Abstract
Hickory (Carya cathayensis Sarg.) is an important economic nut tree in China. However, its long juvenile phase is a deterrent to expanding its cultivation and production to wider areas. To understand the genetic and molecular mechanisms that underlie the reproductive process in hickory trees, CcLFY, a homologue of FLORICAULA/LEAFY, was cloned and its expression patterns were studied during vegetative and reproductive development. The cDNA sequence of CcLFY consisted of 1,442 bp encoding a putative protein of 385 amino acids. The protein consisted of a Pro-rich region, a central acidic domain, a putative Leucine zipper, and a basic region formed by a core of Arg and Lys residues that are critical motifs for transcription factors. sThe amino acid sequence of the CcLFY protein shares 89.2% and 65.6% identities with LFY proteins of Castanea mollissima and Arabidopsis thaliana, respectively. CcLFY was highly expressed in flower buds and leaves, weakly expressed in stems, and was undetectable in roots. In situ hybridization revealed that CcLFY was highly expressed in both immature leaves and flower buds. Expression of CcLFY was initiated during floral transition in the spring, and subsequently, continued to increase, reaching peak levels at 16 days after full bloom (DAFB). Moreover, during this period, the major floral buds formed. Heterologous expression of CcLFY in transgenic tobacco plants induced precocious flowering of growing shoots, and flowers were of normal phenotypes. These results suggested that CcLFY might play a pivotal role in flower initiation and in the development of floral organs in C. cathayensis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Floral transition is one of the major phases in the life cycle of flowering plants. The transition from the vegetative phase to the reproduction phase is controlled by multiple endogenous and environmental cues including hormonal signals, photoperiodic induction and temperature promotion (Mouradov et al. 2002). Four major pathways of flowering have been identified and characterized over recent decades (Mouradov et al. 2002; Simpson and Dean 2002; Liu et al. 2009). First, the photoperiodic pathway involves phytochromes and cryptochromes. The interaction of these photoreceptors with a circadian clock initiates a pathway that eventually results in the expression of the gene CO (CONSTANS). CO acts through other genes to increase the expression of the floral meristem identity gene LFY (LEAFY). Second, in the autonomous and vernalization pathways, flowering occurs either in response to internal signals or to low temperatures. In the autonomous pathway of Arabidopsis, all of the genes associated with the pathway are expressed in the meristem. Third, the carbohydrate, or sucrose, pathway reflects the metabolic state of the plant. Sucrose stimulates flowering in Arabidopsis by increasing LFY expression. Fourth, the gibberellin pathway is required for early flowering and for flowering under noninductive short days (Taiz and Zeiger 2006). All four pathways converge by increasing the expression of the key floral meristem identity gene SOC1 (SUPPRESSOR OF OVEREXPRESSION OF CO1) (Lee and Lee 2010; Ma et al. 2011). Once turned on by SOC1, LFY activates the floral homeotic genes—AP1 (APETALA1), AP3, PI (PISTILLATA), and AG (AGAMOUS)—that are required for the development of floral organs (Du and Pijut 2010; Hou et al. 2011; Li et al. 2011; Liu et al. 2010). While the long day and vernalization pathways respond to light and temperature, the autonomous and gibberellic acid (GA)-dependent pathways monitor the endogenous developmental state of the plant (Moon et al. 2005; Lee and Lee 2010). These signals induce flowering time genes and eventually are integrated on a few genes to form a flower (Zhang et al. 2011). CO, FLC (FLOWERING LOCUS C), FT (FLOWERING LOCUS T), SOC1 and LFY are the key genes in the flowering transition (Moon et al. 2005; Liu et al. 2009; Lee and Lee 2010; Xu et al. 2010; Chang et al. 2011; Ma et al. 2011).
LFY, a floral meristem identity gene, plays a central role in flower development (Coen et al. 1990; Weigel et al. 1992; Blázquez et al. 1997; Shiokawa et al. 2008; Moyroud et al. 2010). LFY integrates signals from multiple flowering pathways, and its expression level eventually determines the exact flowering time (Mouradov et al. 2002; Liu et al. 2009; Lee and Lee 2010). It activates downstream genes that give their unique identities to the floral meristem and floral organ primordia (Liu et al. 2009; Moyroud et al. 2009). Arabidopsis lfy plants form leaflike shoots at the sites of flower formation in wild-type plants and are unable to make the transition to flower development. In contrast, the overexpression of LFY in transgenic plants can induce early flowering (Weigel and Nilsson 1995; Blázquez et al. 1997). LFY homologues are present in a wide range of angiosperms and gymnosperms (Frohlich and Estabrook 2000; Shiokawa et al. 2008), and are even conserved in ferns and bryophytes (Frohlich and Estabrook 2000). Many homologues of LFY have been isolated from different species, especially woody plants such as Populus trichocarpa (Rottmann et al. 2000), Eucalyptus grandis (Southerton et al. 1998; Dornelas et al. 2004), Hevea brasiliensis (Dornelas and Rodriguez 2005a), Pinus caribaea (Mellerowicz et al. 1998; Dornelas and Rodriguez 2005b), Actinidia deliciosa (Walton et al. 2001), Citrus sinensis (Peña et al. 2001), Cedrela fissilis (Dornelas and Rodriguez 2006) and Malus × domestica (Wada et al. 2002).
