Abstract
Background and aims
Phytoextraction is an eco-friendly approach for remediation of heavy metal contaminated soil. The aim is to screen Noccaea caerulescens lines with higher cadmium (Cd) phytoextraction efficiency and investigate differences in Cd species and distribution in the leaves of high and low Cd accumulating lines.
Methods
Biomass production and Cd bioaccumulation capacities of 29 Noccaea caerulescens lines, generated through single-seed-descent from a Cd hyperaccumulating calamine population, were assessed in a pot experiment with a moderately Cd contaminated soil (2.1 mg Cd kg− 1). Synchrotron-based techniques were employed to identify and characterize Cd speciation and distribution in Noccaea caerulescens leaves.
Results
The largest biomass of Noccaea caerulescens reached 5.0 ± 3.3 g (D. W. pot− 1) after 6 months growth. The Cd concentrations in shoots varied from 85 to 203 mg kg− 1. The most efficient line removed 0.64 mg Cd pot− 1 and lowered the total Cd in soil by 30%. Synchrotron-based X-ray absorption spectroscopy showed that the dominant Cd species was Cd-thiol complexes. Cadmium-carboxyl and Cd-phytate/phosphate were present in the leaves of high and low Cd accumulating lines, respectively. Micro X-ray fluorescence microscopy showed cadmium was concentrated in leaf veins.
Conclusions
There are wide variations including both biomass production and Cd accumulation capacity among different lines within the same calamine ecotype of Noccaea caerulescens. Cadmium-thiol complexes play the most important role in Cd detoxification in leaves of Noccaea caerulescens grown in moderately Cd contaminated paddy soil. These findings provide a physiological basis for breeding high Cd accumulation varieties of Noccaea caerulescens.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human activities, such as mining, smelting, waste disposal, and industrial processing cause the redistribution of metals on the earth’s surface. Cadmium (Cd) contamination is widespread and of significant environmental concern due to high bioaccumulation (Zhao et al. 2015; Tóth et al. 2016; Argüello et al. 2019; Wang et al. 2019). The mobility of Cd in soil-plant systems is relatively high, especially in aerobic and low pH conditions (Römkens et al. 2009; de Livera et al. 2011). Cadmium can accumulate in the human body through the food chain and can be retained in the human body for up to 10–30 years (Nordberg et al. 2014). Accordingly, even low levels of chronic Cd exposure can lead to diseases such as osteoporosis and renal dysfunction (Järup et al. 1998; Järup and Åkesson 2009). Cadmium is also associated with cancers of lung, prostate, urinary bladder, renal endometrial and breast (Nawrot et al. 2006; Huff et al. 2007; Järup and Åkesson 2009). Cadmium contamination in agricultural soil has posed a serious threat to food safety and human health. Hence, it is essential to remove Cd from contaminated agricultural soils.
In recent years, great efforts have been made in developing soil remediation techniques, including physical, chemical, and biological methods such as soil isolation, soil washing and phytoextraction (Gray et al. 2006; Bhargava et al. 2012; Bolan et al. 2014; Chaney and Baklanov 2017; Qayyum et al. 2020). Phytoextraction is an eco-friendly soil remediation technique, which uses metal-accumulating plants to remove contaminant metals from soil (Bhargava et al. 2012; Suman et al. 2018). Phytoextraction is realistically only feasible for moderately contaminated soils (Zhao et al. 2022), which are typical of Cd-contaminated agricultural soils in some areas in southern China that often lead to exceedance of the Cd limits in food crops (Zhao et al. 2015). To be efficient, this method requires both high biomass and metal hyperaccumulation (McGrath and Zhao 2003). Therefore, hyperaccumulators are good candidates for phytoextraction, due to their extraordinary ability to accumulate metals in aboveground biomass. Plants that contain over 100 mg kg− 1 cadmium in their dry weight foliar tissue in their natural habitat are defined as Cd hyperaccumulators (Reeves et al. 2018). Nine Cd hyperaccumulators have been identified so far (Krämer 2010; Rosenfeld et al. 2018). Noccaea caerulescens (N. caerulescens, formerly Thlaspi caerulescens) is a well-known Zinc (Zn)/Cd/Nickel (Ni) hyperaccumulator (Baker et al. 1994; Reeves et al. 2001; Assunção et al. 2003; Kozhevnikova et al. 2020). Noccaea caerulescens is particularly abundant in parts of Europe (Krämer 2010), and can accumulate up to 0.1–0.4% Cd in the shoots, which makes it a potential candidate for effective phytoextraction of Cd from Cd-contaminated soil (Robinson et al. 1998; Reeves et al. 2001; Zhao et al. 2003).
Comparisons among different ecotypes of N. caerulescens showed that there are great variations in Cd accumulation abilities (Lombi et al. 2000; Lovy et al. 2013; Jacobs et al. 2018a, b; Kozhevnikova et al. 2020). For example, the Ganges accession (of the Cd hyperaccumulating calamine ecotype found in the Cevennes region in France) usually accumulate several times more Cd in shoots than accessions of other ecotypes, and is also highly Cd tolerant (Lombi et al. 2000). In a field experiment, Jacobs et al. (2018b) found that Cd uptake of Ganges reached 260 g ha− 1 after 12-month growth, while the non-metallicolous accession Wilwerwiltz, from Luxemburg, accumulated 40 g ha− 1. Lombi et al. (2000) compared the bioaccumulation abilities of four N. caerulescens accessions in a pot experiment and found that the Cd accumulation increased with increasing soil Cd concentration, and the highest leaf Cd concentration reached 2,800 mg kg− 1 in the Ganges accession, 5-fold higher than the Zn hyperaccumulating calamine accession Whitesike from the UK. In a hydroponic study, Kozhevnikova et al. (2020) compared the patterns of natural variation in Cd accumulation capacities among 28 accessions of N. caerulescens, and found the accessions from calamine populations showed significantly higher variations than the accessions from non-metallicolous or ultramafic populations. Although studies have shown that the Ganges accession is far superior in Cd accumulation to several other accessions (Lombi et al. 2000, 2002; Jacobs et al. 2018a), it is still unclear whether there is a difference in the Cd accumulation capacities between different individuals of the population in the Cevennes region from where the Ganges accession originated. Thus, a further investigation of the variations in Cd accumulation among different lines from this Cd hyperaccumulating Cevennes population is required.
