Abstract
H+-ATPase subunit c (VHA-c) is involved in the adaptation to environmental stresses, including salt, drought, and heavy metals. However, it remains unclear whether VHA-c can induce a physiological response related to stress tolerance. To investigate this possibility, we generated transgenic tobacco lines overexpressing a V-ATPase subunit c (LbVHA-c1) gene from Limonium bicolor (Bunge) Kuntze. Compared with wild-type (WT) tobacco, superoxide dismutase (SOD) and peroxidase (POD) activities in the transgenic plants were significantly enhanced under salt stress conditions. The level of malondialdehyde (MDA) in the transgenic plants was significantly lower than that in WT plants grown under salt stress conditions. Moreover, the transgenic plants displayed obviously better growth than the WT plants under salt stress. These results suggest that LbVHA-c1 may confer stress tolerance through enhancing POD and SOD activities, and by protecting membranes from damage by decreasing lipid peroxidation under salt stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vacuolar H+-ATPase (VHA) is one of several ATP-dependent protein pumps that are involved in cell growth, vesicle trafficking, and adaptation to environmental stresses, including drought, salt, and heavy metals (Ratajczak 2000). V-ATPase comprises two domains: a cytosolic V1 domain and a membrane V0 domain. The large cytosolic V1 domain, which has eight different subunits (A through H), is involved in ATP hydrolysis that is coupled to the pumping of protons into a compartment via the membrane-bound V0 complex (Padmanaban et al. 2004). V-ATPase subunit c (VHA-c), which contains four transmembrane helices, is a major component of the membrane V0 domain, and is a highly hydrophobic protein. VHA-c is likely to be involved in transporting protons, and the rotation of a ring of six c subunits is necessary for driving proton transport (Hirata et al. 2003).
Previous studies have shown that V-ATPase has diverse functions; furthermore, the expression of V-ATPase is tissue-specific, and can be induced by certain stressors. For example, the transcripts of the V-ATPase c subunit from Acanthus ebracteatus Vah1 is detected in leaf, floral, stem, and root tissues, whereas its expression is lower in stem and root tissues (Ho et al. 2008). Padmanaban et al. (2004) investigated the role of multiple VHA-c genes encoding the 16-kDa subunit of the membrane V0 sector in Arabidopsis. Their results showed that V-ATPase was responsive to light or dark in an organ-specific manner, functions in the root cap under the influence root growth, and is also involved in membrane trafficking and fusion. Seidel et al. (2004) reported that a VHA-c gene from the plant Mesembryanthemum crystallinum is expressed in cytoplasmic vesicles, and interacts with the V-ATPase A subunit in living plant cells. Moreover, VHA-c is found to play an important role in plant stress response. For example, VHA-c genes have been found to be highly induced by salt (Tyagi et al. 2005; Kluge et al. 2003; Lehr et al. 1999; Chen et al. 2002), osmotic (Tyagi et al. 2005; Kluge et al. 2003), heat (Kluge et al. 2003), and low temperature (Tyagi et al. 2005; Kluge et al. 2003) stress, suggesting that it may play a common role in the stress response of plants. Furthermore, the expression of VHA-c can also be stimulated by exogenous application of abscisic acid (ABA), salicylic acid (SA), and calcium (Tyagi et al. 2005), indicating that it is involved in ABA-dependent signal transduction and may also be involved the response to biotic stresses. Although these studies have characterized the expression of VHA-c in response to different stressors, and have found that it is involved in the stress response, there is still little information regarding the physiological changes associated with the abiotic stress tolerance mediated by VHA-c. Analysis of the stress tolerance and physiological responses in plants overexpressing the VHA-c gene would provide a valuable insight into the mechanisms of stress tolerance conferred by VHA-c.
The Plumbaginaceae is family of highly stress-tolerant plants, which includes species tolerant to a wide range of harsh environments (Bouchereau et al. 1999), among which Limonium bicolor (Bunge) Kuntze is a highly salt-tolerant halophyte. The ability of L. bicolor to thrive in saline soils, demonstrates that it has developed molecular and physiological mechanisms that enable it to adapt to salt stress conditions, and has potential utility as a source of genetic determinants of saline tolerance.
