Abstract
Aims
Crop residue amendment is likely to stimulate symbiotic N2 fixation, and clarifying its effect on N2-fixing bacteria, i.e., diazotrophs in the rhizosphere of legume crops, is important for sustainable N management in legume-cereal cropping systems. Therefore, this study aimed to reveal the diazotrophic community composition in the rhizosphere of soybean in response to maize residue amendment.
Methods
Being designed with treatments of maize residue, chemical fertilizer, and non-fertilizer applications, this study deployed the 15N-labeling technology combined with high-throughput sequencing of the nifH gene as a molecular marker for diazotrophs to quantify the symbiotically-fixed N2 in soybean plants and link symbiotically fixed N2 to the diazotrophic community diversity in the rhizosphere.
Results
Residue amendment increased the abundance of diazotrophs and fundamentally altered the composition of its community in the rhizosphere. It increased the relative abundances of Bradyrhizobium and Azohydromonas compared to the chemical fertilizer treatment. The copy number of nifH in the rhizosphere was associated with dissolved organic carbon and N2 fixation.
Conclusions
Residue-induced increase in dissolved organic carbon may provide sufficient carbon sources for diazotroph enrichment and thus enhance nodulation. The maize residue amendment may enrich N2 fixers to facilitate nodulation and subsequent N2 fixation of soybean, highlighting the eco-functional importance of diazotrophs fixing extra N into the rotation system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the decomposition of crop residues in soil, the residue-nitrogen (N) may either become a direct N source to subsequent crops (Meki et al. 2013; Dam et al. 2005), or the residue amendment may indirectly affect N availability by altering symbiotic N2 fixation in legumes. A number of studies reported that wheat residue amendment stimulated nodule formation and development, and consequently N2 fixation in soybean (Argaw 2014; Khaliq and Abbasi 2015; Li et al. 2017). Similarly, our unpublished data indicate that the symbiotically fixed N rather than residue-derived N became the dominant N source in soybean grown under the amendment with maize residue. In contrast, Kadiata et al. (2002) observed that barley straw amendment into a brown soil decreased nodule number and N2 fixation in faba bean compared to chemical N supply. The most probable reason for these discrepancies would be different responses of rhizobia to the residue amendment in the rhizosphere of legumes, as the abundance and the composition of the rhizobial community strongly determine nodulation and N2 fixation (Yu et al. 2018).
Previous studies on the impact of residue amendment on the rhizobium community were mainly conducted using bulk soil, rather than considering its interaction with the roots of legume plants. For instance, Tang et al. (2017) showed that a combination of rice straw and chemical fertilizer increased the diazotrophic richness and diversity in the top 20 cm of a paddy soil. Using 13C stable isotope probing (SIP), Wang et al. (2019) found that some residue-metabolizing bacteria might belong to the diazotrophic community after 28 days of soybean residue amendment into a Mollisol soil. However, few studies have examined the residue-mediating diazotrophic community in the rhizosphere of legumes as this response of the diazotrophic community may substantially influence symbiotic diazotrophs and consequently alter the nodulation and N2 fixation of the subsequent legume crop under residue amendment (Kadiata et al. 2002; Thilakarathna and Raizada 2017).
Using the high-throughput sequencing technology, we investigated the effect of residue amendment on the abundance and community composition of diazotrophs in the rhizosphere of soybean and its association with N2 fixation. We hypothesized that residue amendment would enhance the rhizobium diversity in the rhizosphere of soybean as the residue provides additional carbon substrates for the growth of the diazotrophic community.