In recent years, synchrotron-based techniques such as X-ray absorption spectroscopy (XAS) and X-ray fluorescence microscopy (µ-XRF) are increasingly utilized in soil–plant systems to analyze metal speciation and distribution in biological samples (Kopittke et al. 2017). X-ray absorption spectroscopy includes two complementary techniques: the extended X-ray absorption of fine structure (EXAFS), which provides information on the local environment of investigated atoms in the sample, and X-ray absorption near-edge structure (XANES), which provides information concerning the chemical oxidation stage and coordination geometry of elements in complexes (Gardea-Torresdey et al. 2005). The EXAFS technique is an element-specific method that is particularly suited for analysing the in vivo ligand environment of Cd and Zn in plants (Küpper et al. 2004; Lu et al. 2014). XAS investigations have already been used for the determination of Cd ligand environments in the roots, stems, leaves and seeds of Cd-hyperaccumulating plants (Küpper et al. 2004; Ueno et al. 2005; Vogel-Mikuš et al. 2010; Koren et al. 2013; Isaure et al. 2015) both found Cd-organic acids were the dominant Cd species in N. caerulescens. Species of Cd bound to sulfur ligands (e.g. phytochelatins) were also detected in both leaves and stems in the Ganges accession with proportions of 20–35% (Küpper et al. 2004). A similar Cd ligand environment that Cd-O ligands prevailed over the Cd-S ligands in leaves, was also found in the related Cd hyperaccumulator Noccaea praecox and in Arabidopsis halleri (Koren et al. 2013; Isaure et al. 2015). In addition, distribution of Cd in leaves of N. caerulescens has also been studied, which indicated that most Cd was mainly concentrated at the edge of the leaves and in localized spots of higher concentration distributed throughout leaf surface (Cosio et al. 2005). It is worth noting that these studies on Cd speciation in hyperaccumulators were performed on plants that were grown in hydroponic conditions with very high levels of Cd (Küpper et al. 2004; Cosio et al. 2005; Ueno et al. 2005; Koren et al. 2013; Isaure et al. 2015).
In the present study, Cd bioaccumulation of 29 different N. caerulescens lines generated from individuals collected from the Cd-hyperaccumulating and Cd-hypertolerant population at the site of a former zinc smelter in Les Avinières, South Cevennes, France (Mahieu et al. 2011), was compared in a pot experiment with a moderately Cd-contaminated soil to evaluate the variations in biomass production and Cd accumulation capacity. To further elucidate the Cd detoxification mechanism in leaves of N. caerulescens grown in moderately Cd-contaminated soil, synchrotron radiation techniques, such as bulk XAS coupled with Linear combination fitting (LCF), were used to compare the differences in Cd speciation and distribution in leaves between two high and two low Cd accumulating N. caerulescens lines.
Materials and methods
Plant materials
Twenty-nine lines of Noccaea caerulescens were used. They are derived from 11 individual plants originating from an abandoned zinc smelter site at Les Avinières, France (43° 56’ 11.2’’ N, 3° 40’ 17.2’’ E)(Mahieu et al. 2011), also supposedly the origin of the Ganges accession (Jacobs et al. 2018a). Plants at this site belong to the Cd hyperaccumulating calamine ecotype (Kozhevnikova et al. 2020). Initially, from a set of around 25 seed samples, each sample collected from an individual plant at the site, five progeny plants were grown from each sample. These were numbered “AV-#-#” with the first # indicating the original sampled plant and the second # the plant grown from the seed sample. While several progenies displayed very poor seed set, progeny of 11 original individuals showed at least a few plants with high fecundity in the greenhouse. Most progenies display variation in plant morphology between plants, suggesting at least part of the sampled seed resulted from outbreeding. Plants with moderate to high seed production were harvested, and inbred for two generations of single seed descent, favouring plants with high fecundity and biomass. The seeds of the resulting 29 lines were used for this experiment. Two generations of inbreeding, and selection for fecundity, reduced the initially observed within-line variation, to yield phenotypically similar plants per line, while potentially maintaining the between-line variation.
Pot experiment
A Cd contaminated soil was collected from a paddy field (0–15 cm) in Xiangtan, Hunan Province, China. This paddy field has been suffering from Cd contamination due to rapid industrialization and agricultural intensification over the past four decades. Specifically, the Cd pollution is mainly caused by the discharge of pollutants from nearby chemical industry and steel plants and sewage irrigation in the Xiang River from the 1980s to the 2000s (Li et al. 2018). The soil contains 2.1 and 118 mg kg− 1 of total Cd and Zn, respectively, and the soil pH is 6.4. The concentration of DTPA extractable Cd is 1.1 mg kg− 1. Noccaea caerulescens seeds were germinated in a petri dish filled with moistened vermiculite in the dark. After 10 days, three seedlings of each line were transferred to one pot filled with 1 kg air-dried soil. Each line was replicated in 3 pots. Plants were grown in a greenhouse with the following conditions: temperature 20–25 oC, natural sunlight supplemented with sodium vapour lamps to maintain light intensity > 300 µmol m− 2 s− 1 and a photoperiod of 12 h per day. Plants were watered every 3 days or as needed to maintain adequate soil moisture. The plants were cultured for six months and as they were not vernalized, remained in the rosette stage until harvesting. The plant shoots were harvested, washed with deionized water and blotted dry. Five leaves taken from the centre of rosette from 5 different plants of each line with similar size were immediately freeze-dried for synchrotron-based analysis. The remainder of the above-ground part was oven-dried at 65 oC for 3 days and weighed for biomass before elemental analysis.
Determination of Cd and Zn concentrations in plant samples
Dry plant samples were ground to fine powders and digested with 5 mL HNO3/HClO4 (85:15 v/v) in a heating block. Blanks and a certified reference material (GBW10015-spinach) were included in the digestion for quality control. The concentrations of Cd and Zn were determined using Flame Atomic Absorption Spectrometry (FAAS).
Cadmium speciation and elemental distribution by X-ray absorption spectroscopy (XAS) techniques
The Cd standard spectra used in this study are described in Yan et al. (2016), including 3 complexes of Cd bound to thiol groups (Cd-cysteine, Cd-glutathione and Cd-phytochelatin), 7 complexes of Cd bound to carboxyl groups (Cd-histidine, Cd-citrate, Cd-L-malate, Cd-methionine, Cd-glutamine, Cd-succinate, and Cd-polygalacturonic acid), and Cd-phytate. Freeze-dried plant leaves were ground into fine powders and mounted on sample holders for synchrotron analysis. Cadmium speciation in leaves was analysed by bulk X-ray absorption fine structure (bulk-XAFS) at the Cd K-edge (26,711 eV) at beamline 7 − 3, Stanford Synchrotron Radiation Laboratory (Menlo Park, CA, USA). A Cd metal foil was measured simultaneously in transmission mode as a reference. The energy calibration of each spectrum was conducted by setting the first inflection point of the X-ray absorption near edge structure (XANES) spectrum of the Cd metal foil to 26,711 eV. Each XAFS spectrum was collected from 300 eV below Cd K-edge to k values over 13 Å1 above the edge in fluorescence mode. Three scans were taken for each sample to maximize the signal-to-noise ratio.
All experimental spectra were energy calibrated and merged prior to analysis. The extended X-ray absorption fine structure (EXAFS) analyses were obtained using the SixPack software package and Athena (version 0.9.26). Principal component analysis (PCA) of the EXAFS spectra with k values between 3 and 10 Å−1 was performed to estimate the likely number of Cd-bound species contained in the samples. The SPOIL values were calculated using target transformation (TT) function in SixPack. Linear combination fitting (LCF) was conducted to identify and characterize Cd speciation in each sample, in k-weight 1 with k values ranging from 3 to 10 Å−1. The best fit with the lowest R-factor and reduced chi-square was chosen to represent Cd speciation and the proportion of each species.