Although woody plants receive flowering signals every year, they exhibit vegetative growth during early development. Woody plants begin to flower for the first time after several years, and in some cases, even decades, after sowing. Signals may block the flowering transition through the lower expression of integration genes in the juvenile phase. The longer juvenile phase causes difficulties for in the breeding of woody plants. LFY can shorten the juvenile period. When the LFY gene was transferred to Populus, the time to flowering was reduced from years to months (Weigel and Nilsson 1995). Therefore, biotechnological control of flowering should facilitate the breeding of woody plants (Shiokawa et al. 2008).
Hickory (Carya cathayensis Sarg.) is a member of the walnut family. It is a very well-known nut tree in Eastern Chinaand and is mainly found in the vicinity of Tianmu Mountain (30°18′30″–30°24′55″ N, 119°23′47″–119°28′27″ E). Hickory is very important economically, since its nuts are good sources of oil and nutrients. However, hickory grown from seed needs almost 10 years in the juvenile phase before flowering, which reduces its breeding efficiency and, therefore, its yield. Naturally, shortening the juvenile period and increasing the propagation efficiency represent an intriguing strategy for promoting production. In this paper, we report the cloning and characterization of the hickory LFY homologous gene to explore its application to shortening the juvenile phase.
Material and Methods
Plant Materials and Isolation of Total DNA and Total RNA from Hickory
The experimental material consisted of five clones from 15-year-old hickory (C. cathayensis) trees in the fields of Lin'an City (30°N, 119°W). Terminal buds of short branches of hickory were sampled and 30–50 terminal buds were immediately frozen in liquid nitrogen and stored at about −70°C. Sampling dates are presented as days after full bloom (DAFB), where full bloom is defined as the date when 80% of the terminal flowers on spurs are open. Collections were made from −30 DAFB (26th March 2008) until 22 DAFB (18th May 2008) during the female flowering period. Each frozen sample was ground in a stainless steel blender and then in a stainless steel grinder to give a fine powder. The DNA was extracted by using a modified version of the CTAB method as described by Tai and Tanksley (1990). Total RNA extraction was performed as described by Wang et al. (2000). Isolated DNA and RNA were quantitated using a Nanodrop spectrophotometer.
Scanning Electron Microscopy (SEM)
Terminal buds of short branches of hickory were immediately fixed in 4% paraformaldehyde under vacuum for 24 h, dehydrated in absolute ethanol, and stored at 4°C until needed. For SEM observation, the plant material was initially dissected in absolute ethanol under an Olympus dissecting microscope. The resultant material was critically point-dried with CO2 in a Balzer's drier and further dissected, when necessary. The samples were mounted in metallic stubs with carbon conductive adhesive tape, coated with colloidal gold (40-nm thick) and observed at 10–20 kV using a ZEISS DSM 940 A or a LEO 435 VP scanning electron microscope.
Amplification by PCR and Sequencing
To obtain a fragment of the CcLFY gene, we performed both normal PCR and inverse-PCR using total DNA from hickory. We amplified a DNA fragment that corresponded to the conservative sequence of LFYlike genes. The sequences of primers were the sense primer E1 and antisense primer E2 (Table 1). Amplification by PCR was performed under the following conditions: 94°C for 5 min, 35 cycles of 94°C for 1 min, 57°C for 45 s and 72°C for 1.5 min, and final extension at 72°C for 7 min. We succeeded in amplifying a DNA fragment of 873 bp. This fragment was named CcL873.
To obtain 5′- and 3′-sequences adjacent to CcL873, rapid amplification of cDNA ends (RACE)-PCR was performed using a SMART™ rapid cDNA Amplification Kit (Invitrogen, USA). Adapter primer AP, universal amplification primer (AUAP), specific PCR amplification primer 3GSP1 and nest PCR primer 3GSP2 (Table 1) were designed for 3′-RACE according to the fragment. Amplification by inverse-PCR was performed under the following conditions: 94°C for 3 min, 35 cycles of 94°C for 30 s, 56°C for 30 s and 72°C for 1.5 min, and final extension at 72°C for 7 min. We obtained an amplified DNA fragment of about 3,500 bp that contained the putative region. For 5′-RACE, three primers, 5GSP1 (reverse transcription primer), 5GSP2 (PCR primer) and 5GSP3 (nest PCR primer) (Table 1), were designed from the cloned sequence and the synthesis of first-strand cDNA from total RNA and subsequent PCR with gene-specific primers were performed with a 5′-RACE kit (Version 2.0; Invitrogen). The 5′-RACE procedure yielded an amplified DNA fragment of about 507 bp.
The full-length cDNAs were amplified by the shared 5′ primer sequence (LSP) (designed according to the sequence of 5′-RACE flanking regions) and 3′ primer sequence (RSP) (designed according to the sequence 3′-RACE flanking regions) (Table 1). A fragment of about 1.2-kb cDNA was obtained and subcloned into the pMDT-18 vector and sequenced completely in both directions.
To analyze the genomic structure of the gene, two overlapping genomic fragments were amplified by PCR on genomic DNA. PCR primers Intron1 (I1-1 and I1-2) and Intron2 (I2-1 and I2-2) are shown in Table 1. The sequencing was performed by dideoxy methods using an ABI PRISM 3730 automated sequencer. Sequence analysis was carried out in public databases using BLASTX (Altschul et al. 1997) and the DNAMAN software package. The complete nucleotide and protein sequences of different LFY homologues were retrieved from GenBank (http://www.ncbi.nlm.nih.gov/sites/entrez) and aligned with Clustal W (Thompson et al. 1994). Genetic distance matrixes were obtained from the alignments using MEGA4, and phylogenetic trees were built using DNAMAN software.