Materials and methods
Experimental design and plant growth
A pot experiment was conducted in a glasshouse at the Northeast Institute of Geography and Agroecology (45° 41′ N, 126° 38′ E), Chinese Academy of Sciences, Harbin, China. The temperature in the glasshouse ranged from 24 to 28 °C during the day and from 16 to 20 °C at night. Three treatments, i.e., residue amendment, chemical fertilizer, and non-fertilizer as control, were deployed in this experiment with three replicates. Two plants were grown in each pot (19 cm diameter, 30 cm height) filled with 9 kg of a silty clay Mollisol that was collected from the top 20 cm tillage layer from farming land located on Guangrong Village (47° 23′ N, 126° 51′ E). Approximately 81 mg N kg−1 (equal to 55 kg N ha−1) with 19.2% of 15N and 5 mg kg−1 with 96% of 15N atom-excess in urea were applied for the chemical fertilizer and non-fertilizer treatments, respectively. In the non-fertilizer treatment, the application of 5 mg N kg−1 was considered minimal N input to estimate symbiotic N2 fixation using 15N-labeling technology. Regarding the residue amendment treatment, 22.5 g of above-ground residues of maize (2.65% of 15N atom-excess) was applied (equal to 8 t ha−1). Basal nutrients were thoroughly mixed with the soil before sowing (Jin et al. 2010). The soybean (Glycine max L.) cultivar Dongsheng 1 was used in this study, which has been widely planted in Northeast China (Zhang et al. 2014). In addition, a non-nodulating isogenic soybean genotype, Clark L73-1054, was grown under the same 15N-labeling condition for each treatment to estimate N2 fixation (Elmore and Jackobs, 1986). In each treatment, there were four pots of the non-nodulation soybean genotype (Clark L73-1054).
Harvest and measurements
At the initial pod-filling stage (R5, 64 days after sowing) and the maturity stage (R8, 130 days after sowing), three pots from each treatment were harvested, respectively. The reason for the two harvests was that nodules reached maximal biomass at R5 and total N2 fixation over the growth season can be estimated at R8 (Jin et al. 2010). The harvest at R8 was for N2 fixation measurements only. Shoots were removed at the soil surface, and the root system was separated from the soil. Then, roots from the first harvest were gently shaken to collect rhizosphere soils that adhered to the roots. Approximately 2 g of rhizosphere soil sample that adhered to roots was immediately stored at − 80 °C for DNA extraction. The rest of the soil samples were used for soil biochemical property analysis.
Soil NH4+ and NO3− were extracted from fresh soils in 100 mL of 1 M KCl, and their concentrations were measured using a flow injection auto-analyzer (SKALAR, San++, the Netherlands). Soil pH was measured using a pH meter after shaking the soil-water (1:5 v/v in H2O) suspension for 30 min. Dissolved organic carbon (DOC) in soil was extracted in 100 mL of 0.5 M K2SO4, and determined using an automated TOC Analyzer (Shimadzu, TOC-VCPH, Japan). Olsen P was extracted with 0.5 M NaHCO3 and analyzed colorimetrically (Murphy and Riley, 1962). Available potassium (K) was determined by flame photometry following extraction with 1 M ammonium acetate (Walker and Barber, 1962). Plant materials were dried in an oven at 65 °C for 72 h. The N concentration of plant tissues was analyzed with an EL III analyzer (Elementar Analysensysteme, Hanau, Germany), then the atom% 15N was determined using an isotope ratio mass spectrometer (Deltaplus, Finnigan MAT, Bremen, Germany).
The nodule numbers, weight and density were determined at R5, and the amount of N2 fixed was estimated at R5 and R8, using two pots of Clark L73-1054 in each treatment as the non-N2-fixing control.