Micro X-ray fluorescence (µ-XRF) and micro X-ray absorption near edge structure (µ-XANES) analysis were employed to detect the elemental distributions and Cd speciation at selected hot spots of intact freeze-dried leaves. Data were collected at beamline 20-ID-B, Advanced Photon Source (APS, Chicago, IL, USA). The step size was 8 μm and the dwell time was 1 s. The beam energy was set to 29,000 eV for µ-XRF analysis of Cd, Zn, Mn, and Cu distributions in the leaves. Micro XANES spectra were collected at hot spots, with energy ranging from 100 eV below to 200 eV above the Cd K-edge. Three to four scans were taken at each location to maximize the signal-to-noise ratio. Cadmium speciation in hot spots was identified as described above.
Results
Biomass production in different N. caerulescens lines
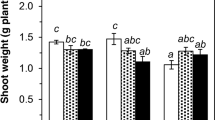
The 29 lines of the Avinières N. caerulescens population showed a 6.5-fold variation in shoot biomass (Fig. 1). The largest shoot biomass was observed in line AV-24-5, with a mean biomass of 5.0 ± 3.3 g per pot. By contrast, the smallest shoot biomass was produced by line AV-21-5, with a mean biomass of 0.8 ± 0.2 g per pot.
Above ground biomass (dry weight) of N. caerulescens lines. Plants were cultivated in a greenhouse for six months. Data represent means ± SE of three pot replicates. The image shows a replicate of line AV-5-1. Four lines selected for synchrotron analysis are marked with black. Different letters indicate a significant difference (Duncan test, P < 0.05)
Cadmium and Zinc accumulation in different N. caerulescens lines
Cd and Zn concentrations in the shoot of the 29 tested N. caerulescens lines were analysed. A 2.4-fold difference was observed in shoot Cd concentrations among the lines, varying from 85 mg kg− 1 in line AV-3-1 to 203 mg kg− 1 in line AV-14-1 (Fig. 2a). A total of 27 lines (93%) had Cd concentrations exceeding the Cd hyperaccumulation concentration criterion (> 100 mg kg− 1) (Krämer 2010), illustrating the Cd-hyperaccumulation property of the population. Furthermore, the Cd bioaccumulation factor (the ratio of shoot Cd concentration to soil Cd concentration) of the tested N. caerulescens lines varied from 40 to 95.
Cadmium (a) and Zinc (b) concentrations and bioaccumulation factors in the shoots of N. caerulescens lines. (c) Relationship between shoot Zn and Cd concentrations. Plants were cultivated in a greenhouse for six months. Data represent means ± SE of three pot replicates. Four lines selected for synchrotron analysis are marked with black. Different letters indicate a significant difference (Duncan test, P < 0.05)
Shoot Zn concentration varied from 460 to 1483 mg kg− 1, with the Zn bioaccumulation factor ranging from 5.4 to 12.6 (Fig. 2b). There was a significant (r = 0.58, P < 0.01) correlation between shoot Zn and Cd concentrations among the 29 lines of N. caerulescens (Fig. 2c). In addition, a significant correlation (r = 0.46, P < 0.01) between shoot Zn concentration and shoot biomass was observed (Fig. 3A), whereas there was no significant correlation between shoot Cd concentration and shoot biomass (r = 0.003, P > 0.05) (Fig. 3B).
Cadmium and Zinc removal efficiency by N. caerulescens
The removal of Cd and Zn from soil by N. caerulescens lines was calculated as the products of shoot Cd or Zn concentration and shoot biomass (Fig. 4). The amount of Cd removed by the shoots varied from 0.09 to 0.64 mg pot− 1, accounting for 4.3–30.5% of the total Cd in the soil (Fig. 4a). The amount of Zn removal varied from 0.39 to 4.55 mg pot− 1, accounting for 0.4–3.8% of the total Zn in the soil (Fig. 4b).
Total extracted (a) Cd and (b) Zn from a pot filled with 1 kg of soil by N. caerulescens lines. The soil has 2.1 mg kg-1 Cd and 118 mg kg-1 Zn. Data represent means ± SE of three pot replicates. Four lines selected for synchrotron analysis are marked with black. Different letters indicate a significant difference (Duncan test, P < 0.05)
Cadmium speciation in N. caerulescens leaves
To investigate the Cd speciation in N. caerulescens leaves, two high Cd accumulating lines (AV-5-1 and AV-6-1) and two low Cd accumulating lines (AV-8-5 and AV-15-1) were selected for synchrotron analysis. Leaves from the centre of rosette were sampled for Cd speciation analysis using EXAFS. Total Cd concentration in the leaf samples varied from 103 to 197 mg kg− 1. The EXAFS spectra of the reference standards are shown in Fig. S1. All Cd standards were divided into three ligand groups: thiol, phytate/phosphate, and carboxyl, as the spectral differences between the compounds within each group were small. Principal component analysis (PCA) of the EXAFS spectra from the leaf samples showed that the cumulative variance reached a minimum at the second component, with the fitting of two standards accounting for 98% of the variance (Table S1). Based on this information, two groups of standards were used in linear combination fitting (LCF) of the EXAFS spectra of leaf samples. Linear combination fitting is influenced by k weight. A higher k weight will amplify the data at higher k ranges (over 7). Metals (e.g., Fe, Mn) have a larger impact on the EXAFS spectrum at higher k space (7–12 Å−1), while light elements are more impacted by scattering (i.e., shoulder features and oscillations) at low k values (3–5 Å−1). In the present study, the most important nearest neighbouring scattering atoms for Cd are light elements, such as oxygen, phosphorus, or sulphur. For this reason, a k-weight of 1 was set in the present study. The best fits for the Cd EXAFS spectra in the leaf samples are shown in Fig. 5. In all four lines of N. caerulescens, the majority of Cd (61–69%) in the leaves was complexed with thiol compounds. The remainder was complexed with carboxy ligands in the two high Cd accumulating lines (AV-5-1 and AV-6-1), but with phytate/phosphate in the two low Cd accumulating lines (AV-8-5 and AV-15-1).
Elemental distribution and µ-XANES analyses
Micro-X-ray fluorescence maps of Cd, Cu, Mn, Zn, and Fe distribution in leaf sections of two high Cd accumulating lines (AV-5-1 and AV-6-1) and a low Cd accumulating line (AV-15-1) were obtained (Fig. 6a-c). All three lines showed generally similar distribution patterns of the five metals analysed. Cadmium was mainly distributed in the veins (Fig. 6a-c). There were also hot spots near the vein where Cd was locally concentrated. Cadmium was also concentrated at the edge of the leaf in the low Cd accumulating line AV-15-1 (Fig. 6c). The distribution patterns of Cu and Mn were similar to that of Cd, whereas Zn showed a diffused distribution throughout the leaves.