Southern Blot Analysis
Southern blotting was performed as described by Sambrook et al. (1989) using genomic DNA digested with MseI, VspI, KpnI, and BamHI and blotted on a Hybond-N Plus membrane (Roche). The CcLFY probe used in Southern experiments was an 873-bp PCR product obtained using the primers E1 and E2 and a CcLFY cDNA clone as template. Hybridization conditions, washing stringencies and detection conditions were those suggested by the kit manufacturer (DTG-High Primer DNA Labeling and Detection Starter KitII, Roche, Germany). The hybridized DNA was immunologically detected with antidigoxigenin-AP and visualized with NBT/BCIP (Sangon).
Real-time RT-PCR
We performed real-time RT-PCR with total RNA from root, shoot, leaf and terminal bud of hickory using the Qiagen RNeasy Plant Mini Kit (Qiagen, Hilden, Germany). Overall, the procedures for real-time RT-PCR and the detection of genomic DNA contamination were carried out as described by Bustin et al. (2010) and Zheng et al. (2010). The isolated RNA yield and purity were calculated to ensure that none of the RNA had significant impurities that may have affected reverse transcription and/or amplification. Five micrograms of RNA was used for cDNA synthesis using oligo dT-primer and Superscript II Rnase-Reverse Transcriptase (Invitrogen) according to the manufacturer's instructions. Amplification of cDNA was performed in the presence of gene-specific primers and the SYBR Green PCR master mix (Applied Biosystems, Foster City, CA, USA) in MicroAmp Optical 96-well reaction plates with optical covers using an ABI Prism 7000 Sequence Detector (Applied Biosystems). Each sample was analyzed in biological triplicate, using individual plants and treatments to test for reproducibility. The reaction conditions were 50°C for 2 min, 94°C for 10 min, and then 40 cycles of 94°C for 15 s and 60°C for 1 min. All cDNA samples were included in triplicate in all assays. Primers were designed using Primer express software (Applied Biosystems). Relative quantification of gene expression data was carried out with the 2−ΔΔCT or comparative CT method (Livak and Schmittgen 2001), where the threshold cycle (CT) indicates the cycle number at which the amount of amplified transcript reaches a fixed threshold. Expression levels were normalized with the CT values obtained for the hickory actin. The gene-specific primers for the actin gene (Act1 and Act2) and CcLFY gene (RT1 and RT2) are shown in Table 1.
In Situ Hybridization
The collected shoots of short branches in different developmental stages of hickory were fixed in 3.7% formaldehyde, 5% acetic acid, and 50% ethanol for 5 h. The material was dehydrated with ethanol, cleared with Histoclear, embedded in paraffin (Paraplast Plus) and processed as described by Dornelas et al. (1999, 2000). The template for the CcLFY digoxigenin-labeled riboprobes was the 873-bp cDNA fragment cloned in pGEM-T vector using E1 and E2 primers. Probes were labeled using DIG labeling mix (Roche) according to the manufacturer's protocol. Signal was detected by a colorimetric assay using antiDIG coupled to alkalyne phosphatase and NBT/BCIP as a substrate. The hybridized sections were observed immediately and photographed under a Zeiss Axiovert 35 microscope.
Construction of Expression Vector
The KpnI–SacI fragment of CcLFY was generated by PCR with primers CL1 and CL2 (Table 1). This fragment was cloned into the pGEM-T Easy vector to yield pGEM-T-CcLFY, which was then sequenced. The fragment was introduced into the vector pCAMBIA1301 with CaMV35S promoter and 35S polyA, which had been digested with KpnI and SacI, and the product was designated as pCAM–CcLFY, which was used for the transformation of tobacco.
Transformation of Tobacco
Leaf sections from Nicotiana tabacum cultivar yuyan 86 were transformed as described previously (Yamada et al. 2003). Transformed plants were grown on Murashige and Skoog medium with 100 mg l−1 kanamycin under long-day conditions (18-h photoperiod) at 26°C. Antibiotic-resistant plants were maintained as transgenic lines and plantlets were transplanted to soil.
Results
Reproductive Development in C. cathayensis
Based on our analysis by SEM and the morphological observation of bourse shoot apices, five morphologically distinct stages, from vegetative development to floral organ initiation, were defined (Figs. 1 and 2), which is the same as in a study on female flower development (Huang et al. 2007). Initially, at −30 DAFB, the exterior appearance of pistillate flower bud shows no differentiation from leaf bud before flower-bud initiation, and all bourse shoot meristems are small, flat and narrow (Fig. 1a). In the second stage, all bourse shoot meristems become more broad and the top tends to assume a hemisphere shape at −23 DAFB, but the exterior appearance does not change (Fig. 1b). At −20 DAFB, three flower primordia are formed (Fig. 1c). Stage 4 begins with the initiation of a bract that subtends the apical meristem itself; the flower bud is ambiguous and every tiny flower forms a total husk and three small husks at around −17 DAFB (Fig. 1d). During stage 5, the tiny flower begins to develop, and all meristems have begun floral organ initiation from −4 DAFB to 22 DAFB (Fig. 2).