DNA extraction and quantitative PCR analysis
Soil DNA was extracted from 0.5 g fresh soil using a Fast DNA SPIN Kit for Soil (Qbiogene Inc., Carlsbad, CA, USA). As the nifH genes are the most representative sequences for the diazotrophic community, the copy number of nifH genes were determined using primers nifH-F (5′-AAA GGY GGWATC GGYAAR TCCA CCA C-3′) and nifH-R (5′-TTG TTS GCS GCRTAC ATS GCC ATC AT-3′) (Rösch et al. 2002) in a LightCycler® 480 system (Roche Applied Science, Basel, Switzerland). The copy number of the bacterial 16S rRNA gene was also determined using the universal primers 338-F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806-R (5′- GGACTACHVGGGTWTCTAAT-3′) to target the V3–V4 region of the prokaryotic 16S rRNA gene. In the qPCR reaction (20 μL) containing 1 μL DNA template, 10 μL of SYBR Premix Ex TaqTM (Takara, Dalian, China), 1 μL of 10 μM each primer, and 7.0 μL of sterilized MilliQ water, qPCR amplification was initiated at 95 °C for 30 s, followed by 35 cycles of 95 °C for 5 s and 60 °C for 30 s and 5 °C for 30 s. We used a regression equation for converting the cycle threshold value (Ct) to the number of nifH gene ranged from 3.1 × 105 to 9.6 × 105 gene copies per μL, which were in the standard range from 4.4 × 102 to 4.4 × 108 gene copies per μL. The bacterial 16S rRNA gene copies of samples ranged from 9.1 × 107 to 1.7 × 108, which were within the standard scale from 6.0 × 102 to 6.0 × 108 gene copies per μL. Then, the copy number per μL in the PCR reaction system was converted to the copy number per gram of soil (Sun et al. 2015).
Illumina MiSeq sequencing
Illumina MiSeq sequencing was performed by creating the amplicon libraries of the nifH gene and 16S rRNA gene with the same primers, respectively. All the remaining high-quality sequences were clustered into operational taxonomic units (OTU) based on a 97% similarity level with UPARSE (Edgar 2013). Representative sequences from each OTU were selected and aligned with the FunGene database (http://fungene.cme.msu.edu/). Then, using GenBank (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to determine the taxonomic identity of each phylotype, a random subset of 9402 and 19,244 sequences were selected based on the minimum sequences across samples for the nifH gene and the 16S rRNA gene, respectively. The raw sequences obtained in this study were deposited into the NCBI database under the accession number PRJNA626399.
Calculations and statistical analysis
Atom% 15N excess was calculated according to the natural 15N abundance in the air, i.e., 15Nair = 0.36647 atom% (Werner and Brand 2001). Isotopic ratios of N in plants that was derived from the atmosphere due to N2 fixation (%Ndfa) were calculated as follows (Rennie and Dubetz 1986; Li et al. 2016):
where fs and nfs indicated fixing and non-fixing (Clark L73-1054) (Elmore and Jackobs 1986) soybean genotypes, respectively.
The amount of N2 fixed per plant was calculated as:
where Nplant was the N content of the shoot.
According to the integrated high-throughput absolute quantification (iHAAQ) method, the absolute abundance of each specific genus in the diazotrophic community was estimated using the copy number of the nifH gene multiplied by the relative abundance of the genus from high-throughput sequencing (Lou et al. 2018). Statistical significance was analyzed using the SPSS software (version 19.0). One-way ANOVA was conducted to reveal the differences in soil properties, alpha diversity, and relative abundance of diazotrophic taxa among the treatments. The Pearson correlations of the relative and absolute abundances of diazotrophic taxa with soybean nodulation and the environmental properties factors were visualized on heatmaps. In addition, the correlations of alpha diversity indices with the nodulation and environmental properties were estimated as well.
Principal coordinate analysis (PCoA) was based on a Bray-Curtis distance matrix; the hierarchy clustering analysis was performed based on the weighted UniFrac distance. The PCoA and clustering analysis VIF RDA were calculated using the “vegan” and “GUniFrac” packages in the R platform (version 3.2.5) (R Development Core Team 2016).
In order to assess the impact of residue amendment on the network of diazotrophic communities in the rhizosphere, a co-occurrence network analysis was performed by calculating pairwise correlations between diazotrophic OTUs of all samples using the R package “psych” (Revelle, 2017). The OTUs with a relative abundance >0.1% and correlations between those with r > 0.8 and P ≤ 0.05 were included in the network. Gephi v.0.9.2 was used for co-occurrence network visualization and module analysis (Bastian et al. 2009).