Elemental distribution of Cd, Cu, Mn and Zn in leaves of N. caerulescens line AV-5-1 (a), AV-6-1 (b), and AV-15-1 (c) using µ-XRF mapping. (d) µ-XANES spectra at Cd-K-edge of Cd standards (in solid lines) and different spots taken in 5 A, 5B, 6 A, 6B, 15 A and 15B, and LCF results (in dotted lines). Bars (White line) = 1 mm
The µ-XANES spectra at the Cd K-edge were collected at Cd hot spots in the µ-XRF maps of the plant leaves. The best fits obtained from LCF and the proportions of Cd speciation in each hot spot are shown in Fig. 6d; Table 1, respectively. In hot spots of the two high Cd lines, the majority (54–70%) of the Cd was bound to thiol groups, with the remainder (30–46%) being bound to carboxyl groups. By contrast, in hot spots of the low Cd content line AV-15-1, nearly half of the Cd was bound to phytate/phosphate, while the other half was bound to thiol groups. These results are consistent with Cd speciation in the bulk samples of whole leaves (Fig. 5).
Discussion
Noccaea caerulescens is a well-known hyperaccumulator of Zn, Ni and Cd (Reeves et al. 2001; Assunção et al. 2003; Krämer 2010; Kozhevnikova et al. 2020). It is typically found as small, scattered populations on non-metalliferous soils (Besnard et al. 2009) or as large populations on abandoned mine spoils containing high concentrations or Zn and/or Cd. Few populations grow on ultramafic soils (Gonneau et al. 2017). There is considerable variation among different populations of this species in their metal accumulation ability, especially for Cd (Lombi et al. 2001; Gonneau et al. 2014; Sterckeman et al. 2017). Currently three different edaphic ecotypes are distinguished, formed by non-metallicolous, calamine and ultramafic populations (Koshevnikova et al., 2020). The Ganges accession from southern France has a particularly high Cd accumulation (Lombi et al. 2001; Sterckeman et al. 2017). The exact origin of this accession is obscure (“collected in the vicinity of St. Laurent le Minier”), and may well be from the large population present at the Les Avinières former smelter site. Variation within populations has not been investigated extensively. In the present study, we found that 29 lines of the Avinières population of N. caerulescens varied substantially in biomass production and accumulation of Cd and Zn in the shoots (Figs. 1 and 2). These results suggest that there is substantial genetic variability among N. caerulescens lines of the same population. These differences can be useful in improving biomass production in N. caerulescens. Plant biomass production is a complex trait depending on numerous genes and environmental conditions (Demura and Ye 2010). Under the growth conditions in the present study, the largest shoot biomass produced by line AV-24-5 was weighed at 1.67 g dry matter (DM) per plant (5.0 g per pot with 3 plants), which could theoretically yield up to 1.5 t DM ha− 1 with a sowing density of 90 plants m− 2 in field conditions (McGrath et al. 2000; Simmons et al. 2015). Although the biomass produced by N. caerulescens lines in the present study may not be enough to fully decontaminate Cd-contaminated soils within a reasonable time, N. caerulescens has the potential to produce up to 10 t dry matter ha− 1 with appropriate genotypes and sowing density (McGrath et al. 2000; Zhao et al. 2003; Koopmans et al. 2008; Sterckeman et al. 2019). Some fields trials have also demonstrated that hyperaccumulators with optimized agronomic strategies have great potential to be applied in remediation of metal contaminated soil. For example, Deng et al. (2016) optimized the phytoextraction efficiency of Zn/Cd hyperaccumulator Sedum plumbizincicola in metal contaminated soil by two agronomic strategies of intercropping with maize and plant densities through two long-term (8 years) field experiments. Results indicated that S. plumbizincicola at an appropriate planting density and intercropped with maize can achieve high remediation efficiency to contaminated soil without affecting the cereal crop productivity. In a large scale field experiment on a moderately contaminated soil in southern China, S. plumbizincicola produced 2–6 t ha− 1 dry biomass per season and reduced soil Cd from 0.6 to 0.3 mg kg− 1 within 2–3 years (Hu et al. 2019; Simmons et al. 2015) conducted a field trial to investigate the potential of N. caerulescens to phytoextract localized Cd/Zn contamination in Thailand and suggested that optimizing the use of fungicidal sprays, acidic soil pH, planting density and increasing the effective cropping period will increase rates of Cd and Zn removal enough to facilitate practical Cd phytoextraction from rice paddy soils.
Cadmium and Zn concentration in the shoots of the 29 N. caerulescens lines ranged from 85 mg kg− 1 to 203 mg kg− 1 and 460 to 1483 mg kg− 1, respectively, reflecting the natural variability of Cd and Zn accumulation among N. caerulescens lines. The significant correlation between shoot Cd and Zn concentrations (Fig. 2c) suggests a common pathway of uptake and transport shared by the two metals. The variation in Cd and Zn accumulation could be caused by variation in the expression of metal transporter genes. A previous study showed that NcZNT1 is a plasma membrane transporter with a high affinity for Zn and a low affinity for Cd and is possibly involved in Zn and Cd uptake in N. caerulescens (Pence et al. 2000), and in transgenic Arabidopsis thaliana expressing NcZNT1 (Lin et al. 2016), albeit a later study suggested that NcZNT1 is involved in Zn and not Cd uptake and translocation in N. caerulescens (Milner et al. 2012). Another transporter, NcNRAMP1, is involved in the influx of Cd across the endodermal plasma membrane and may play a role in Cd flux into the stele and root-to-shoot Cd translocation (Milner et al. 2014). Contribution of NcNRAMP1 to the increased Cd hyperaccumulation in the high Cd-accumulating Ganges accession appears to be due to the higher expression level than that in the low Cd-accumulating Prayon accession (Milner et al. 2014). The iron transporter NcIRT1 may also contribute to Cd uptake. Previous studies have shown that Fe deficiency enhanced Cd uptake and the expression of NcITR1 in the Ganges accession (Lombi et al. 2002), and NcIRT1 from Ganges also showed Cd transport activity in yeast assay (Halimaa et al. 2019). NcHMA3 is a tonoplast-localized transporter responsible for Cd sequestration in leaf vacuoles, which showed sevenfold higher expression in the high Cd-accumulating Ganges accession than in the low Cd-accumulating Prayon accession (Ueno et al. 2011). The gene copy number and expression of NcHMA4 among N. caerulescens populations were also reported to be associated with Cd tolerance and accumulation (Craciun et al. 2012). Whether the expression levels of NcZNT1, NcNRAMP1, NcIRT1, NcHMA3 and NcHMA4 vary among the different lines of N. caerulescens used in the present study remains to be investigated.
Interestingly, there is no trade-off between shoot Cd and Zn concentrations and shoot biomass. In fact, there was a significant positive correlation between shoot Zn concentration and shoot biomass (Fig. 3 A), suggesting that N. caerulescens lines producing larger biomass may also have a higher ability to accumulate Zn. Our results are consistent with the study of Sterckeman et al. (2017), who also found no trade-off between biomass production and metal concentrations in N. caerulescens. Gonneau et al. (2014) reported no trade-off in the calamine and non-metalliferous populations of N. caerulescens, but substantial trade-off in the serpentine populations, which are very Cd sensitive (Koshevnikova et al., 2020). These findings suggest that metal accumulation and plant biomass are genetically independent, and that selection and breeding for large biomass would not lead to decreased concentrations of Zn and Cd, at least for the calamine populations such as those used in the present study. In a study spanning three generations of N. caerulescens, Sterckeman et al. (2019) showed that pure-line selection could improve Cd and Zn accumulation capacities, but the high biomass traits could not be fixed readily due to the complex genetic basis of biomass production and the interactions between genotype and environmental conditions.
