Abstract
Fertilization treatments can promote the growth and development of tree plants. In the present study, we evaluated the combined effects of different doses of nitrogen, phosphorous, and potassium on the blossoming and the expression of five flowering-associated genes, including BpMADS1, BpMADS3, BpMADS5, BpSPL1, and BpSPL2. The results showed that balanced NPK (nitrogen, phosphorous, and potassium) fertilization cannot only promote the transition from juvenility to maturity but also can increase the inflorescence production of white birch. Furthermore, some fertilizing treatments not only increase the expression levels of all the five flowering-associated genes, but also change their expression patterns during the growth season. These results indicated that NPK fertilization have remarkable effects on flowering of white birch.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitrogen (N), phosphorus (P), and potassium (K) are essential macronutrients for plant growth and development. Application of NPK (nitrogen, phosphorous, and potassium) fertilizers can improve the growth condition of the soil and promote the growth and development of tree plants. Furthermore, improved mineral nutrition in plants is often considered to induce flowering. NPK fertilization has been reported to promote the transition from juvenility to maturity (Zimmerman 1971; Aldwinckle 1975) and increase floral induction (McArthur and Eaton 1989).
Flower formation in plants is regulated by a network of many genes, most of which encode transcription factors. Many of them belong to the MADS-box gene family, such as AP1 (Yanofsky 1995), LEY (Simon et al. 1996; Guo and Qing 2008), SEPALLATA (Pelaz et al. 2000; Honma and Goto 2001), SOC1 (Ferrario et al. 2004), FLC (Poduska et al. 2003; Rouse et al. 2002), AGL11 (Xu et al. 2010), AGAMOUS (Yanofsky et al. 1990; Liu et al. 2010), and FUL (Yanofsky 1995). Plant MADS-box proteins contain a DNA-binding (M), an intervening (I), a keratin-like, and a C-terminal C-domain; thus, plant MADS-box proteins are of the MIKC type (Saedler et al. 2001). Some of these genes are involved in the transition of the vegetative apical meristem to an inflorescence meristem and further to a floral meristem, which finally results in the determination and development of floral organs. BpMADS1, BpMADS3, and BpMADS5 are the three MADS-box genes in birch, and they are similar in sequence to the genes of SEPALLATA3, AP1, and FUL from Arabidopsis, respectively. All these genes play roles in flower formation (Elo et al. 2001; Lemmetyinen et al. 2004). SQUAMOSA promoter-binding proteins (SBP)-box genes were first characterized as SBPs that regulated the expression of MADS-box genes in early flower development in Antirrhinum majus (Klein et al. 1996). Since then, SBP-box genes have been identified in many plants such as moss, silver birch, Arabidopsis, and maize. These genes play critical roles in regulating flower and fruit development as well as other physiological processes (Moreno et al. 1997; Cardon et al. 1999; Lannenpaa et al. 2004; Arazi et al. 2005). BpSPL1 and BpSPL2 are the 2 SBP-box genes in birch. SBP-domain protein binding sites were found in the promoters of Arabidopsis APETALA1 and Antirrhinum SQUAMOSA; in silver birch, a putative SBP-domain binding element has been observed in the promoter of BpMADS5. These 2 SBP-box genes in birch are involved in the regulation of flower development (Lannenpaa et al. 2004; Wei 2006).
White birch (Betula platyphylla) is one of the most economically important forest trees in the boreal region in China. Birch is a monoecious tree with separate male and female inflorescences (Macdonald and Mothersill 1986). The development of birch inflorescences starts about a year before the opening of flowers, but the anthesis takes place only during the following spring, after overwintering. The male inflorescences begin to develop early in the spring 1 year before flowering. In Harbin, the male inflorescences emerge around early June. Development of the female inflorescences begins in June–July, and the inflorescences overwinter inside buds, which open the following May (Li 2004). Birch trees are very large and long-lived trees with a juvenile period of 5–15 years. White birch genetic improvement is gaining more attention, but its long generation time severely hinder the efforts in classical breeding and genetic studies (Wei et al. 2010). The study of birch flower development is important from the point of view of both breeding and molecular biotechnology.