Results
The richness and diversity of the diazotrophic community
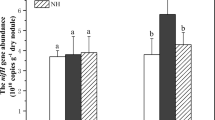
The coverage values of all samples were more than 99%, revealing that the number of sequences was sufficient to represent the diazotrophic diversity. There was no significant difference in the OTU richness index (ACE and Chao1) among the three treatments (Table 1). However, the Simpson index significantly decreased in the residue amendment and chemical fertilizer treatments compared to the non-fertilizer treatment (Table 1). Residue amendment significantly increased the copy numbers of nifH gene and bacterial 16S rRNA gene compared to the non-fertilizer and chemical fertilizer treatments (Table 1, Figure S1).
Diazotrophic and bacterial community structures
A PCoA plot was presented for diazotrophic community structure discrimination (Fig. 1). The first (x-axis) and the second (y-axis) principal coordinates together explained 81.1% variation of the diazotrophic community. The diazotrophic community under the residue amendment treatment was separated from the chemical fertilizer treatment. The hierarchical clustering tree also revealed that there was fundamental dissimilarity among the three treatments. The most abundant genus of diazotrophs was Bradyrhizobium, and its abundance was significantly different among the three treatments with 61.8%, 52.9% and 69.1% in the residue amendment, chemical fertilizer, and non-fertilizer treatments, respectively (Fig. 2). The second abundant genus was Azohydromonas, and its abundance was significantly higher under residue amendment than under the non-fertilizer and chemical fertilizer treatments. The relative abundance of each OTU in those genera is detailed in Table S1.
The entire network of the diazotrophic community was parsed into seven major modules (Figure S2), and the OTU numbers under three treatments in each module indicated how the module was specific to those treatments. In module 6, the OTU number under the residue amendment was more than any other treatment, indicating that the residue amendment likely dominated the keystone nodes in this module.
The rhizobacterial community structure was significantly (Adonis, P=0.005) different among the treatments (Figure S3). The phyla Proteobacteria, Actinobacteria, Acidobacteria, and Chloroflexi accounted for 77.8–80.5% of the rhizobacterial community. The residue amendment and chemical fertilizer treatments significantly increased the relative abundance of Proteobacteria but decreased the relative abundance of Acidobacteria and Chloroflexi compared to the non-fertilizer treatment (Figure S4).
Soybean nodulation and N2 fixation
The amount of fixed N by soybean plants in the chemical fertilizer treatment was much less (P ≤ 0.05) than the residue amendment or non-fertilizer treatment (Table 2). The nodule density and fixed N per nodule at the R5 stage in the residue amendment treatment were 67.3% and 107% higher than the chemical fertilizer treatment, but no difference was found compared to the non-fertilizer treatment. Although the non-fertilizer treatment increased the nodule number and fixed N at the R5 stage compared to the residue amendment and chemical fertilizer treatments, the residue amendment increased the fixed N per nodule and total fixed N at the R8 stage compared to the non-fertilizer treatment.
Soil chemical properties and its association to diazotrophic community
Residue amendment increased the concentrations of available K and DOC in the rhizosphere by 30.0% and 53.6%, respectively, compared to the non-fertilizer treatment, but decreased pH compared to the fertilizer and non-fertilizer treatment (Table S2). Compared to the non-fertilizer treatment, the residue amendment and chemical fertilization treatment significantly increased available K, and the chemical fertilization treatment also significantly increased NO3−-N compared to the residue amendment and non-fertilizer treatments (P ≤ 0.05).