Reproductive development in Carya cathayensis. All pictures are images obtained by scanning electron microscopy (SEM). a Formation of vegetative meristems in an apical bud collected during early spring at −30 DAFB. b Early development of flower meristem at −23 DAFB. c Flower meristem at −20 DAFB. d Early floral meristem development at −17 DAFB. lf leaf primordia, bp bract primordium, fp flower primordium, mf flower meristem, ma apical meristem, gy female flower bud primordium. Bars: (a, d) 50 μm; (b, c) 100 μ
Cloning and Sequence Analyses of CcLFY
A full-length cDNA clone from the young leaves of hickory, named CcLFY (GenBank accession number: DQ989225), was identified by using the RACE method. The completed cDNA of CcLFY was 1,442-bp long and encoded a putative protein of 385 amino acids (Fig. 3). The predicted molecular weight (MW) of CcLFY protein is 43.5 kDa. Based on the amino acid sequence alignment, CcLFY showed high homology to LFY homolog genes of trees, especially to CMLFY of chestnut, which has 89.2% amino acid sequence similarity to CMLFY (Castanea mollissima). The putative protein CcFLY showed 88%, 83.7%, 83.6% and 65.6% identity to TroLFY (Trochodendron aralioides), PTLF (P. trichocarpa), SdLFY (Salix discolor) and LFY (Arabidopsis), respectively (Fig. 3). Moreover, alignment of the deduced amino acid sequence of CcLFY and its homologs from other organisms revealed that CcLFY shared two major conserved regions with other members of the LFY family: one in the N-terminal end and the other in the C-terminal region (Fig. 3). Further structural analysis of CcLFY revealed that the deduced amino acid sequence of CcLFY included several transcriptional function-related domains such as a proline-rich region, a central acidic domain, a putative Leu-zipper, and a basic region that consists of a core of Arg and Lys residues in the C-terminal region (Fig. 3).
Multiple sequence alignment of plant FLORICAULA/LEAFY. Identical amino acid residues in this alignment are shaded in blue, and similar amino acid residues are shaded in red. The sequences included in the analysis have the following accession numbers: Carya cathayensis DQ989225 (CcLFY), Castanea mollissima ABB83126 (CMLFY), Salix discolor AAO73539 (SdLFY), Arabidopsis thaliana B38104 (LFY), Trochodendron aralioides AAF77118 (TroLFY), and Populus trichocarpa AAB51533 (PTLF)
Evolutionary Position of CcLFY
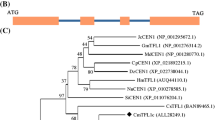
To understand the relationships among plant LFYs, we constructed a phylogenetic tree of aligned amino acid sequences using the DNAMAN software program. A phylogenetic analysis showed that CcLFY in C. cathayensis is in a distinct class of the angiosperm LFY family (Fig. 4). Based on the above alignment and previously reported results, the C termini of the LFY family are highly conserved throughout evolution in Angiosperms, Gymnosperms, Ferns and Mosses, which suggests that CcLFY probably also functioned as a transcription factor, like LFY in Arabidopsis. As in previous results (Shiokawa et al. 2008), the present phylogenetic analysis showed that after Arabidopsis, LFY evolves into a higher subfamily in the angiosperm super-family. As indicated in Fig. 4, CcLFY is closely homologous to CMLFY (Angiosperms), which was derived from C. mollissima.
Phylogenetic analysis of Angiosperm FLORICAULA/LEAFY amino acid sequences by the Neighborhood Joining Bootstrap method (Bootstrap analysis with 1,000 replicates). The sequences included in the analysis have the following accession numbers: Populus trichocarpa AAB51533 (PTLF), Salix discolor AAO73539 (SdLFY), Carya cathayensis DQ989225 (CcLFY), Castanea mollissima ABB83126 (CMLFY), Platanus racemosa AAF77610 (PlaraLFY), Vitis vinifera AAN14527 (VFL), Petunia × hybrida O22621 (ALF), Nicotiana tabacum Q40504 (NFL1), Nicotiana tabacum Q40505 (NFL2), Antirrhinum majus AAA62574 (FLO), Malus × domestica BAB83096 (AFL1), Malus × domestica BAB83097 (AFL2), Pisum sativum AAB88139 (UNI), Acacia auriculiformis AY229891 (AAL), Acacia mangium AAO64448 (AML), Arabidopsis thaliana B38104 (LFY), Jonopsidium acaule AAF00503 (VcLFY1), Jonopsidium acaule AAO73066 (VcLFY2), Brassica oleracea CAA79166(BOFH), Eucalyptus globulus O64953(ELF1), Lycopersicon esculentum AAF66101(FLSFL), and Cucumis sativus AAC64705.1(CFL)
Transcript Levels of CcLFY Determined by Real-Time RT-PCR
To gain insight into the role of CcLFY, CcLFY cDNA levels were analyzed by real-time RT-PCR in samples prepared from different tissues of mature hickory such as leaves, stems, roots and flower buds. Transcripts for the gene were present in most tissues of adult hickory. The CcLFY gene was most highly expressed in flower buds, whereas it was barely detectable in roots and stems (Fig. 5). We also isolated total RNA from various developmental stages of the apical floral buds of short branches and analyzed cDNA for the CcLFY gene using real-time RT-PCR (Fig. 6). With the development of apical floral buds, expression of the CcLFY gene increased and reached a peak at −16 DAFB. Over the next 16 days, the expression decreased sharply (Fig. 6).
In Situ Hybridization Analysis of CcLFY mRNA
Real-time RT-PCR showed that CcLFY was highly expressed in the vegetative bud. To more exactly pinpoint the tissues that express CcLFY during flowering, we used RNA in situ hybridization. The CcLFY sense and antisense transcripts were hybridized to longitudinal sections of vegetative and reproductive meristems at the apical floral buds of short branches in various developmental stages. The results from hybridization with the antisense probe indicated that expression of CcLFY is mainly localized in the shoot apical meristem (SAM) and leaf primordia (Fig. 7). CcLFY was strongly expressed over the leaf primordia and the whole SAM in the early floral buds at −26 DAFB (Fig. 7a). The expression of CcLFY continued to increase in the leaf primordia and SAM after the initiation of floral development (Fig. 7b). At −12 DAFB, a large quantity of CcLFY accumulated and reached a peak in the apices of SAM (Fig. 7c). A low level of CcLFY expression was detected in the primordia of sepal, leaf and pistil, flower bracts and vascular cambium. However, no expression was found in the mature leaf primordia (Fig. 7c, d, e). No hybridization signal was obtained with the CcLFY sense RNA probe (Fig. 7f).