In the present investigation, we studied a VHA-c (LbVHA-c1) gene from L. bicolor. Transgenic tobacco plants overexpressing LbVHA-c1 were generated. The physiological parameters related to salt tolerance were compared between transgenic and wild-type (WT) plants under normal growth and stress conditions. Our results may provide insights on the mechanistic details of salt tolerance conferred by VHA-c, which correlate with the physiological changes observed.
Material and Methods
Plant Expression Vector Construction and Tobacco Transformation
A VHA-c (LbVHA-c1) gene (GQ404375) was cloned from a cDNA library of L. bicolor (Wang et al. 2008). The transmembrane prediction of the LbVHA-c1 was performed using HMMTOP (http://www.enzim.hu/hmmtop/index.html). To amplify the ORF of the LbVHA-c1 gene, primers were designed as follows, forward primer: 5′-ATCGTCTAGAATGTCTTCTACCTTCAGTGGCG-3′, and reverse primer: 5′-ATCGGGTACCTTAATCAGCTCTTGACTGGCCA-3′. XbaI and KpnI cutting sites were contained in the forward and reverse primers, respectively. The PCR amplified product was digested with XbaI and KpnI, and then ligated into a pROKII vector that was also digested with XbaI and KpnI. The recombinant vector (Fig. 1a) was transformed into the Agrobacterium EHA105 for plant transformation.
Detection of the transgene by Southern and Northern analysis. a Diagram of the T-DNA region of the vector pROKII-LbVHA-c1 used for transformation. Nos-P, promoter of the nopaline synthase gene; Nos-T, terminator of the nopaline synthase gene; 35S-P, CaMV 35S promoter; LbVHA-c1, coding region of LbVHA-c1 gene. b Detection of the transgene from kanamycin-resistant lines by Southern analysis. c Analysis of the exogenous LbVHA-c1 expression of the transgenic lines by Northern blot; WT, non-transformed plants; 1–5, independently transformed plant lines, 1 through 5
Transgenic tobacco was generated using an Agrobacterium-mediate method. When the kanamycin-resistant adventitious shoots grew to about 2 cm in length, we transferred them to the rooting medium (1/2 MS + 0.5 mg/l NAA + 50 mg/l Kanamycin + 300 mg/l Cefotaxime) for rooting.
Southern and Northern Blot Analyses
For Southern analysis, total DNA (30 μg) from each transgenic lines (T0) and WT was digested with XbaI and KpnI, fractionated by electrophoresis on a 1.0% agarose gel, denatured with NaOH, blotted on a Hybond N+-membranes (Amersham, USA). Hybridization and detection were performed following the instructions of the manufacturer (DIG High Prime DNA Labeling and Detection Starter Kit I, Roche). For Northern analysis, total RNA (20 μg) from each sample was dissolved in denaturing buffer, separated on formaldehyde agarose gels, transferred to Hybond N+ membranes (Amersham, Pharmacia Biotech, USA), and fixed by UV cross-linking (254 nm, 8 min). Membranes were prehybridized at 68°C for 2 h, and then hybridized with labeled probe at 68°C for 12 h. Detection of hybridization was performed following the instructions of manufacturer (Dig Northern Starter Kit, Roche).
Salt Treatment of LbVHA-c1 Transgenic Plants
Tobacco plantlets (T0) from WT and two randomly selected transgenic lines (lines 2 and 3) were employed for NaCl tolerance testing. At least three plantlets from each line were used in each experiment. The well-watered plants were treated with 0.6% (w/v) NaCl for 0 (without stress, control), 1 and 4 days, and the leaves from each treatment were harvested for analysis. Each experiment was performed at least in triplicate.
Analysis of Superoxidase, Peroxidase Activity, and Soluble Protein Content
Superoxide dismutase (SOD) activity of each sample was measured according to the method described by Wang et al. (2010). For determination of peroxidase (POD) activity, 1.5 ml of phosphate buffer (0.01 mol/l, pH 7.2) was added to 0.05–0.1 g ground tobacco material, incubated at 4°C for 30 min, and centrifuged. Twenty microliters of supernatant was diluted to 500 μl with water, and was added with 0.5 ml of 0.8% H2O2, 0.5 ml of 0.1 mol/l phosphate buffer (pH 7.2), and 0.5 ml of 0.1 mol/l Guaiacol buffer, and then incubated at 30°C for 8 min. Absorbance was measured at 470 nm. POD activity was defined as: A POD = (A 470*V)/WTv, where A 470 is the absorbance at 470 nm, V is the total volume of enzyme, W is the fresh weight of the sample, and v is the volume of the enzyme used in the reaction.