The Mantel test showed that pH, NO3−-N, and DOC were significant factors in shifting the diazotrophic community structures (Table S3). Redundancy analysis indicated that the shift of the diazotrophic community in response to residue amendment was associated positively with DOC concentrations and negatively with pH and NO3−-N in the rhizosphere (Fig. 3a). Pearson’s correlation analyses showed that the nifH gene copy number was negatively correlated with the soil pH and positively correlated with DOC, N2 fixation per nodule, and N2 fixation at R8 (Table 3). The relative abundances of the genera were significantly correlated with some soil properties. The relative abundance of Bradyrhizobium was negatively correlated with the soil NO3−-N concentration. The abundance of Anaeromyxobacter was negatively correlated with the available K concentration. The N2 fixation at R8 was positively correlated with Azohydromonas and Geobacter (Fig. 3b). In addition, the absolute abundance of Bradyrhizobium was significantly (P < 0.05) correlated with DOC and N2 fixation at R5 and R8 (Figure S5).
a) Redundancy analysis of soil diazotrophic communities in the chemical fertilizer, residue amendment, and non-fertilizer treatments; b) The heatmap of correlation of soil properties and N2 fixation at the initial pod-filling (R5) and maturity (R8) stages with the relative abundances of the diazotrophic genera. * and ** represent P ≤ 0.05 and P ≤ 0.01, respectively
Discussion
Residue amendment significantly increased the copy number of nifH genes compared to the chemical fertilizer and non-fertilizer treatments and decreased the Simpson index of the diazotrophic community compared to the non-fertilizer treatment (Table 1). This finding was consistent with other studies on the increased diazotrophic diversity of soil in response to the amendment of wheat and rice residues (Tang et al. 2017). This result could be attributed to the increase of labile C in the residue-amended soil, being beneficial to the growth of the nifH-expressing bacteria which are generally heterotrophic or mixotrophic (Rahav et al. 2016). A significant increase in the concentration of DOC and a positive correlation between DOC and nifH copy number were observed in this study (Tables 3 and S2). Thus, the increased abundance of diazotrophic bacteria under residue amendment likely enhances their infectious opportunities to soybean plants to produce more nodules and fix more N2 (Table 2). While we found a stimulation of N2 fixation and increased abundance of diazotrophs with increased DOC, del Valle et al. (2020) found a negative correlation between the addition rate of organic matter to soil and N2 fixation in alfalfa, which was attributed to the decrease of the lifetime of flavonoids. Flavonoids, derived from root exudates, trigger rhizobium Nod gene synthesis necessary to initiate nodulation (Peck et al. 2006; Zhang et al. 2009). It is possible that different types of DOC have various effects on diazotrophs and the ability to nodulate with legumes. Nevertheless, Maarastawi et al. (2019) found that the amendment of rice residue reduced root exudate consumption by microorganisms because the residue provided an additional C source for microorganisms. These results imply that flavonoids may be retained longer with the decreasing decomposition of root exudates in the residue treatment. However, further investigations on flavonoid retention in the rhizosphere and how flavonoids interact with diazotrophs in response to reside amendment are warranted to verify these assumptions.