The aim of the present study was to show the influence of NPK fertilization on the transition from juvenility to maturity and on the production of inflorescences in white birch. To this end, we studied the effects of NPK fertilization on the expression levels and temporal expression profiles of five flowering-associated genes and aimed to identify the functions of these genes in flower formation.
Materials and Methods
Plant Growth and Fertilization Treatment
White birch seedlings from a half-sib family were grown in plastic containers (length, 50 cm; width, 40 cm; depth, 40 cm) filled with mixture of turf peat and sand (3:1 v/v) in a greenhouse at the Northeast Forestry University. The amounts of N, P, and K applied to each group of seedlings between 2006 and 2007 are given in Table 1. After birch germination in early May, the birch seedlings were fertilized every 15 days till the end of September each year. The fertilization treatment period was divided into 3 parts: spring (May 1–June 30), summer (July 15–August 14), and autumn (September 1–30); the NPK ratios for different treatment groups were kept constant in each period. At each fertigation, 2-year-old birch plants were given 0.5 L of nutrient solution per seedling and 3- and 4-year-old birch plants were given 1 L of nutrient solution. The amount of fertilizer was adjusted by increasing the fertilizer doses according to the increasing demand of the plants during the 2 years of growth. The NPK fertilization treatment used in this study was consulted with our previous research (Li et al. 2009).
Plant Materials
In 2007, apical buds from lateral branches were harvested in the beginning of each month from May to September. For each sampling, one bud was collected from each seedling. In total, 36 buds from each treatment group were collected and immediately frozen in liquid nitrogen. All the samples were kept at −80°C until use.
RNA Isolation and cDNA synthesis
Total RNA was extracted from buds of B. platyphylla seedlings by using a cetyltrimethylammonium bromide (CTAB) method. In brief, bud material was ground into powder with a pestle in a mortar containing liquid nitrogen. The powder was mixed with 600 μl of extraction buffer (0.1 M TRIS, 1.4 M NaCl, 0.01 M EDTA, 20% CTAB (w/v), and pH 8) and 100 μl of β-mercaptoethanol. This mixture was spun for 30 s. After addition of 600 μl of chloroform/phenol (1:1), the mixture was centrifuged at 12,000×g for 5 min at 4°C. One volume of 4 M LiCl solution was added to the supernatant, and the mix was incubated at 4°C for 30 min. After centrifugation (12,000×g, 15 min, 4°C), the pellet was suspended in 100 μl of sterile water. To precipitate total RNA, 30 μl of 3 M sodium acetate (pH 5.6) and 300 μl of absolute ethanol were added, and left for 1 h at −80°C. After centrifugation (12,000×g, 15 min, 4°C), the pellet was washed with 1 ml of 70% ethanol (v/v), then centrifuged at 12,000×g for 5 min at 4°C. The resulting pellet was dried and suspended in sterile water. To remove DNA contamination, total RNA was then treated with RNase-free DNase (TaKaRa) according to the manufacturer’s protocol. The quantity and the quality of the RNA samples were analyzed using a spectrophotometer (Eppendorf) and by running test gels with ethidium bromide staining. First-strand cDNA was synthesized from 1 μg of total RNA using a BD Smart™ rapid amplification of cDNA ends (RACE) cDNA Amplification Kit (BD Biosciences) and diluted with 10-fold before used in real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR) assays.
Primer Design and qRT-PCR Analysis
Gene-specific primers for the qRT-PCR analysis were designed using the Primer Premier Version 5.0 (Table 2). The 18S rRNA was used as internal reference, with the primer sequence as follows: forward, 5′-ATCTTGGGTTGGGCAGATCG-3′; reverse, 5′-CATTACTCCGATCCCGAAGG-3′. RT-PCR was repeated three times for each sample with SYBR-Green Real-time PCR Master Mix (Toyobo) on a DNA Engine Opticon 2 (MJ Research) in accordance with the manufacturer’s recommendations. Each reaction mixture contained 1 μl of diluted cDNA template, 500 nM of each primer, and 2× SYBR-Green Real-time PCR Master mix in a final volume of 12 μl. The amplification was performed as the following cycling parameters: 94°C for 30 s followed by 45 cycles at 94°C for 12 s, 60°C for 30 s, 72°C for 40 s, and 1 s at 78.5°C for plate reading (Gao et al. 2010). Specific primers for 18S rRNA were used as the internal controls for the normalization of the RNA steady-state level, and the relative changes in gene expression were quantified using the \( 2^{{ - \Delta \Delta {\rm{CT}}}} \) method (Livak and Schmittgen 2001).