Analysis of Malondialdehyde Content
The samples were ground into a fine powder under liquid nitrogen. Trichloroacetic acid (10%, 3 ml) was added to about 0.2 g of the powder, mixed, and incubated at room temperature for 30 min. After centrifugation, 2 ml of the supernatant was transferred to a new tube, and was added with TBA (2 ml, 0.6%). The mixture was heated in boiling water for 15 min, cooled immediately and centrifuged. Absorbance values were determined for the supernatant at wavelengths of 532 and 450 nm, with water as the background. The formula for the calculation of malondialdehyde (MDA) content was: \( {\hbox{MDA}}\,{\hbox{content}}\left[ {\mu {\hbox{mol}} \cdot {{\hbox{g}}^{ - {1}}}\left( {\hbox{FW}} \right)} \right] = \left( {{6}.{45}\,{\hbox{O}}{{\hbox{D}}_{{532}}} - 0.{\hbox{56O}}{{\hbox{D}}_{{45}0}}} \right)/{\hbox{W}} \).
Comparison of Relative Weight Gain Between Transgenic and WT Plants
For measuring relative weight gain (RWG), the plantlets (T0), which had similar weight, were divided into two groups, of which one group was used directly for weight measurement. The weight of root and aerial part of plantlets in this group were measured, and these data were as initial weights. The other group was treated with 0.6% (w/v) NaCl for 22 days, and the weight of root and aerial part were measured; these data were used as final values. Each group contained three seedlings from each transgenic line and WT plants. The weight gain of each transgenic or WT line was calculated as its final value subtracted from its original weight. The RWG were calculated as weight gain/initial weight.
Salt Tolerance Test of T1 Seedling Tobacco
To evaluate the salt tolerance of T1 transgenic plants, seeds of transgenic line 3 were grown planted on MS medium (containing kanamycin, 50 mg/l), and the WT tobacco seeds were also planted on MS medium without kanamycin as a control. After the kanamycin-resistant seedling germination, the transgenic and WT plants with similar size were transferred to normal MS medium and MS medium containing 0.8% (W/V) of NaCl. After growth for 30 days, the root length and height from transgenic line and WT plants were measured and compared.
Results
Transmembrane Helices Prediction and Generation of Transgenic Tobacco Plants
HMMTOP predicted that there are four transmembrane helices in the LbVHA-c1 protein, and that these are located at amino acid residue positions 11–35, 56–80, 99–117, and 130–154. Tobacco explants were infected with Agrobacterium EHA105 containing the LbVHA-c1 gene, and were selected on medium supplemented with kanamycin (50 mg/l). After growth for 3 weeks, five independent kanamycin-resistant shoots were generated. Southern blot analysis indicated that all transgenic lines showed the expected hybridization signal, whereas the WT plants did not, suggesting that the exogenous LbVHA-c1 had been integrated into the genome of the transgenic plants (Fig. 1b). Northern blot analysis showed that all transgenic lines displayed a distinct band consistent with the predicted LbVHA-c1 mRNA, whereas no hybridization signal was detected from the WT plants (Fig. 1c). Collectively, these results indicate that the LbVHA-c1 gene was successfully integrated into, and expressed in, the five transgenic tobacco lines.
Analyses of SOD Activity in Transgenic and WT Plants
Compared with WT plants, the SOD activity in transgenic plants was markedly elevated under normal growth conditions (Fig. 2a). Following salt stress, the SOD activity in both transgenic and WT plants increased; however, the SOD activities in transgenic plants were all higher than those in WT plants, particularly in plants stressed for 4 days, at which time the transgenic lines had significantly (P < 0.05) higher SOD activity than the WT plants (Fig. 2a). These results showed that overexpression of LbVHA-c1 can increase SOD activity in transgenic plants.