In situ localization of CcLFY transcripts during vegetative and reproductive growth of Carya cathayensis at −26 DAFB (a), −16 DAFB (b), −12 DAFB (c), −4 DAFB (d) and 0 DAFB (e). All sections are longitudinal. All hybridizations were done with the antisense probe, except in (f), where the corresponding sense probe was used. The hybridization signal with CcLFY is observed as a blue precipitate. am apical meristem, ap axillary bud primordium, gy female flower bud primordium, bp bract primordium. Bar: (a–c and e–f) 346 μm; (d) 200 μm
Genetic Transformation Analysis of CcLFY
To analyze the effect of CcLFY on flowering, we generated 35S::CcLFY transgenic tobacco that expressed the 1158 bp PCR-amplified fragment of CcLFY. Overall, we obtained 38 independent T0 plants that harbored the CcLFY gene as determined by PCR. Wild-type and 35S::CcLFY transgenic plants were grown in a growth chamber. As a result of the transformation, 35S::CcLFY transgenic plants produced higher levels of CcLFY mRNA in their leaves than the control lines. Compared with the wild-type, all of these 35S::CcLFY transgenic plants produced oval leaves during the seedling stage (Fig. 8), and flowered 10–12 days earlier than the wild-type plants, approximately 90 days after sowing (Fig. 9). The 35S::CcLFY transgenic plants had a normal floral phenotype.
Discussion
A better understanding of the process of flower development is critical for the establishment of breeding programs. In woody perennials, the mechanism that regulates flowering is remarkably different from that in herbaceous species, with regard to long juvenile phases, bud dormancy and flower transition (Dornelas and Rodriguez 2005a). It is crucial that we understand the regulation of the flowering process in woody perennials for the management and improvement of woody species. In the present study, we succeeded in isolating and characterizing CcLFY from hickory, which encodes for a transcription factor that regulates floral meristem identity. The predicted protein of CcLFY has 385 amino acid residues with conserved structures that are characteristic of most LFY proteins. CcLFY had high amino acid sequence homology (89.2%) to a chestnut LFY protein (Fig. 3). An alignment analysis among CcLFY and other LFY homologues showed that the gene structure and splicing sites are highly conserved among the angiosperms. Most angiosperm LFY proteins have a prolinerich region within about the first 40 amino acids. In addition, two highly conserved regions are present in both CcLFY and other LFY homologues (Fig. 3). One is located at the amino (N) region of the proteins and the other is located in the carboxyl (C) region. The prolinerich region, as suggested by Coen et al. (1990), plays a critical role in transcriptional activation. However, Maizel et al. (2005) suggested that the C-terminal domain plays an essential role in DNA-binding activity and regulates the expression of downstream floral homologs by binding the sequences in their enhancers (Weigel and Nilsson 1995; Souer et al. 1998; Southerton et al. 1998; Lohmann and Weigel 2002). These regions are also conserved in CcLFY. These results indicate that the CcLFY protein possesses conserved domains that exhibit the same transcriptional regulating functions as the other angiosperm and gymnosperm homologs of LFY proteins.
To understand the evolutionary relationships between LFY genes, we constructed a phylogenetic tree of LFY amino acid sequences (Fig. 4). As expected, groups are formed by the sequences from Angiosperms (Fig. 4) and the genetic relationship between monocotyledon and gymnosperm is closer than that between dicotyledon and gymnosperm (data not shown). Southern blot analysis indicated that the CcLFY gene exists in the C. cathayensis genome as a single copy (Fig. 10). Generally, angiosperm species have one copy of the LFY gene while gymnosperm species have two copies. However, some angiosperm species such as apple (Wada et al. 2002), tobacco (Ahearn et al. 2001) and maize (Bomblies et al. 2003) have more than two copies. Among these copies of LFY genes, only one has been well characterized and no function was found for the other copy (Dornelas et al. 2004; Southerton et al. 1998). Frohlich and Estabrook (2000) reported that the copy number of the LFY gene in angiosperms tends to decrease. Therefore, the copy number of LFY homologous genes can, to a certain extent, also reflect the evolutionary relationship between plants.