Data Collection and Statistical Analysis
After 2 years of fertilization treatment, we counted the number of male and female inflorescences (catkins) in spring and July in 2008. We also counted the number of plants with inflorescences and calculated the ratio of the mature and total trees in each treatment group. Statistical analysis of the data was performed using Statistical Package for Social Sciences (SPSS) version 16.0.1 (SPSS Inc., USA) software. To evaluate the potential effects of the different fertilization treatments on the production of male and female inflorescences, we used the analysis of variance to test differences between the results for different treatments in seedlings of different ages. Pearson correlation coefficients were calculated to characterize the relationships between the productions of inflorescences and the total relative expression levels of the five flowering-associated genes in 5 months.
Results
Number of Inflorescences Induced by Different Fertilization Treatments
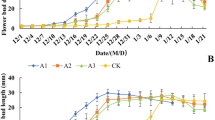
To better understand the relationship between fertilization and blossoming of white birch, we investigated the numbers of male and female inflorescences in the different treatment groups. Group A, which contained 144 2-year-old birch seedlings, was divided into four subgroups that were treated with different fertilizers as shown in Table 1. Group B, which contained the same number of 3-year-old birch seedlings was treated with the protocol mentioned above. The four treatments for group A were named as A0 (control), A1, A2, and A3, respectively. The four treatments for group B seedlings were named B0 (control), B1, B2, and B3. After 2 years of fertilization treatment, the average and median inflorescence numbers for each subgroup were determined. The differences in the number of inflorescences among the 4 treatment subgroups (including control) of the seedlings of different ages were significant (P < 0.01) (Table 3). For the seedlings in group A, the number of male and female inflorescences responded markedly to the addition of fertilizer. In subgroup A3, the average numbers of male and female inflorescences per plant were 98 and four, respectively, while in subgroup A0 (control), the average numbers of male and female inflorescences per plant were only 1 and 0, respectively (Fig. 1a). Fertilization also had a major effect on increasing the numbers of inflorescences in seedlings in group B (Fig. 1b). The average number of male and female inflorescences per plant in subgroup B2 were respectively 8.43-fold and 3.67-fold higher than those in the control subgroup. Treatment B2 and B3 were more effective than treatment B1, and the difference between the effects of treatments B2 and B3 was not significant, although the fertilizer dosage in treatment B3 was higher than that in treatment B2.
Ratios of the Mature Trees and Total Trees Under Different Fertilization Treatments
To better understand the influence of fertilization on the transition from juvenility to maturity of birch seedlings, we counted the number of plants with inflorescences and calculated the ratio between the number of mature and total trees in each treatment group (Fig. 2). For group A, the ratios in subgroups A0 (control), A1, A2, and A3 were 3%, 22%, 69%, and 92%, respectively. The ratios of subgroups in group A were directly proportional to the dosage of fertilizer. For group B, the ratio of subgroups B1, B2, and B3 were 94%, 94%, and 97%, respectively, and the differences between these ratios were insignificant. The ratio in subgroup B0 (control; 33%) was much lower than those in the treatment groups.
Total Expression of the Five Flowering-Associated Genes Under Different Fertilization Treatments in 5 Months
In order to study the relationship between the fertilization treatments and the expression of the five flowering-associated genes (BpMADS1, BpMADS3, BpMADS5, BpSPL1 and BpSPL2), we investigated the relative expression levels of these genes in the growth season (from May to September). For subgroups of group A, the tree-fertilization treatments, particularly treatment A3, dramatically increased the total expression levels of each gene, which was consistent with the results of the influences of different fertilization treatments on the number of inflorescences in the next year (Fig. 3a). For group B, the total expression levels of the five genes in the treatment groups were higher than those in the control group, except the expression of BpMADS3 under treatment B1 (Fig. 3b). For BpMADS1, treatment B2 was most effective, but for BpMADS3 and BpSPL1, treatment B3 was much better than the others. However, for BpMADS5 and BpSPL2, there was no significant difference among the three treatments. In summary, treatment B2 or B3 was the most effective treatment for the seedlings in group B, and this was consistent with the result of the influences of different fertilization treatments on the number of inflorescences in the next year. Correlation analysis also showed that the total expression levels of these five genes were positively correlated with the numbers of inflorescences in the next year (Table 4).