In addition to the increased abundance of diazotrophs, the residue also substantially altered the composition of the diazotroph community. Bradyrhizobium and Azohydromonas were likely to be major members in the diazotrophic community in response to maize residue amendment, as the relative abundances of these two genera were more than 50% in the diazotrophic community and greater in the residue treatment than in the chemical fertilizer treatment. Moreover, Bradyrhizobium has been reported as the universal and predominant rhizobia for soybean in China (Man et al. 2008; Salas et al. 2019). The greater absolute abundance of Bradyrhizobium in the residue amendment treatment compared to the chemical fertilizer treatment (Figure S6) and the significant association of the relative and absolute abundance of Bradyrhizobium with N2 fixation (Figs. 3; S5) indicated that the diazotrophic members would be pivotal to the stimulation of N2 fixation by the residue amendment. Wang et al. (2019) also proposed that the process of wheat residue decomposition may alter the composition of the diazotrophic community, leading to the difference in N2 fixation. Azohydromonas is a rhizosphere inhabitant that has been found associated with legume nodules, where it consumes hydrogen released during N2 fixation inside nodules (Tkacz et al. 2020). Moreover, in response to residue amendment, the increased abundance of these N2-fixing genera would increase their chances of being the dominant symbiont within the soybean nodules. Nevertheless, the relative abundances of these genera were not associated with the concentration of nutrients except NO3−-N (Fig. 3), suggesting that residue-induced changes in nutrient status were not the major contributor to the composition change. The high concentration of NO3−-N in the N-sufficient soils has been found to suppress the growth of N2 fixers and subsequent N2 fixation (Fan et al. 2019; Feng et al. 2018). Interestingly, the residue amendment may impact rhizobium communication, which is associated with nodulation. It has been shown that the N mineralization of crop residue would regulate the rhizobium communication with legumes (Kadiata et al. 2002; Thilakarathna and Raizada 2017) and rhizobium-produced Nod-factors greatly influence rhizobium infection efficiency in legumes (Huang et al. 2014; Laranjo et al. 2014). Moreover, the residue-induced change (the domination of residue-originated OTUs) in a module of the network of the diazotrophic community (Figure S2) further indicates the potential shift of the N2-fixing-relevant function of the diazotrophic community since species groups clustering in the same module are considered to contribute similar functions (Rottjers and Faust 2018), and keystone nodes are crucial components of networks driving community composition and functions (Banerjee et al. 2018; Zhou et al. 2021).
The increased concentration of DOC under residue amendment, compared to the non-fertilizer control, resulted in the supply of additional C sources for diazotrophs to enhance N2 fixation as indicated with stronger N2 fixation per nodule in this treatment especially during the late growth stage from R5 to R8 (Table 2). Moreover, DOC was positively associated with the diazotrophic community (Fig. 3a) and absolute abundance of Bradyrhizobium (Figure S6), implying that Bradyrhizobium was able to access more available organic C to fix N2 during the late growth stage in the residue treatment. Thus, DOC derived from residues would be a major factor regulating diazotroph community composition as well as the temporal function of nodulation.
Conclusions
In Mollisols amended with maize residue, the community structure of diazotrophs was altered in the rhizosphere of soybean. The dominant genera of Bradyrhizobium and Azohydromonas were more abundant in the residue amendment than in the chemical fertilizer treatment. Since these genera were predominant rhizobia infecting soybean and significantly associated with N2 fixation, we postulated that the residue amendment stimulated the N2 fixation through increasing their abundances, as the concentration of dissolved organic C was positively correlated with the copy numbers of nifH genes and of Bradyrhizobium. Residue amendment increased the concentration of dissolved organic C and thus enriched diazotrophic bacteria and likely enhanced their ability to nodulate and fix N2 in soybean plants. Future research may elucidate how residue amendment mediates the communication between rhizobia and Nod gene expression during nodulation of legumes.