Bradley et al. (1996) reported that LFY/FLO is not detected during the early vegetative phase in snapdragon (Antirrhinum majus), and its expression is confined to newly emerging floral primordia. In the rubber tree (H. brasiliensis), Dornelas and Rodriguez (2005b) also found that HbLFY expression was only restricted to reproductive tissues, and that HbLFY transcripts accumulated in the floral buds and floral organs, and in the vegetative-to-reproductive transition apex. A similar suggestion has been proposed by Bomblies et al. (2003) and Rottmann et al. (2000) for Populus and maize, respectively. Moreover, the seasonal alternating expression of LFY homologues has been reported for other woody species such as apple, kiwifruit, and grape (Walton et al. 2001; Carmona et al. 2002; Wada et al. 2002). However, Kelly et al. (1995) reported that LFY/NFL is expressed constitutively in tobacco (N. tabacum), with expression in the vegetative phase including emerging leaf primordia. Southerton et al. (1998) reported that ELF1, a homolog of LFY, is expressed strongly in the early floral primordium and then successively in the primordia of sepals, petals, stamens and carpels. It is also expressed in the leaf primordia, and in young leaves of adult and juvenile trees. Wada et al. (2002) reported that there are two orthologues of FLORICAULA/LEAFY in apple (Malus × domestica Borkh.): AFL1, which is expressed only in the floral bud during the transition from vegetative to reproductive growth, and AFL2, which is expressed in vegetative shoot apex, floral buds, floral organs and root. The same results in other woody species including E. grandis showed that EgLFY, the E. grandis homolog of the Arabidopsis gene LFY, is expressed in both reproductive and vegetative tissues (Dornelas et al. 2004). In our study, CcLFY expression was detected in reproductive tissues (Figs. 5, 6 and 7). The expression of CcLFY increased as organs expanded and the highest level of CcLFY expression corresponded to the time of flower meristem formation (Fig. 7). These results suggest that the expression of CcLFY might play an important role in the process of flower initiation in hickory tree, as do other angiosperm and gymnosperm homologs of most LFYlike genes.
We showed that all of these 35S:CcLFY transgenic tobacco plants produced oval leaves during the seedling stage compared with wild-type (Fig. 8). Kyozuka et al. (1998) also reported that transgenic Arabidopsis plants containing 35S-RFL, the FLO/LFY homolog in rice, had a variety of morphological abnormalities in vegetative organs. Lee et al. (1997) reported lobed leaves in transgenic Arabidopsis carrying 35S-UFO, and this phenotype required the presence of functional LFY. They also reported that a low level of LFY RNA expression is present in young leaf primordia of Arabidopsis. Their results and ours suggest that LFY plays an unknown role in the development of leaves. The expression of CcLFY may disturb the function of endogenous LFY in leaves. It will be interesting to see whether FLO/LFY homologs from other plant species cause phenotypes similar to 35S::CcLFY when they are ectopically expressed in tobacco plants. Although the conservation of sequences indicates that CcLFY and FLO/LFY arose from a common ancestral gene, diversification of both their functions and the regulation of their expression have occurred during evolution.
To better understand the biological role of CcLFY during flower development, we investigated the effect of a gymnosperm CcLFY gene on angiosperm flowering by introducing the CcLFY sequence into the tobacco genome. Although we observed normal flowers on 35S::CcLFY transgenic plants, the early-flowering phenotype observed in 35S::CcLFY transgenic tobacco plants (Fig. 9) was similar to that in transgenic tobacco plants with CjNdly from Cryptomeria japonica plants (Shiokawa et al. 2008) and with a chimeric NFL1 gene (Ahearn et al. 2001), and in transgenic Arabidopsis plants with NLY from radiata pine (Mouradov et al. 1998) and with ELF1 from Eucalyptus plants (Dornelas et al. 2004). Wada et al. (2002) also reported that transgenic Arabidopsis which overexpressed AFL2 of apple (Malus × domestica Borkh.) showed accelerated flowering and directly gave rise to several solitary flowers from rosette axils. AFL1 had similar effects, but the phenotypes of transgenic Arabidopsis with AFL1 were weaker than those with AFL2. These results suggested that CcLFY can act as a meristem identity gene in angiosperms, similar to the AFL, NLY and CjNdly genes isolated from gymnosperms.
To study how CcLFY specifically recognizes its target DNA sequences, we constructed a structural model based on SWISS-MODEL Workspace (http://swissmodel.expasy.org). We found that CcLFY has a seven-helix fold (Fig. 11), similar to Arabidopsis LFY (Hamès et al. 2008). Hamès et al. (2008) crystallized LFY-C from Arabidopsis in complex with DNA, and the structure they identified showed a novel protein fold, made of seven alpha helices, which bind DNA as a cooperative dimer, to form base-specific contacts in both the major and minor grooves. This cooperative binding mechanism was proposed to contribute to the sharp induction of flowering (Moyroud et al. 2009).
Our results showed that CcLFY may play a pivotal role in the transition from the vegetative to the reproductive phase in C. cathayensis, which is consistent with other reports (Weigel et al. 1992; Blázquez et al. 1997). The photoperiod and vernalization contribute to the expression of CcLFY and may integrate external signals into the decision to flower. An increased understanding of flower development in C. cathayensis should eventually contribute to improving breeding efficiency and increasing products through biotechnological manipulation. If we can control meristem-identity genes such as CcLFY with the use of reverse genetics, we might be able to further characterize the role of CcLFY during reproductive development in hickory tree.