Temporal Expression of the Five Flowering-Associated Genes Under Fertilization and Non-fertilization
Temporal gene expression profiles in a given biological process can often provide more insights into how gene expression levels evolve in time and how genes are dependent in a given biological process (Luan and Li 2003). Therefore, we evaluated the time-course expression profiles of the five flowering-associated genes at May, June, July, August, and September under the conditions of fertilization treatment and non-fertilization treatment using real-time PCR. The temporal expression profiles of BpMADS1, BpMADS3, BpMADS5, and BpSPL1 of the unfertilized seedlings in group A were similar (Fig. 4a–d). Their expression levels all peaked in early July and early September. Treatment A3 induced high expression levels of the four genes in early June. However, the expression profile of BpSPL2 in the unfertilized group A seedlings was different from those of the other four genes: BpSPL2 expression level kept increasing from early May to September (Fig. 4e). The expression of BpSPL2 was induced by treatment A3, and reached a peak in early June and early August. In the unfertilized group B seedlings (Fig. 4a–e), the expressions of the five genes peaked in early September, then decreased, and again peaked at early June (BpSPL2) or July (BpMADS1, BpMADS3, BpMADS5, and BpSPL1). However, the peak levels of the five genes induced by treatment B3 markedly decreased in early September.
Discussion
Improved mineral nutrition is often considered a flower-inducing treatment.For instance, Zimmerman (1971) was able to reduce the time to first flowering in tea crabapple (Malus hupehensis Rehd.) seedlings from 3 years to 9.5 months by growing them continuously under extended photoperiods in a greenhouse along with a weekly treatment of 20–20–20 (N–P–K) water-soluble fertilizer. Aldwinckle (1975) obtained nearly identical results with apple. Zimmerman (1972) considered this flower induction response to be largely a result of faster growth. Kolar and Senkova (2008) reported that flowering might be promoted by a separate induction pathway, by changes in sensitivity to day length or by processes associated with changes in leaf growth and development during nutrient stress. Our results showed that NPK fertilization treatments increased the ratio of mature trees and total trees in white birch, suggesting that rational fertilization can promote floral induction. Although all ratios of the mature and total trees in subgroups A3, B1, B2, and B3 exceeded 90%, the fertilizer dose of treatment B1, which contained the lowest amount of fertilizer, for 5-year-old seedlings was less than that of treatment A3. These results suggested that the fertilizer dosage required to induce maturity in over 90% of the seedlings much higher for 4-year-old birch seedlings than that for 5-year-old birch seedlings. We also found that treatment B1 was less effective for increasing the production of inflorescence than treatment B2 and treatment B3 were, although the effects of these treatments on the ratio of the mature trees and total trees were not markedly different. These findings suggested that rational NPK fertilization cannot only promote the transition from juvenility to maturity but also can increase inflorescence production in white birch.
The development of birch inflorescences starts about a year before the opening of flowers, but the anthesis takes place only during the following spring, after overwintering. Therefore, the expression of these genes might be correlated with the yield of the next year, which was consistent well with our correlation analysis (Table 4). Although treatment B1 was as effective for promoting the transition from juvenility to maturity as treatment B2 and treatment B3 were, its ability to increase the production of inflorescence was obviously lower than that of the other two treatments. On the basis of this finding, we could conclude that if all the three fertilization treatments (B1, B2, and B3) elevated the expression level of the target gene and the differences in the effects were not prominent, the genes, such as BpMADS5 and BpSPL2, were possibly involved in flower initiation. However, if treatment B2 or treatment B3 was the optimum treatment and if its influence was better than that of treatment B1, then the genes, such as BpMADS1, BpMADS3, or BpSPL1, were probably linked to both flower initiation and inflorescence as well as flower development, or just to inflorescence and flower development. This finding was consistent with the previous studies, which reported that BpMADS1 participates both in inflorescence and flower formation (Lemmetyinen et al. 2004), and that BpMADS3 and BpMADS5 might be involved in the determination of the identity of the inflorescence or flower meristem (Elo et al. 2001). BpSPL1 is similar to Arabidopsis SPL3, except the unique characteristic that BpSPL1 does not contain an intron typical to all other known SBP-box genes studied thus far (Lannenpaa et al. 2004). BpSPL2, which is similar to Arabidopsis SPL4, was cloned using RACE (Wei 2006). Although both genes are involved in the regulation of flower development in birch, their functions remain to be explained.