Comparison of SOD, POD, and MDA level between the T0 transgenic and WT plants. The plants were treated with 0.6% (w/v) NaCl for 0, 1, and 4 days, their SOD, POD activity, and MDA content were measured. a Comparison of SOD activity between the transgenic and WT plants. b Comparison of POD activity between the transgenic and WT plants. c Analysis of MDA content in transgenic and WT plants. *Significant (P < 0.05) difference with WT plants
Comparison of POD Activity Between Transgenic and WT Plants
The results demonstrated that there is no difference in POD activity between the transgenic lines and WT plants under normal growth conditions (Fig. 2b). Under salt stress, POD activity in both WT and transgenic plants was increased in plants stressed for 1 and 4 days, although the increase in POD activity was more marked in the transgenic plants (Fig. 2b). After 1 and 4 days under salt stress conditions, the POD activity in transgenic plants was significantly (P < 0.05) higher than that in WT plants (Fig. 2b). These results indicated that overexpression of LbVHA-c1 contributes to the enhancement of POD activity in transgenic plants under salt stress.
Analyses of MDA Levels in Transgenic and WT Plants
The MDA content of transgenic and WT plants was assayed both before and after the imposition of salt stress (Fig. 2c). The changes in the patterns of MDA content were different between transgenic and WT plants. In WT plants, the MDA content increased continuously after stress imposition; however, MDA content in transgenic plants was increased after stress for 1 day, but decreased after stress for 4 days (Fig. 2c). There was no obvious difference in MDA content between transgenic and WT plants before the plants were subjected to stress. However, the MDA content in the transgenic lines was lower than that in the WT plants under stress, particularly after stress for 4 days, at which time the MDA content in the transgenic lines was significantly (P < 0.05) lower than that in WT plants (Fig. 2c). Therefore, these results suggest that LbVHA-c1 contributes to a reduction in membrane lipid peroxidation under conditions of salt stress.
Relative Weight Gain Analyses
The RWG of root and aerial parts was determined and compared between transgenic and WT plants after salt stress for 22 days (Fig. 3). The results showed that the RWG of the roots of two transgenic lines was more than 70% higher than that of WT plants, whereas the RWG of the aerial parts of two transgenic lines was more than twice that of WT plants (Fig. 3), indicating that the transgenic plants grew better than WT plants under salt stress conditions. These results suggest that salt tolerance of LbVHA-c1-transformed plants was markedly improved compared with that of WT plants.
Comparison of the Growth of T1 Seedlings Between an LbVAH-c1-transformed Line and WT Tobacco
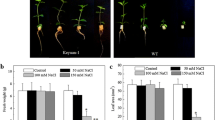
The growth of T1 transgenic and WT plants was compared under normal growth conditions and following exposure to 0.8% NaCl stress. The results showed that compared with WT plants, the average root length of transgenic plants was reduced, but that the average height of transgenic plants was slightly increased under normal growth conditions (Fig. 4). However, both the height and root length of transgenic plants were higher than those of the WT plants after exposure to 0.8% NaCl (Fig. 4). These results suggest that the salt tolerance of T1 LbVHA-c1-transformed plants was improved compared with that of WT plants.
Comparison of the growth of T1 seedlings between LbVHA-c1 transformed line 3 and WT tobacco under salt stress. a Growth of T1 transgenic seedlings and WT tobacco under normal growth condition or salt stress condition. b Comparison of height and root length between T1 transgenic and WT tobacco under normal growth condition or salt stress condition
Discussion
Plants usually generate reactive oxygen species (ROS) when exposed to different adverse environmental conditions, such as salt, drought, or cold. ROS cause secondary oxidative stress that can damage cellular macromolecules; generate lesions in DNA; damage cellular structure, lipids, and membranes; and affect protein synthesis and stability (Leshem et al. 2007; Wang et al. 2005). Therefore, ROS scavenging is a very important process for plants under adverse stress conditions. Plants possess a series of ROS-scavenging enzymes, including SOD, POD, and CAT. Among these, both SOD and POD are key enzymes in the plant ROS scavenging system. SODs can catalyze the dismutation of superoxide radicals to hydrogen peroxide and oxygen that play a central role in protecting aerobic organisms from the damage induced by oxygen toxicity (Bennicelli et al. 1998). Our results showed that SOD activity in transgenic plants was higher than in WT plants, whether exposed to salt stress or not (Fig. 2a). These results suggest that overexpression of LbVHA-c1 positively regulates plant SOD activity. Among all the antioxidative enzymes, PODs play key roles in cellular ROS detoxification, and are widely distributed in organisms (Passardi et al. 2004). Plant PODs play an important role in a variety of biological processes, including hydrogen peroxide detoxification, stress responses, lignin biosynthesis, and hormonal signaling (Østergaard et al. 1998). PODs can efficiently catalyze H2O2 to water and radicals (A*) using organic or inorganic substrates (HA): H2O2 + 1HA → 2 H2O + 2A* (Dunford 1991), and have high antioxidative activity. Our results showed that there was no difference in POD activity between transgenic and WT plants before the imposition of salt stress (Fig. 2b), indicating that H+-ATPase does not directly regulate POD activity. Under salt stress conditions, the POD activity in WT plants was slightly induced; however, in transgenic plants, POD activity was markedly induced and was significantly higher than in WT plants (Fig. 2b). Since the POD activity of plants overexpressing LbVHA-c1 is enhanced only after exposure to salt stress, these results suggest that overexpression of LbVHA-c1 may protect POD from damage and/or stimulate POD activity under salt stress. The highly induced POD and SOD activities in the LbVHA-c1-transformed plants suggest that overexpression of LbVHA-c1 may improve salt tolerance by enhancing ROS detoxification under salt stress.