References
Ahearn KP, Johnson HA, Weigel D, Wagner DR (2001) NFL1, a Nicotiana tabacum LEAFY-like gene, controls meristem initiation and floral structure. Plant Cell Physiol 42:1130–1139
Altschul J, Madden TL, Schffer AA, Zhang J, Zhang Z, Miler W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Blázquez MA, Soowal LN, Lee I, Weigel D (1997) LEAFY expression and flower initiation in Arabidopsis. Development 124:3835–3844
Bomblies K, Wang RL, Ambrose BA, Schmidt RJ, Meeley RB, Doebley J (2003) Duplicate FLORICAULA/LEAFY homologs zfl1 and zfl2 control inflorescence architecture and flower patterning in maize. Development 130:2385–2395
Bradley D, Carpenter R, Copsey L, Vincent C, Rothstein S, Coen ES (1996) Control of inflorescence architecture in Antirrhinum. Nature 379:791–797
Bustin SA, Beaulieu JF, Hugget J, Jaggi R, Kibenge FSB, Olsvik PA, Penning LC, Toegel S (2010) MIQE precis: practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol Biol 11:74–78
Carmona MJ, Cubas P, Martinez-Zapater JM (2002) VFL, the grapevine FLORICAULA/LEAFY ortholog, is expressed in meristematic regions independently of their fate. Plant Physiol 130:68–77
Chang L, Wu L, Chen Y, Ku L, Yang S, Zhang S, Wang X, Wei X (2011) Expression and functional analysis of the ZCN1 (ZmTFL1) gene, a TERMINAL FLOWER 1 homologue that regulates the vegetative to reproductive transition in maize. Plant Mol Biol Rep. doi:10.1007/s11105-011-0317-2
Coen ES, Romero JM, Elliot R (1990) FLORICAULA: a homeotic gene required for flower development in Antirrhinum majus. Cell 63:1311–1322
Dornelas MC, Rodriguez APM (2005a) A FLORICAULA/LEAFY gene homolog is preferentially expressed in developing female cones of the tropical pine Pinus caribaea var caribaea. Gen Mol Biol 28:299–307
Dornelas MC, Rodriguez APM (2005b) The rubber tree (Hevea brasiliensis MuellArg) homologue of the LEAFY/FLORICAULA gene is preferentially expressed in both male and female floral meristems. J Exp Bot 56:1965–1974
Dornelas MC, Rodriguez APM (2006) The tropical cedar tree (Cedrela fissilis Vell, Meliaceae) homolog of the Arabidopsis LEAFY gene is expressed in reproductive tissues and can complement Arabidopsis leafy mutants. Planta 223:306–314
Dornelas MC, Wittich PE, von Recklinghausen IR, van Lammeren AAM, Kreis M (1999) Characterization of three novel members of the Arabidopsis SHAGGY-related protein kinases (ASK) multigene family. Plant Mol Biol 39:137–147
Dornelas MC, Van Lammeren AA, Kreis M (2000) Arabidopsis thaliana SHAGGY-related protein kinases (AtSK11 and 12) function in perianth and gynoecium development. Plant J 21:419–429
Dornelas MC, Amaral WN, Rodriguez APM (2004) EgLFY, the Eucalyptus grandis homolog of the Arabidopsis gene LEAFY is expressed in reproductive and vegetative tissues. Braz J Plant Physiol 16:105–114
Du N, Pijut PM (2010) Isolation and characterization of an AGAMOUS homolog from Fraxinus pennsylvanica. Plant Mol Biol Rep 28:344–351
Frohlich MW, Estabrook GF (2000) Wilkinson support calculated with exact probabilities: an example using Floricaula/LEAFY amino acid sequences that compares three hypotheses involving gene gain/loss in seed plants. Mol Biol Evol 17:1914–1925
Hamès C, Ptchelkine D, Grimm C, Thevenon E, Moyroud E, Gérard F, Martiel JL, Benlloch R, Parcy F, Müller CW (2008) Structural basis for LEAFY floral switch function and similarity with helix-turn-helix proteins. EMBO J 27:2628–2637
Hou JH, Gao ZH, Zhang Z, Chen SM, Ando T, Zhang JY, Wang XW (2011) Isolation and characterization of an AGAMOUS homologue PmAG from the Japanese apricot (Prunus mume Sieb. et Zucc.). Plant Mol Biol Rep 29:473–480
Huang Y, Xia G, Wang Z, Zheng B, Liang J, Huang J (2007) Studies on anatomy of development of female flower in Carya cathayensis Sarg. Acta Agriculturae Universitatis Jiangxiensis 29(5):723–726
Kelly AJ, Bonnlander MB, Meeks-Wagner DR (1995) NFL, the tobacco homolog of FLORICAULA and LEAFY, is transcriptionally expressed in both vegetative and floral meristems. Plant Cell 7:225–234
Kyozuka J, Konishi S, Nemoto K, Izawa T, Shimamoto K (1998) Down-regulation of RFL, the FLO/LFY homolog of rice, accompanied with panicle branch initiation. Proc Natl Acad Sci USA 95(5):1979–1982
Lee J, Lee I (2010) Regulation and function of SOC1, a flowering pathway integrator. J Exp Bot 61:2247–2254
Lee I, Wolfe DS, Nilsson O, Weigel D (1997) A LEAFY co-regulator encoded by UNUSUAL FLORAL ORGANS. Curr Biol 7(2):95–104
Li H, Liu F, Liu G, Wang S, Guo X, Jing J (2011) Molecular cloning and expression analysis of 13 MADS-Box genes in Betula platyphylla. Plant Mol Biol Rep. doi:10.1007/s11105-011-0326-1
Liu C, Thong Z, Yu H (2009) Coming into bloom: the specification of floral meristems. Development 136:3379–3391
Liu X, Anderson JM, Pijut PM (2010) Cloning and characterization of Prunus serotina AGAMOUS, a putative flower homeotic gene. Plant Mol Biol Rep 28:193–203
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Lohmann JU, Weigel D (2002) Building beauty: the genetic control of floral patterning. Dev Cell 2:135–142