The plant metal bioaccumulation factor is also an important factor affecting the efficiency of phytoextraction. Under the experimental conditions of the present study, the efficiency of Cd phytoextraction was much greater than that of Zn phytoextraction. One reason is that the soil used in the present study was not contaminated by Zn. These results suggest that N. caerulescens has great phytoremediation potential for Cd-contaminated soil. On average, of all N. caerulescens lines tested, the percentage of the total Cd removed from soil by the shoots was 13.2%. The best performing line of N. caerulescens removed 30.5% of the total soil Cd. Therefore, phytoextraction of Cd from moderately contaminated soils is feasible, which is in agreement with previous model calculations (Zhao et al. 2003). In the present study, the Cd bioaccumulation factor of the tested N. caerulescens lines varied from 40 to 95, which is consistent with previous studies (Yanai et al. 2006; Maxted et al. 2007; Lovy et al. 2013). The higher the bioaccumulation factor, the more efficient the phytoextraction of Cd by N. caerulescens. The large variation (7-fold) among the 29 lines of N. caerulescens in the Cd phytoextraction efficiency suggests considerable scope for genotype improvement by line selection and subsequent interline cross progeny selection. Although the Cd bioaccumulation factor is also soil pH and soil Cd dependent, this parameter could be used to select the populations or the individuals most suitable for Cd extraction.
In addition to the variation in Cd accumulation, differences in Cd speciation between high- and low-Cd accumulating lines of N. caerulescens were also observed through synchrotron-based EXAFS (Fig. 5). Approximately two thirds of the leaf Cd were complexed with thiol compounds in all lines of N. caerulescens investigated (Fig. 4). However, the remaining one third of Cd was found to be complexed with carboxyl groups, likely to be organic acids, in the high-Cd accumulating lines, but with phytate/phosphate in the low-Cd accumulating lines. This difference was confirmed by both bulk EXAFS and µ-XANES. The results suggest that the complexation of Cd with organic acids may become more important with increasing Cd accumulation in the leaves of N. caerulescens. Previously, Vogel-Mikuš et al. (2010) found that Cd was coordinated to phytate in the embryonic tissues of Noccaea praecox, while Monsant et al. (2011) reported Zn-phytate complexes in N. caerulescens. To our knowledge, it is the first time that Cd-phytate/phosphate was detected in the leaves of relatively low-Cd accumulating lines of N. caerulescens plants. These results indicate that differences in the detoxification mechanism are likely to exist between high- and low-Cd accumulating lines within the Avinières population of N. caerulescens. While N. caerulescens in greenhouse conditions is largely autogamous, especially in natural, high-density, calamine populations in the field, there can be high incurrence of cross-fertilisation, which will support maintenance of high levels of heterozygosity and genetic variation in the populations (Mousset et al. 2016), explaining the variation found in the Avinières population.
The dominance of Cd-thiol complexes in the present study is also surprising, as previous reports using either XAS or 113Cd NMR showed Cd-O (Cd-organic acids) to be the dominant species in the leaves of N. caerulescens plants (Küpper et al. 2004; Ueno et al. 2005). Huguet et al.(2012), using EXAFS spectroscopy analysis, showed that Cd was predominantly bound to COOH/OH groups of organic acids in the leaves of Arabidopsis halleri, while Cd bound to thiol groups was found as a secondary species (less than 25%). Similar results were observed in Cd hyperaccumulator N. praecox, where the Cd-O ligands prevailed over the Cd-S ligands in bulk leaf tissues of N. praecox treated with different Cd salts and concentrations (Koren et al. 2013). Isaure et al. (2015) also found that the proportion of Cd-O ligands increased in the Cd-hyperaccumulating A. halleri and Cd tolerant progenies from the cross between A. halleri and the non-tolerant and non-hyperaccumulating relative A. lyrata, suggesting the binding of Cd with O ligands was associated with Cd tolerance. Küpper et al. (2004) found that only about a third of the Cd in the young and mature leaves of N. caerulescens was complexed with thiol ligands. However, both studies grew N. caerulescens (Ganges) in hydroponic culture supplied with high levels of Cd (50–100 µM) and, as a result, much higher concentrations of Cd were accumulated in the leaves (approximately 20–100 times higher than those obtained in our study). Moreover, the plants were suffering from Cd toxicity in the study of Küpper et al. (2004). However, in the non-hyperaccumulator Brassica juncea, Salt et al. (1997) showed that Cd was mainly associated with S ligands (60%) in the seedling after 36 h of 1 µM Cd exposure. It is reasonable to argue that thiol compounds provide stronger but more costly ligands for Cd complexation that would be suitable at relatively low levels of Cd accumulation, as observed in our study and the study by Salt et al. (1997). In contrast, organic acids provide weaker and low cost ligands for Cd complexation at very high levels of Cd accumulation. Thus, Cd speciation observed in our study represents N. caerulescens plants growing on slightly contaminated soils.
With respect to the distribution of Cd in leaves, Cosio et al. (2005) showed that Cd was concentrated at the edge of N. caerulescens leaves, and there were hot spots on the leaf surface. Cadmium imaging using laser ablation inductively coupled plasma mass spectrometry also showed increased Cd concentration on the leaf edges of N. caerulescens (Callahan et al. 2016). These results are consistent with the findings of the present study. The elemental distribution pattern was similar in the three leaves from different lines (Fig. 6), which showed that Cd, Cu, and Mn were co-localized at the veins. This similarity indicates that likely the same accumulation pathway is shared by these three elements. Though Zn and Cd may also share a similar uptake and transport mechanism, the distribution of Zn was more diffuse than Cd (Fig. 6). This was possibly due to the significantly higher Zn concentration in N. caerulescens leaves. At lower Zn concentrations, Zn also localizes mainly to the veins (van der Zee et al. 2021).
Conclusions
Significant differences existed in Cd accumulation and biomass production among different hyperaccumulator N. caerulescens lines of the Les Avinières population. Selection for higher Cd accumulation and biomass production lines of Cd hyperaccumulating calamine populations could provide more efficient N. caerulescens for soil Cd phytoextraction. The EXAFS analysis identified that Cd-thiol and Cd-carboxyl complexes exist in leaves of high Cd accumulating lines while Cd-thiol and Cd-phytate/phosphate complexes are present in leaves of low Cd accumulating lines. In addition, cadmium-thiol complexes were the most dominant species in the N. caerulescens leaves (61–69%) when plants were grown on a moderately Cd-contaminated soil. These new results show that the mechanisms of Cd storage and detoxification in N. caerulescens differ from what was previously found.