In adverse environments, plants usually accumulate excessive ROS, and the accumulated ROS will, in turn, cause lipid peroxidation in plant biomembranes (Sunkar et al. 2003). MDA is an end product of free radical chain reactions and lipid peroxidation in biomembranes. The MDA level can reflect the level of lipid peroxidation in biomembranes, and also indirectly reflects the extent of membrane injury (Fang and Liu 2006). Therefore, MDA content is an important index for the evaluation of plant injury under stress conditions. In the present study, our results showed that the MDA content of transgenic plants was significantly lower than that in WT plants exposed to salt stress for 4 days (Fig. 2c), suggesting that, compared with WT plants, lipid peroxidation in biomembranes was highly decreased and that the membranes were slightly damaged in the LbVHA-c1-transformed plants under salt stress. Both SOD and POD are important ROS-scavenging enzymes. Our data showed that compared with WT plants, POD and SOD activities in transgenic plants were significantly enhanced under salt stress, implying that ROS-scavenging ability may be increased in transgenic plants. Therefore, the decreased lipid peroxidation in transgenic plants may be due to the enhanced ROS-scavenging ability conferred by the overexpression of LbVHA-c1.
In conclusion, our results showed that transformation with LbVHA-c1 can markedly improve the salt tolerance of plants. Under conditions of salt stress, both POD and SOD activities were significantly enhanced in transgenic plants compared with WT plants. Moreover, the MDA content of transgenic plants was significantly lower than that in WT plants under salt stress conditions. These results suggest that, in addition to the role that VHA-c may play in maintaining intracellular pH and ion homeostasis, it can also improve plant tolerance to salt stress by elevating SOD and POD activities and reducing lipid peroxidation in biomembranes.
References
Bennicelli RP, Stepniewski W, Zakrzhevsky DA, Balakhnina TI, Stepniewska Z, LiPiec J (1998) The effect of soil aeration on superoxide dismutase activity, malondialdehyde level, pigment content and stomatal diffusive resistance in maize seedlings. Environ Exp Bot 39:203–211
Bouchereau A, Duhazéa C, Martin-Tanguya J, Guéganb JP, Larher F (1999) Improved analytical methods for determination of nitrogenous stress metabolites occurring in Limonium species. J Chromatogr A 836:209–221
Chen X, Kanokporn T, Zeng Q, Wilkins TA, Wood AJ (2002) Characterization of V-type H(+)-ATPase in the resurrection plant Tortula ruralis: accumulation and polysomal recruitment of proteolipid c subunit in response to salt-stress. J Exp Bot 53(367):225–232
Dunford HB (1991) In: Everse J, Everse KE, Grisham MB (eds) Peroxidases in chemistry and biology. CRC Press, Boca Raton, Horseradish peroxidase: Structure and kinetic properties. pp 1–23
Fang F, Liu GT (2006) Protective effects of compound FLZ on β-amyloid peptide-(25–35)-induced mouse hippocampal injury and learning and memory impairment. Acta Pharmacol Sin 27:651–658
Hirata T, Iwamoto-Kihara A, Sun-Wada GH, Okajima T, Wada Y, Futai M (2003) Subunit rotation of vacuolar-type proton pumping ATPase: relative rotation of the G as to c subunit. J Biol Chem 278:23714–23719
Ho CL, Nguyen PD, Harikrishna JA, Rahim RA (2008) Sequence analysis and characterization of vacuolar-type H+-ATPase proteolipid transcript from Acanthus ebracteatus Vah1. DNA Seq 19(1):73–77