Ma G, Ning G, Zhang W, Zhan J, Lv H, Bao M (2011) Overexpression of Petunia SOC1-like gene FBP21 in tobacco promotes flowering without decreasing flower or fruit quantity. Plant Mol Biol Rep 29:573–581
Maizel A, Busch MA, Tanahashi T, Perkovic J, Kato M, Hasebe M, Weigel D (2005) The floral regulator LEAFY evolves by substitutions in the DNA binding domain. Science 308:260–263
Mellerowicz EJ, Horgan K, Walden A, Coker A, Walter C (1998) PRFLL—a Pinus radiata homologue of FLORICAULA and LEAFY is expressed in buds containing vegetative shoot and undifferentiated male cone primordia. Planta 206:619–629
Moon J, Lee H, Kim M, Lee I (2005) Analysis of flowering pathway integrators in Arabidopsis. Plant Cell Physiol 46:292–299
Mouradov A, Glassic T, Hamdorf B, Murphy L, Fowler B, Marla S, Teasdale RD (1998) NEEDLY, a Pinus radiata ortholog of FLORICAULA/LEAFY genes, expressed in both reproductive and vegetative meristems. Proc Natl Acad Sci USA 95:6537–6542
Mouradov A, Cremer F, Coupland G (2002) Control of flowering time: interacting pathways as a basis for diversity. Plant Cell 14:S111–S130
Moyroud E, Tichtinsky G, Parcy F (2009) The LEAFY floral regulators in Angiosperms: conserved proteins with diverse roles. J Plant Biol 52:177–185
Moyroud E, Kusters E, Monniaux M, Koes R, Parcy F (2010) LEAFY blossoms. Trends Plant Sci 15:346–352
Peña L, Martin-Trillo M, Juárez J, Pina JA, Navarro L, Martínez-Zapater JM (2001) Constitutive expression of Arabidopsis LEAFY or APETALA1 genes in citrus reduces their generation time. Nat Biotechnol 19:263–267
Rottmann WH, Meilan R, Sheppard LA, Brunner AM, Skinner JS, Ma C, Cheng S, Jouanin L, Pilate G, Strauss SH (2000) Diverse effects of over-expression of LEAFY and PTLF, a poplar (Populus) homolog of LEAFY/FLORICAULA, in transgenic poplar and Arabidopsis. Plant J 22:235–245
Sambrook J, Fritsch FP, Maniatis T (1989) Molecular cloning. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Shiokawa T, Yamada S, Futamura N, Osani K, Murasugi D, Shinohara K, Kawai S, Morohoshi N, Katayama Y, Kajita S (2008) Isolation and functional analysis of the CjNdly gene, a homolog in Cryptomeria japonica of FLORICAULA/LEAFY genes. Tree Physiol 28:21–28
Simpson GG, Dean C (2002) Arabidopsis, the Rosetta stone of flowering time? Science 296:285–289
Souer E, Vander K, Kloos D, Spelt C, Bliek M (1998) Genetic control of branching pattern and floral identity during Petunia inflorescence development. Development 125:733–742
Southerton SG, Marshall H, Mouradov A (1998) Eucalyptus MADS-box genes expressed in developing flowers. Plant Physiol 118:365–372
Tai TH, Tanksley SD (1990) A rapid and inexpensive method for isolation of total DNA from dehydrated plant tissue. Plant Mol Biol Rep 8:297–303
Taiz L, Zeiger E (2006) Plant physiology, 4th edn. Sinauer Associates Inc., Sunderland MA
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Wada M, Cao QF, Kotoda N, Soejima J, Masuda T (2002) Apple has two orthologues of FLORICAULA/LEAFY involved in flowering. Plant Mol Bio 49:567–577
Walton EF, Podivinsky E, Wu RM (2001) Bimodal pattern of floral gene expression over the two seasons that kiwifruit flowers develop. Physiol Plant 111:396–404
Wang SX, Hunter W, Plant A (2000) Isolation and purification of functional total RNA from woody branches and needles of Sitka and white spruce. Biotechniques 28:292–296
Weigel D, Nilsson O (1995) A developmental switch sufficient for flower initiation in diverse plants. Nature 377:495–500
Weigel D, Alvarez J, Smyth DR, Yanofsky MF, Meyerowitz FM (1992) LEAFY controls floral meristem identity in Arabidopsis. Cell 4:910–913
Xu J, Zhong X, Zhang Q, Li H (2010) Overexpression of the GmGAL2 gene accelerates flowering in Arabidopsis. Plant Mol Biol Rep 28:704–711
Yamada S, Kajita S, Shiokawa T, Morohoshi N (2003) Isolation and functional analysis of the promoter sequence of the Cryj1 gene, which encodes a major allergenic protein in the pollen of Japanese cedar (Cryptomeria japonica). Plant Biotechnol 20:241–245
Zhang A, Qiu L, Huang L, Yu X, Lu G, Cao J (2011) Isolation and characterization of an anther-specific polygalacturonase gene, BcMF16, in Brassica campestris ssp. chinensis. Plant Mol Biol Rep 2011. doi:10.1007/s11105-011-0341-2
Zheng BS, Chu HL, Jin SH, Huang YJ, Wang ZJ, Chen M, Huang JQ (2010) cDNA-AFLP analysis of gene expression in hickory (Carya cathayensis) during graft process. Tree Physiol 30:297–303
Acknowledgements
This work was supported by the National Natural Science Foundation of China (30872047, 31170637 and 31070604), the initial project of the National Basic Research Program of China (2011CB111510), the Zhejiang Provincial Natural Science Foundation of China (Z307534 and Y305331), the Key Project of the Department of Science and Technology of Zhejiang Province (2007C12023) and the Innovation Team Project of Zhejiang A & F University (Class B) (2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Z.J., Huang, J.Q., Huang, Y.J. et al. Cloning and Characterization of a Homologue of the FLORICAULA/LEAFY Gene in Hickory (Carya cathayensis Sarg). Plant Mol Biol Rep 30, 794–805 (2012). https://doi.org/10.1007/s11105-011-0389-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-011-0389-z