References
Argüello D, Chavez E, Lauryssen F, Vanderschueren R, Smolders E, Montalvo D (2019) Soil properties and agronomic factors affecting cadmium concentrations in cacao beans: A nationwide survey in Ecuador. Sci Total Environ 649:120–127. https://doi.org/10.1016/j.scitotenv.2018.08.292
Assunção AGL, Schat H, Aarts MGM (2003) Thlaspi caerulescens, an attractive model species to study heavy metal hyperaccumulation in plants. New Phytol 159:351–360. https://doi.org/10.1046/j.1469-8137.2003.00820.x
Baker AJM, Reeves RD, Hajar ASM (1994) Heavy metal accumulation and tolerance in British populations of the metallophyte Thlaspi caerulescens J. & C. Presl (Brassicaceae). New Phytol 127:61–68. https://doi.org/10.1111/j.1469-8137.1994.tb04259.x
Besnard G, Basic N, Christin P-A, Savova-Bianchi D, Galland N (2009) Thlaspi caerulescens (Brassicaceae) population genetics in western Switzerland: is the genetic structure affected by natural variation of soil heavy metal concentrations? New Phytol 181:974–984. https://doi.org/10.1111/j.1469-8137.2008.02706.x
Bhargava A, Carmona FF, Bhargava M, Srivastava S (2012) Approaches for enhanced phytoextraction of heavy metals. J Environ Manage 105:103–120. https://doi.org/10.1016/j.jenvman.2012.04.002
Bolan N, Kunhikrishnan A, Thangarajan R, Kumpiene J, Park J, Makino T, Kirkham MB, Scheckel K (2014) Remediation of heavy metal(loid)s contaminated soils – To mobilize or to immobilize? J Hazard Mater 266:141–166. https://doi.org/10.1016/j.jhazmat.2013.12.018
Callahan DL, Hare DJ, Bishop DP, Doble PA, Roessner U (2016) Elemental imaging of leaves from the metal hyperaccumulating plant Noccaea caerulescens shows different spatial distribution of Ni, Zn and Cd. RSC Adv 6:2337–2344. https://doi.org/10.1039/C5RA23953B
Chaney RL, Baklanov IA (2017) Chapter Five - Phytoremediation and Phytomining: status and promise. In: Cuypers A (ed) Advances in Botanical Research. Academic, Cambridge
Cosio C, DeSantis L, Frey B, Diallo S, Keller C (2005) Distribution of cadmium in leaves of Thlaspi caerulescens. J Exp Bot 56:765–775. https://doi.org/10.1093/jxb/eri062
Craciun AR, Meyer C-L, Chen J, Roosens N, De Groodt R, Hilson P, Verbruggen N (2012) Variation in HMA4 gene copy number and expression among Noccaea caerulescens populations presenting different levels of Cd tolerance and accumulation. J Exp Bot 63:4179–4189. https://doi.org/10.1093/jxb/ers104
de Livera J, McLaughlin MJ, Hettiarachchi GM, Kirby JK, Beak DG (2011) Cadmium solubility in paddy soils: Effects of soil oxidation, metal sulfides and competitive ions. Sci Total Environ 409:1489–1497. https://doi.org/10.1016/j.scitotenv.2010.12.028
Demura T, Ye Z-H (2010) Regulation of plant biomass production. Curr Opin Plant Biol 13:298–303. https://doi.org/10.1016/j.pbi.2010.03.002
Deng L, Li Z, Wang J, Liu H, Li N, Wu L, Hu P, Luo Y, Christie P (2016) Long-term field phytoextraction of zinc/cadmium contaminated soil by Sedum plumbizincicola under different agronomic strategies. Int J Phytoremediation 18:134–140. https://doi.org/10.1080/15226514.2015.1058328
Gardea-Torresdey JL, Peralta-Videa JR, de la Rosa G, Parsons JG (2005) Phytoremediation of heavy metals and study of the metal coordination by X-ray absorption spectroscopy. Coord Chem Rev 249:1797–1810. https://doi.org/10.1016/j.ccr.2005.01.001
Gonneau C, Genevois N, Frérot H, Sirguey C, Sterckeman T (2014) Variation of trace metal accumulation, major nutrient uptake and growth parameters and their correlations in 22 populations of Noccaea caerulescens. Plant Soil 384:271–287. https://doi.org/10.1007/s11104-014-2208-4
Gonneau C, Noret N, Godé C, Frérot H, Sirguey C, Sterckeman T, Pauwels M (2017) Demographic history of the trace metal hyperaccumulator Noccaea caerulescens (J. Presl and C. Presl) F. K. Mey. in Western Europe. Mol Ecol 26:904–922. https://doi.org/10.1111/mec.13942
Gray CW, Dunham SJ, Dennis PG, Zhao FJ, McGrath SP (2006) Field evaluation of in situ remediation of a heavy metal contaminated soil using lime and red-mud. Environ Pollut 142:530–539. https://doi.org/10.1016/j.envpol.2005.10.017
Halimaa P, Blande D, Baltzi E, Aarts MGM, Granlund L, Keinänen M, Kärenlampi SO, Kozhevnikova AD, Peräniemi S, Schat H, Seregin IV, Tuomainen M, Tervahauta AI (2019) Transcriptional effects of cadmium on iron homeostasis differ in calamine accessions of Noccaea caerulescens. Plant J 97:306–320. https://doi.org/10.1111/tpj.14121
Hu P, Zhang Y, Dong B, Gao W, Cheng C, Luo Y, Christie P, Wu L (2019) Assessment of phytoextraction using Sedum plumbizincicola and rice production in Cd-polluted acid paddy soils of south China: A field study. Agric Ecosyst Environ 286:106651. https://doi.org/10.1016/j.agee.2019.106651
Huff J, Lunn RM, Waalkes MP, Tomatis L, Infante PF (2007) Cadmium-induced cancers in animals and in humans International. J Occup Environ Health 13:202–212. https://doi.org/10.1179/oeh.2007.13.2.202
Huguet S, Bert V, Laboudigue A, Barthès V, Isaure M-P, Llorens I, Schat H, Sarret G (2012) Cd speciation and localization in the hyperaccumulator Arabidopsis halleri. Environ Exp Bot 82:54–65. https://doi.org/10.1016/j.envexpbot.2012.03.011
Isaure M-P, Huguet S, Meyer C-L, Castillo-Michel H, Testemale D, Vantelon D, Saumitou-Laprade P, Verbruggen N, Sarret G (2015) Evidence of various mechanisms of Cd sequestration in the hyperaccumulator Arabidopsis halleri, the non-accumulator Arabidopsis lyrata, and their progenies by combined synchrotron-based techniques. J Exp Bot 66:3201–3214. https://doi.org/10.1093/jxb/erv131
Jacobs A, De Brabandere L, Drouet T, Sterckeman T, Noret N (2018a) Phytoextraction of Cd and Zn with Noccaea caerulescens for urban soil remediation: influence of nitrogen fertilization and planting density. Ecol Eng 116:178–187. https://doi.org/10.1016/j.ecoleng.2018.03.007
Jacobs A, Drouet T, Noret N (2018b) Field evaluation of cultural cycles for improved cadmium and zinc phytoextraction with Noccaea caerulescens. Plant Soil 430:381–394. https://doi.org/10.1007/s11104-018-3734-2
Järup L, Åkesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmcol 238:201–208. https://doi.org/10.1016/j.taap.2009.04.020