In this study, the temporal expression profiles of the five flowering-associated genes in unfertilized plants during the annual growing period were studied. We found that the expression levels of BpMADS1, BpMADS3, BpMADS5, and BpSPL1 in groups A and B seedlings reached o peak level in early July and September. In early July, the male inflorescences emerge, while female inflorescences start to develop but remain inside the buds during the winter. We examined apical buds from lateral branches, and BpMADS1, BpMADS3, BpMADS5, and BpSPL1 were expressed in the developing female inflorescence (Elo et al. 2001; Lannenpaa et al. 2004; Lemmetyinen et al. 2004). Therefore, the expression level of the genes in early July might be associated with female inflorescence. Treatment A3 induced high expression levels of the genes in early June, suggesting that fertilization could accelerate growth and development of birch. Another interesting phenomenon in our study is that the peak expression levels of the five genes in early September dramatically decreased in subgroup B3, implying that these five genes may be involved in flowering. The phenomenon in subgroup B3 is still needed to be explained. Therefore, further studies are needed to determine their functions in birch.
References
Aldwinckle HS (1975) Flowering of apple seedlings 16–20 months after germination. HortScience 10:124–126
Arazi T, Talmor-Neiman M, Stav R, Riese M, Huijser P, Baulcombe DC (2005) Cloning and characterization of micro-RNAs from moss. Plant J 43:837–848
Cardon G, Hohmann S, Klein J, Nettesheim K, Saedler H, Huijser P (1999) Molecular characterisation of the Arabidopsis SBP-box genes. Gene 237:91–104
Elo A, Lemmetyinen J, Turunen ML, Tikka L, Sopanen T (2001) Three MADS-box genes similar to APETALA1 and FRUITFULL from silver birch (Betula pendula). Physiol Plant 112:95–103
Ferrario S, Busscher J, Franken J, Gerats T, Vandenbussche M, Angenent GC, Immink RG (2004) Ectopic expression of the Petunia MADS box gene UNSHAVEN accelerates flowering and confers leaf-like characteristics to floral organs in a dominant negative manner. Plant Cell 16:1490–1505
Gao C, Wang Y, Liu G, Wang C, Jiang J, Yang C (2010) Cloning of ten peroxidase (POD) genes from Tamarix hispida and characterization of their responses to abiotic stress. Plant Mol Biol Rep 28:77–89
Guo JL, Qing Y (2008) Molecular cloning and expression analysis of a LFY homologous gene from potato. Plant Mol Biol Rep 26:324–334
Honma T, Goto K (2001) Complexes of MADS-box proteins are sufficient to convert leaves into floral organs. Nature 409:525–529
Klein J, Saedler H, Huiser P (1996) A new family of DNA binding proteins includes putative transcriptional regulators of the Antirrhinum majus floral meristem identity gene SQUAMOSA. Mol Gen Genet 250:7–16
Kolar J, Senkova J (2008) Reduction of mineral nutrient availability accelerates flowering of Arabidopsis thaliana. J Plant Physiol 165:1601–1609
Lannenpaa M, Janonen I, Holtta-Vuori M, Gardemeister M, Porali I, Sopanen T (2004) A new SBP-box gene BpSPL1 in silver birch (Betula pendula). Physiol Plant 120:491–500
Lemmetyinen J, Hassinen M, Elo A, Porali I, Keinonen K, Mäkelä H, Sopanen T (2004) Functional characterisation of SEPALLATA3 and AGAMOUS orthologues in silver birch. Physiol Plant 121:149–162
Li TH (2004) The morphological and anatomic study and analysis on change of endogenous hormones during Birch reproductive development. MD dissertation, Northeast Forestry University, Harbin, China
