yu Cheng, Dr. Hui Liang and Mrs. Yuxiang Wen from the Institute of Genetics and Developmental Biology, Chinese Academy of Science for their helpful suggestions and observation of GFP with the Laser Confocal Scanning Microscope. The authors are grateful to Dr. Hongjie Li from the Institute of Crop Science, Chinese Academy of Agriculture Science, and Dr. Ling Yuan from the Department of Plant and Soil Sciences, University of Kentucky for their critical reviews of this manuscript. The research was supported by the grants of Major Transgenic Organism Breeding Projects from Chinese Ministry of Agriculture (2009ZX08003-017B and 2011ZX08003-001) and Shanxi International Cooperation Project (2012081005-1).
Author information
Authors and Affiliations
Corresponding authors
Additional information
X. Wang and J. Bai contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, X., Bai, J., Liu, H. et al. Overexpression of a Maize Transcription Factor ZmPHR1 Improves Shoot Inorganic Phosphate Content and Growth of Arabidopsis under Low-Phosphate Conditions. Plant Mol Biol Rep 31, 665–677 (2013). https://doi.org/10.1007/s11105-012-0534-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-012-0534-3