Järup L, Berglund M, Elinder CG, Nordberg G, Vanter M (1998) Health effects of cadmium exposure–a review of the literature and a risk estimate. Scand J Work Environ Health 24:1–51. http://www.jstor.org/stable/40967243
Koopmans GF, Römkens PFAM, Fokkema MJ, Song J, Luo YM, Japenga J, Zhao FJ (2008) Feasibility of phytoextraction to remediate cadmium and zinc contaminated soils. Environ Pollut 156:905–914. https://doi.org/10.1016/j.envpol.2008.05.029
Kopittke PM, Wang P, Lombi E, Donner E (2017) Synchrotron-based X-ray approaches for examining toxic trace metal(loid)s in soil–plant systems. J Environ Qual 46:1175–1189. https://doi.org/10.2134/jeq2016.09.0361
Koren Å, Arčon I, Kump P, Nečemer M, Vogel-Mikuš K (2013) Influence of CdCl2 and CdSO4 supplementation on Cd distribution and ligand environment in leaves of the Cd hyperaccumulator Noccaea (Thlaspi) praecox. Plant Soil 370:125–148. https://doi.org/10.1007/s11104-013-1617-0
Kozhevnikova AD, Seregin IV, Aarts MGM, Schat H (2020) Intra-specific variation in zinc, cadmium and nickel hypertolerance and hyperaccumulation capacities in Noccaea caerulescens. Plant Soil 452:479–498. https://doi.org/10.1007/s11104-020-04572-7
Krämer U (2010) Metal hyperaccumulation in plants Annual. Rev Plant Biology 61:517–534. https://doi.org/10.1146/annurev-arplant-042809-112156
Küpper H, Mijovilovich A, Meyer-Klaucke W, Kroneck PMH (2004) Tissue- and age-dependent differences in the complexation of cadmium and zinc in the cadmium/zinc hyperaccumulator Thlaspi caerulescens (Ganges Ecotype) revealed by x-ray. Absorpt Spectrosc Plant Physiol 134:748–757. https://doi.org/10.1104/pp.103.032953
Li X, Zhao Z, Yuan Y, Wang X, Li X (2018) Heavy metal accumulation and its spatial distribution in agricultural soils: evidence from Hunan province, China. RSC Adv 8:10665–10672
Lin Y-F, Hassan Z, Talukdar S, Schat H, Aarts MGM (2016) Expression of the ZNT1 zinc transporter from the metal hyperaccumulator Noccaea caerulescens confers enhanced zinc and cadmium tolerance and accumulation to Arabidopsis thaliana. PLoS One 11:e0149750. https://doi.org/10.1371/journal.pone.0149750
Lombi E, Tearall KL, Howarth JR, Zhao F-J, Hawkesford MJ, McGrath SP (2002) Influence of iron status on cadmium and zinc uptake by different ecotypes of the hyperaccumulator. Thlaspi caerulescens Plant Physiology 128:1359–1367. https://doi.org/10.1104/pp.010731
Lombi E, Zhao FJ, Dunham SJ, McGrath SP (2000) Cadmium accumulation in populations of Thlaspi caerulescens and Thlaspi goesingense. New Phytol 145:11–20. https://doi.org/10.1046/j.1469-8137.2000.00560.x
Lombi E, Zhao FJ, McGrath SP, Young SD, Sacchi GA (2001) Physiological evidence for a high-affinity cadmium transporter highly expressed in a Thlaspi caerulescens ecotype. New Phytol 149:53–60. https://doi.org/10.1046/j.1469-8137.2001.00003.x
Lovy L, Latt D, Sterckeman T (2013) Cadmium uptake and partitioning in the hyperaccumulator Noccaea caerulescens exposed to constant Cd concentrations throughout complete growth cycles. Plant Soil 362:345–354. https://doi.org/10.1007/s11104-012-1291-7
Lu L, Liao X, Labavitch J, Yang X, Nelson E, Du Y, Brown PH, Tian S (2014) Speciation and localization of Zn in the hyperaccumulator Sedum alfredii by extended X-ray absorption fine structure and micro-X-ray fluorescence. Plant Physiol Biochem 84:224–232. https://doi.org/10.1016/j.plaphy.2014.10.004
Mahieu S, Frérot H, Vidal C, Galiana A, Heulin K, Maure L, Brunel B, Lefèbvre C, Escarré J, Cleyet-Marel J-C (2011) Anthyllis vulneraria/Mesorhizobium metallidurans, an efficient symbiotic nitrogen fixing association able to grow in mine tailings highly contaminated by Zn, Pb and Cd. Plant Soil 342:405–417. https://doi.org/10.1007/s11104-010-0705-7
Maxted AP, Black CR, West HM, Crout NMJ, McGrath SP, Young SD (2007) Phytoextraction of cadmium and zinc from arable soils amended with sewage sludge using Thlaspi caerulescens: Development of a predictive model. Environ Pollut 150:363–372. https://doi.org/10.1016/j.envpol.2007.01.021
McGrath S, Dunham S, Correll R (2000) Potential for phytoextraction of zinc and cadmium from soils using hyperaccumulator plants. Phytoremediation of contaminated soil and water. CRC Press, Boca Raton
McGrath SP, Zhao F-J (2003) Phytoextraction of metals and metalloids from contaminated soils. Curr Opin Biotechnol 14:277–282. https://doi.org/10.1016/S0958-1669(03)00060-0
Milner MJ, Craft E, Yamaji N, Koyama E, Ma JF, Kochian LV (2012) Characterization of the high affinity Zn transporter from Noccaea caerulescens, NcZNT1, and dissection of its promoter for its role in Zn uptake and hyperaccumulation. New Phytol 195:113–123. https://doi.org/10.1111/j.1469-8137.2012.04144.x
Milner MJ, Mitani-Ueno N, Yamaji N, Yokosho K, Craft E, Fei Z, Ebbs S, Clemencia Zambrano M, Ma JF, Kochian LV (2014) Root and shoot transcriptome analysis of two ecotypes of Noccaea caerulescens uncovers the role of NcNramp1 in Cd hyperaccumulation. Plant J 78:398–410. https://doi.org/10.1111/tpj.12480
Monsant AC, Kappen P, Wang Y, Pigram PJ, Baker AJM, Tang C (2011) In vivo speciation of zinc in Noccaea caerulescens in response to nitrogen form and zinc exposure. Plant Soil 348:167. https://doi.org/10.1007/s11104-011-0887-7
Mousset M, David P, Petit C, Pouzadoux J, Hatt C, Flaven É, Ronce O, Mignot A (2016) Lower selfing rates in metallicolous populations than in non-metallicolous populations of the pseudometallophyte Noccaea caerulescens (Brassicaceae) in southern France. Ann Botany 117:507–519. https://doi.org/10.1093/aob/mcv191
Nawrot T, Plusquin M, Hogervorst J, Roels HA, Celis H, Thijs L, Vangronsveld J, Van Hecke E, Staessen JA (2006) Environmental exposure to cadmium and risk of cancer: a prospective population-based study. Lancet Oncol 7:119–126. https://doi.org/10.1016/S1470-2045(06)70545-9
Nordberg GF, Fowler BA, Nordberg M (2014) Handbook on the toxicology of metals. Academic, Cambridge
Pence NS, Larsen PB, Ebbs SD, Letham DLD, Lasat MM, Garvin DF, Eide D, Kochian LV (2000) The molecular physiology of heavy metal transport in the Zn/Cd hyperaccumulator Thlaspi caerulescens. Proc Natl Acad Sci 97:4956–4960. https://doi.org/10.1073/pnas.97.9.4956