References
Argaw A (2014) Symbiotic effectiveness of inoculation with Bradyrhizobium isolates on soybean [Glycine max (L.) Merrill] genotypes with different maturities. Springerplus 3:753
Banerjee S, Schlaeppi K, van der Heijden MGA (2018) Keystone taxa as drivers of microbiome structure and functioning. Nat Rev Microbiol 16:567–576
Bastian M, Heymann S, Jacomy M (2009) Gephi: an open source software for exploring and manipulating networks” in Proceedings of the third international conference on weblogs and social media conference, Vol. 8, San Jose, CA; pp 361–362
Dam RF, Mehdi BB, Burgess MSE, Madramootoo CA, Mehuys GR, Callum IR (2005) Soil bulk density and crop yield under eleven consecutive years of corn with different tillage and residue practices in a sandy loam soil in Central Canada. Soil Tillage Res 84:41–53
Del Valle I, Webster TM, Cheng HY, Thies JE, Kessler A, Miller MK, Ball ZT, MacKenzie KR, Masiello CA, Silberg JJ, Lehmann J (2020) Soil organic matter attenuates the efficacy of flavonoid-based plant-microbe communication. Sci Adv 6:eaax8254
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998
Elmore RW, Jackobs JA (1986) Yield and nitrogen yield of sorghum intercropped with nodulating and nonnodulating soybeans. Agron J 78:780–782
Fan K, Delgado-Baquerizo M, Guo X, Wang D, Wu Y, Zhu M, Yu W, Yao H, Zhu Y, Chu H (2019) Suppressed N fixation and diazotrophs after four decades of fertilization. Microbiome 7:143
Feng M, Adams JM, Fan K, Shi Y, Sun R, Wang D, Guo X, Chu H (2018) Long-term fertilization influences community assembly processes of soil diazotrophs. Soil Biol Biochem 126:151–158
Huang XF, Chaparro JM, Reardon KF, Zhang RF, Shen QR, Vivanco JM (2014) Rhizosphere interactions: root exudates, microbes, and microbial communities. Botany 92:281–289
Jin J, Wang G, Liu X, Mi L, Li Y, Xu Y, Herbert SJ (2010) Genetic improvement of yield shapes the temporal and spatial root morphology of soybean (Glycine max) grown in north-East China. New Zeal J Crop Hort 38:177–188
Kadiata BD, Yan F, Schubert S (2002) Nodulation and N2 fixation of faba bean (Vicia faba L.) after soil amendment with crop residue. J Plant Nutr Soil Sc 165:725–731
Khaliq A, Abbasi MK (2015) Soybean response to single or mixed soil amendments in Kashmir, Pakistan. Agron J 107:887–895
Laranjo M, Alexandre A, Oliveira S (2014) Legume growth-promoting rhizobia: an overview on the Mesorhizobium genus. Microbiol Res 169:2–17
Li YS, Liu XB, Wang GH, Yu ZH, Mathesius U, Liu JD, Stephen JH, Jin J (2016) Shift in origin of plant nitrogen alters carbon and nitrogen assimilation during reproductive stages of soybean grown in a Mollisol. Crop Pasture Sci 67:872–880
Li YS, Yu ZH, Liu XB, Mathesius U, Wang GH, Tang CX, Wu JJ, Liu JJ, Zhang SQ, Jin J (2017) Elevated CO2 increases nitrogen fixation at the reproductive phase contributing to various yield responses of soybean cultivars. Front Plant Sci 8:1546
Lou J, Yang L, Wang H, Wu L, Xu J (2018) Assessing soil bacterial community and dynamics by integrated high-through put absolute abundance quantification. Peer J 6:e4514
Maarastawi SA, Frindte K, Bodelier PLE, Knief C (2019) Rice straw serves as additional carbon source for rhizosphere microorganisms and reduces root exudate consumption. Soil Biol Biochem 135:235–238
Man CX, Wang H, Chen WF, Sui XH, Wang ET, Chen WX (2008) Diverse rhizobia associated with soybean grown in the subtropical and tropical regions of China. Plant Soil 310:77–87
Meki MN, Atwood JD, Norfleet LM, Williams JR, Gerik TJ, Kiniry JR (2013) Corn residue removal effects on soybean yield and nitrogen dynamics in the upper Mississippi River basin. Agroecol Sust Food 37:379–400
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Peck MC, Fisher RF, Long SR (2006) Diverse flavonoids stimulate NodD1 binding to nod gene promoters in Sinorhizobium meliloti. J Bacteriol 188:5417–5427