Kluge C, Lamkemeyer P, Tavakoli N, Golldack D, Kandlbinder A, Dietz KJ (2003) cDNA cloning of 12 subunits of the V-type ATPase from Mesembryanthemum crystallinum and their expression under stress. Mol Membr Biol 20(2):171–183
Lehr A, Kirsch M, Viereck R, Schiemann J, Rausch T (1999) cDNA and genomic cloning of sugar beet V-type H+-ATPase subunit A and c isoforms: evidence for coordinate expression during plant development and coordinate induction in response to high salinity. Plant Mol Biol 39(3):463–475
Leshem Y, Seri L, Levine A (2007) Induction of phosphatidylinositol 3-kinase-mediated endocytosis by salt stress leads to intracellular production of reactive oxygen species and salt tolerance. Plant J 51:185–197
Østergaard L, Pedersen AG, Jespersen HM, Brunak S, Welinder KG (1998) Computational analyses and annotations of the Arabidopsis peroxidase gene family. FEBS Lett 433:98–102
Padmanaban S, Lin X, Perera I, Kawamura Y, Sze H (2004) Differential expression of vacuolar H+-ATPase subunit c genes in tissues active in membrane trafficking and their roles in plant growth as revealed by RNAi. Plant Physiol 134(4):1514–1526
Passardi F, Longet D, Penel C, Dunand C (2004) The class III peroxidase multigenic family in rice and its evolution in land plants. Phytochemistry 65:1879–1893
Ratajczak R (2000) Structure, function and regulation of the plant vacuolar H(+)-translocating ATPase. Biochim Biophys Acta 1465:17–36
Seidel T, Kluge C, Hanitzsch M, Ross J, Sauer M, Dietz KJ, Golldack D (2004) Colocalization and FRET-analysis of subunits c and a of the vacuolar H+-ATPase in living plant cells. J Biotechnol 112(1–2):165–175
Sunkar R, Bartels D, Kirch HH (2003) Overexpression of a stress inducible aldehyde dehydrogenase gene from Arabidopsis thaliana in transgenic plants improves stress tolerance. Plant J 35:452–464
Tyagi W, Rajagopal D, Singla-Pareek SL, Reddy MK, Sopory SK (2005) Cloning and regulation of a stress-regulated Pennisetum glaucum vacuolar ATPase c gene and characterization of its promoter that is expressed in shoot hairs and floral organs. Plant Cell Physiol 46(8):1411–1422
Wang FZ, Wang QB, Kwon SY, Kwak SS, Su WA (2005) Enhanced drought tolerance of transgenic rice plants expressing a pea manganese superoxide dismutase. J Plant Physiol 162:465–472
Wang YC, Ma H, Liu GF, Zhang DW, Ban QY, Zhang GD, Xu CX, Yang CP (2008) Generation and analysis of expressed sequence tags from a NaHCO3-treated L. bicolor cDNA library. Plant Physiol Biochem 46(11):977–986
Wang YC, Qu GZ, Li HY, Wu YJ, Wang C, Liu GF, Yang CP (2010) Enhanced salt tolerance of transgenic poplar plants expressing a manganese superoxide dismutase from Tamarix androssowii. Mol Biol Rep 37(2):1119–1124
Acknowledgments
This work was supported by Fundamental Research Funds for the Central Universities (DL09DA01), the National Natural Science Foundation of China (No. 30972387), Genetically modified organisms breeding major projects (2009ZX08009-098B), and National High Technology Research and Development Program of China (863 Program) (2009AA10Z107).
Author information
Authors and Affiliations
Corresponding author
Additional information
C. Xu and L. Zheng have equally contributed to this work.
Rights and permissions
About this article
Cite this article
Xu, C., Zheng, L., Gao, C. et al. Ovexpression of a Vacuolar H+-ATPase c Subunit Gene Mediates Physiological Changes Leading to Enhanced Salt Tolerance in Transgenic Tobacco. Plant Mol Biol Rep 29, 424–430 (2011). https://doi.org/10.1007/s11105-010-0247-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-010-0247-4