Qayyum S, Khan I, Meng K, Zhao Y, Peng C (2020) A review on remediation technologies for heavy metals contaminated soil. Cent Asian J Environ Sci Technol Innov 1:21–29. https://doi.org/10.22034/cajesti.2020.01.03
Reeves RD, Baker AJM, Jaffré T, Erskine PD, Echevarria G, van der Ent A (2018) A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol 218:407–411. https://doi.org/10.1111/nph.14907
Reeves RD, Schwartz C, Morel JL, Edmondson J (2001) Distribution and metal-accumulating behavior of Thlaspi caerulescens and associated metallophytes in France. Int J Phytoremediation 3:145–172. https://doi.org/10.1080/15226510108500054
Robinson BH, Leblanc M, Petit D, Brooks RR, Kirkman JH, Gregg PEH (1998) The potential of Thlaspi caerulescens for phytoremediation of contaminated soils. Plant Soil 203:47–56. https://doi.org/10.1023/A:1004328816645
Römkens PFAM, Guo HY, Chu CL, Liu TS, Chiang CF, Koopmans GF (2009) Prediction of cadmium uptake by brown rice and derivation of soil–plant transfer. models to improve soil protection guidelines Environmental Pollution 157:2435–2444. https://doi.org/10.1016/j.envpol.2009.03.009
Rosenfeld CE, Chaney RL, Martínez CE (2018) Soil geochemical factors regulate Cd accumulation by metal hyperaccumulating Noccaea caerulescens (J. Presl & C. Presl) F.K. Mey in field-contaminated soils. Sci Total Environ 616–617: 279–287. https://doi.org/10.1016/j.scitotenv.2017.11.016
Salt DE, Pickering IJ, Prince RC, Gleba D, Dushenkov S, Smith RD, Raskin I (1997) Metal accumulation by aquacultured seedlings of Indian Mustard. Environ Sci Technol 31:1636–1644. https://doi.org/10.1021/es960802n
Simmons RW, Chaney RL, Angle JS, Kruatrachue M, Klinphoklap S, Reeves RD, Bellamy P (2015) Towards practical cadmium phytoextraction with Noccaea caerulescens International. J Phytorem 17:191–199. https://doi.org/10.1080/15226514.2013.876961
Sterckeman T, Cazes Y, Gonneau C, Sirguey C (2017) Phenotyping 60 populations of Noccaea caerulescens provides a broader knowledge of variation in traits of interest for phytoextraction. Plant Soil 418:523–540. https://doi.org/10.1007/s11104-017-3311-0
Sterckeman T, Cazes Y, Sirguey C (2019) Breeding the hyperaccumulator Noccaea caerulescens for trace metal phytoextraction: first results of a pure-line selection. Int J Phytoremediation 21:448–455. https://doi.org/10.1080/15226514.2018.1537250
Suman J, Uhlik O, Viktorova J, Macek T (2018) Phytoextraction of heavy metals: A promising tool for clean-up of polluted environment? Front Plant Sci 9. https://doi.org/10.3389/fpls.2018.01476
Tóth G, Hermann T, Da Silva MR, Montanarella L (2016) Heavy metals in agricultural soils of the European Union with implications for food safety. Environ Int 88:299–309. https://doi.org/10.1016/j.envint.2015.12.017
Ueno D, Ma JF, Iwashita T, Zhao F-J, McGrath SP (2005) Identification of the form of Cd in the leaves of a superior Cd-accumulating ecotype of Thlaspi caerulescens using 113Cd-NMR. Planta 221:928–936. https://doi.org/10.1007/s00425-005-1491-y
Ueno D, Milner MJ, Yamaji N, Yokosho K, Koyama E, Clemencia Zambrano M, Kaskie M, Ebbs S, Kochian LV, Ma JF (2011) Elevated expression of TcHMA3 plays a key role in the extreme Cd tolerance in a Cd-hyperaccumulating ecotype of Thlaspi caerulescens. Plant J 66:852–862. https://doi.org/10.1111/j.1365-313X.2011.04548.x
van der Zee L, Corzo Remigio A, Casey LW, Purwadi I, Yamjabok J, van der Ent A, Kootstra G, Aarts MGM (2021) Quantification of spatial metal accumulation patterns in Noccaea caerulescens by X-ray fluorescence image processing for genetic studies. Plant Methods 17:86. https://doi.org/10.1186/s13007-021-00784-9
Vogel-Mikuš K, Arčon I, Kodre A (2010) Complexation of cadmium in seeds and vegetative tissues of the cadmium hyperaccumulator Thlaspi praecox as studied by X-ray absorption spectroscopy. Plant Soil 331:439–451. https://doi.org/10.1007/s11104-009-0264-y
Wang P, Chen H, Kopittke PM, Zhao F-J (2019) Cadmium contamination in agricultural soils of China and the impact on food safety. Environ Pollut 249:1038–1048. https://doi.org/10.1016/j.envpol.2019.03.063
Yan J, Wang P, Wang P, Yang M, Lian X, Tang Z, Huang C-F, Salt DE, Zhao FJ (2016) A loss-of-function allele of OsHMA3 associated with high cadmium accumulation in shoots and grain of Japonica rice cultivars. Plant Cell Environ 39:1941–1954. https://doi.org/10.1111/pce.12747
Yanai J, Zhao F-J, McGrath SP, Kosaki T (2006) Effect of soil characteristics on Cd uptake by the hyperaccumulator Thlaspi caerulescens. Environ Pollut 139:167–175. https://doi.org/10.1016/j.envpol.2005.03.013
Zhao F-J, Ma Y, Zhu Y-G, Tang Z, McGrath SP (2015) Soil contamination in China: current status and mitigation strategies Environmental. Sci Technol 49:750–759. https://doi.org/10.1021/es5047099
Zhao F-J, Tang Z, Song J-J, Huang X-Y, Wang P (2022) Toxic metals and metalloids: Uptake, transport, detoxification, phytoremediation, and crop improvement for safer food. Mol Plant 15:27–44. https://doi.org/10.1016/j.molp.2021.09.016
Zhao FJ, Lombi E, McGrath SP (2003) Assessing the potential for zinc and cadmium phytoremediation with the hyperaccumulator Thlaspi caerulescens. Plant Soil 249:37–43. https://doi.org/10.1023/A:1022530217289
Acknowledgements
This work was supported by the grants from the Natural Science Foundation of China (31972500). We thank Yanli Wang and Corrie Hanhart, and the WUR-Unifarm greenhouse technicians, for their excellent help in propagating the N. caerulescens lines.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Juan Barcelo.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(PPTX 107 KB)
Rights and permissions
About this article
Cite this article
Yan, J., Tang, Z., Fischel, M. et al. Variation in cadmium accumulation and speciation within the same population of the hyperaccumulator Noccaea caerulescens grown in a moderately contaminated soil. Plant Soil 475, 379–394 (2022). https://doi.org/10.1007/s11104-022-05373-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05373-w