R Development Core Team (2016) R: a language and environment for statistical computing. The R Foundation for Statistical Computing, Vienna. Available at http://www.R-project.org/
Rahav E, Giannetto MJ, Bar-Zeev E (2016) Contribution of mono and polysaccharides to heterotrophic N2 fixation at the eastern Mediterranean coastline. Sci Rep 6:27858
Rennie RJ, Dubetz S (1986) Nitrogen-15-determined nitrogen fixation in field-grown chickpea, lentil, fababean, and field pea. Agron J 78:654–660
Revelle W (2017) Procedures for personality and psychological research. Northwestern University, Evanston
Rösch C, Mergel A, Bothe H (2002) Biodiversity of denitrifying and dinitrogen-fixing bacteria in an acid forest soil. Appl Environ Microbiol 68:3818–3829
Rottjers L, Faust K (2018) From hairballs to hypotheses-biological insights from microbial networks. FEMS Microbiol Rev 42:761–780
Salas A, Tortosa G, Hidalgo-Garcia A, Delgado A, Bedmar EJ, Richardson DJ, Gates AJ, Delgado MJ (2019) The hemoglobin Bjgb from Bradyrhizobium diazoefficiens controls NO homeostasis in soybean nodules to protect symbiotic nitrogen fixation. Front Microbiol 10:2915
Sun RB, Zhang XX, Guo XS, Wang DZ, Chu HY (2015) Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol Biochem 88:9–18
Tang YF, Zhang MM, Chen AL, Zhang WZ, Wei WX, Sheng R (2017) Impact of fertilization regimes on diazotroph community compositions and N2-fixation activity in paddy soil. Agric Ecosyst Environ 247:1–8
Thilakarathna MS, Raizada MN (2017) A meta-analysis of the effectiveness of diverse rhizobia inoculants on soybean traits under field conditions. Soil Biol Biochem 105:177–196
Tkacz A, Bestion E, Bo Z, Hortala M, Philip S, Poole (2020) Influence of plant fraction, soil, and plant species on microbiota: a multikingdom comparison. mBio 11:e02785–e02719
Walker JM, Barber SA (1962) Absorption of potassium and rubidium from the soil by corn roots. Plant Soil 17:243–259
Wang YH, Yu ZH, Li YS, Wang GH, Tang CX, Liu XB, Liu JJ, Xie ZH, Jin J (2019) 13C-DNA-SIP distinguishes the prokaryotic community that metabolizes soybean residues produced under different CO2 concentrations. Front Microbiol 10:2184
Werner RA, Brand WA (2001) Referencing strategies and techniques in stable isotope ratio analysis. Rapid Commun Mass Spectrom 15:501–519
Yu Z, Li Y, Wang G, Tang C, Wang Y, Liu J, Liu X, Jin J (2018) Elevated CO2 alters the abundance but not the structure of diazotrophic community in the rhizosphere of soybean grown in a Mollisol. Biol Fert Soils 54:877–881
Zhang J, Subramanian S, Stacey G, Yu O (2009) Flavones and flavonols play distinct critical roles during nodulation of Medicago truncatula by Sinorhizobium meliloti. Plant J 57:171–183
Zhang W, Wang G, Wang M, Liu X, Feng Z (2014) Responses of soybean cultivar Dongsheng-1 to different O3 concentrations in Northeast China. Environ Sci 35:1473–1478 (in Chinese)
Zhou L, Zhou Y, Tang X, Zhang Y, Jang KS, Székely AJ, Jeppesen E (2021) Resource aromaticity affects bacterial community successions in response to different sources of dissolved organic matter. Water Res 190:116776
Funding
The project was funded by the National Natural Science Foundation of China (41771326), the National Key Research and Development Program of China (2017YFD0300300), and the “Touyan” Program of Heilongjiang province and Youth Innovation Promotion Association of Chinese Academy of Sciences (2019233).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial Responsibility: Euan K. James
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOC 688 kb)
Rights and permissions
About this article
Cite this article
Xie, Z., Yu, Z., Li, Y. et al. Linking rhizospheric diazotrophs to the stimulation of soybean N2 fixation in a Mollisol amended with maize straw. Plant Soil 463, 279–289 (2021). https://doi.org/10.1007/s11104-021-04904-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-021-04904-1