Li TF, Jiang J, Wang L, Zhu ZB, Mu HZ, Yang CP, Liu GF (2009) The effects on the growth of different families of Betula Platyphylla seedling caused by prescription fertilization. Sci Silvae Sinicae 45:60–64
Liu X, Anderson JM, Pijut PM (2010) Cloning and characterization of Prunus serotina AGAMOUS, a putative flower homeotic gene. Plant Mol Biol Rep 28:193–203
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 -ΔΔCT method. Methods 25:402–408
Luan Y, Li H (2003) Clustering of time-course gene expression data using a mixed-effects model with B-splines. Bioinformatics 19:474–482
Macdonald AD, Mothersill DH (1986) Shoot development in Betula papyrifera.VI. Development of the reproductive structures. Can J Bot 65:466–475
McArthur DAJ, Eaton GW (1989) Cranberry growth and yield response to fertilizer and paclobutrazol. Sci Hortic 38:131–146
Moreno MA, Harper LC, Krueger RW, Dellaporta SL, Freeling M (1997) Liguleless1 encodes a nuclear-localized protein required for induction of ligules and auricles during maize leaf organogenesis. Genes Dev 11:616–628
Pelaz S, Ditta GS, Baumann E, Wisman E, Yanofsky MF (2000) B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 405:200–203
Poduska B, Humphrey T, Redweik A, Grbic V (2003) The synergistic activation of FLOWERING LOCUS C by FRIGIDA and a new flowering gene AERIAL ROSETTE 1 underlies a novel morphology in Arabidopsis. Genetics 163:1457–1465
Rouse DT, Sheldon CC, Bagnall DJ, Peacock WJ, Dennis ES (2002) FLC, a repressor of flowering, is regulated by genes in different inductive pathways. Plant J 29:183–191
Saedler H, Becker A, Winter KU, Kirchner C, Theissen G (2001) MADS-box genes are involved in floral development and evolution. Acta Biochemica Pologica 48:351–358
Simon R, Igeno MI, Coupland G (1996) Activation of floral meristem identity genes in Arabidopsis. Nature 384:59–62
Wei JC (2006) Construction of suppression subtracted cDNA libraries of Betula platyphylla in flower phase and cloning of genes related to flower. Ph.D. dissertation, Northeast Forestry University, Harbin, China
Wei Z, Zhang K, Yang C, Liu G, Liu G, Lian L, Zhang H (2010) Genetic linkage maps of Betula platyphylla Suk based on ISSR and AFLP markers. Plant Mol Biol Rep 28:169–175
Xu J, Zhong X, Zhang Q, Li H (2010) Overexpression of the GmGAL2 gene accelerates flowering in Arabidopsis. Plant Mol Biol Rep 28:704–711
Yanofsky MF (1995) Floral meristems to floral organs: genes controlling early events in Arabidopsis flower development. Annu Rev Plant Physiol Plant Mol Biol 46:167–188
Yanofsky MF, Ma H, Bowman JL, Drews GN, Feldmann KA, Meyerowitz EM (1990) The protein encoded by the Arabidopsis homeotic gene AGAMOUS resembles transcription factors. Nature 346:35–39
Zimmerman RH (1971) Flowering in crab apple seedlings: methods of shortening the juvenile phase. J Am Soc Hort Sci 96:404–411
Zimmerman RH (1972) Juvenility and flowering in woody plants: a review. HortScience 7:447–455
Acknowledgments
This work has been supported by Special Research Funds for Public Welfare Forestry (No. 200904039) and Graduate assistantship of Northeast Forestry University (No. GRAM09).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, S., Jiang, J., Li, T. et al. Influence of Nitrogen, Phosphorus, and Potassium Fertilization on Flowering and Expression of Flowering-Associated Genes in White Birch (Betula platyphylla Suk.). Plant Mol Biol Rep 29, 794–801 (2011). https://doi.org/10.1007/s11105-010-0281-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-010-0281-2