Abstract
Background and aims
Understanding the variability in water availability in agroforestry systems in rain-fed orchards is vital for optimizing orchard management in semiarid areas. However, few studies have examined the soil capacity of water stock and supply in these systems over multiple years. We aim at (i) characterizing several soil physical properties related to water availability and inter-annual dynamics of soil water content and (ii) exploring their response to meteorological conditions and root distribution.
Methods
Jujube (Ziziphus jujuba Mill.) intercropped with the fodder species canola (Brassica napus L.) (JFCS), jujube intercropped with daylily (Hemerocallis fulva L.) (JDLS), and a jujube orchard with clean tillage (JCS) were established on the Loess Plateau, China. Soil physical properties (including soil bulk density, soil hydraulic conductivity, soil field capacity, and soil porosity), soil water content and fine root data were collected over the period 2014–2017.
Results
Compared to JCS-Tree, the field capacity was significantly increased both in the JFCS-Tree and JDLS-Tree treatments, while soil capillary porosity increased significantly only in the JFCS-Tree. Compared to JCS-Inter-row, the JFCS-Inter-row and JDLS-Inter-row exhibited significantly decreased soil bulk density, and increased field capacity, saturated hydraulic conductivity, and improved soil porosity, but the non-capillary porosity in the JDLS-Inter-row treatment were not significantly modified. Compare to JCS-Tree treatment, the soil water at 0–60 cm significantly increased under JFCS-Tree and JDLS-Tree in four years. However, due to the deeper fine root distribution for both tree and crop under JDLS-Inter-row, the soil water content at 60–180 cm in JDLS-Inter-row significantly decreased more than JFCS-Inter-row and JCS-Inter-row.
Conclusions
The introduced crop modified the soil physical properties and soil water content, indirectly under trees and directly between inter-rows through the role of fine roots, thereby changing the orchard environment in semiarid areas. Agroforests can generally improve water condition at shallow soil layers compared to monocultural plantations, although such an effect may be accompanied with lower water stock at deeper soil layers in inter-rows, depending on crop species chosen.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drylands account for around 40% of all terrestrial ecosystems, and nearly 38% of the world’s population would be affected by climate change (Huang et al. 2016a). In particular, changes in precipitation and temperature have been identified as a concern for agricultural production in dryland agriculture (Slingo et al. 2005; Bradford et al. 2017; Huang et al. 2017). Typically, agricultural production in certain regions may be at risk in the future due to increased competition for water and more variability in extreme rainfall and drought (Altieri 2002; Altieri and Nicholls 2017). This issue not only threatens food production but could also jeopardize the many small farmers who depend on rain-fed agriculture for their livelihoods (Verdin et al. 2005; Zhang and He 2016). Agroforestry systems, as traditional intensive land use, are playing an important role in combating climate change and increasing system stability. Recently, many studies have demonstrated that agroforestry may be more climate-smart and resilient to climatic extremes than other agricultural methods and deliver more socioeconomic benefits (Altieri and Nicholls 2017; Sida et al. 2018). However, in environmentally fragile areas (e.g., arid and semiarid zones and hilly regions), establishing effective agroforestry is still a challenge because of the restrictions and limitations of economic conditions and water availability.
Soil physical properties are widely considered to be the key factors reflecting the effect of soil management (Merwin et al. 1994; Steele et al. 2012; Liu et al. 2013). It may be better to examine soil physical properties that indicate the capacity of a soil to function within an ecosystem or land-use area and to sustain biological productivity, maintain environmental quality, and promote plant and animal health (Doran and Parkin 1994). Agroforestry is a successful and sustainable soil management approach, providing an alternative to conventional tillage in orchards (Ramos et al. 2010; King and Berry 2005; Tahir et al. 2016; Palese et al. 2014; Huang et al. 2016a, b; Li et al. 2007; Schwab et al. 2015; Abdulai et al. 2018). It could alter many aspects of soil physical properties, such as soil bulk density and soil porosity, which are essential soil structure favorable to crop root growth and soil water holding capacity (Xu and Zhang 2004; Liu et al. 2013). Meantime, the increase of soil saturated hydraulic conductivity and field capacity is essential to the faster downward movement of water (Klik et al. 1998; Siriri et al. 2005), improving water retention and increasing plant available water content (Bilek 2007). These characteristics reflect the fundamental aspect of soil performances, which indeed are altered by the management practices (Steele et al. 2012; Schwab et al. 2015; Sun et al. 2018; Liu et al. 2013). Li et al. (2007) suggested that intercropped with herbage in an apple orchard, the soil bulk density reduced, the soil porosity increased, and the soil water-holding capacity enhanced greatly. Long-term use of winter rye cover crop significantly increased both the field capacity by 10–11% and plant available water by 21–22% (Basche et al. 2016). Nevertheless, these benefits are subject to the choice and management of relevant cover crops, to ensure that they promote beneficial ecosystem service while circumscribing above-ground and below the ground competition (Liu et al. 2013; Palese et al. 2014). Meanwhile, indicators of soil physical properties that respond on relatively long scales (e.g., several years) to change in management are critical for evaluating agroforestry system functioning (Schwab et al. 2015; Basche et al. 2016). However, these studies focus on the soil physical properties change in the entire ecosystem, little attention has been paid on the soil physical properties difference between understory and inter-row which were affected by crops in an agroforestry system.
In arid and semiarid areas, water availability in orchards will always be the main focus of attention. Inter-row vegetation consumes a substantial amount of water, which may not be supplemented by precipitation, causing a water shortage during tree growth (Li et al. 2007; Bai et al. 2018). Li et al. (2007) studied the grass in apple orchards and found that the impact of grass on soil water content depends on the type of rainfall. Moreover, their research suggested that grass increased soil water content under trees during normal years but decreased soil water content in drought years. Abdulai et al. (2018) suggested that cocoa agroforestry is less resilient than cocoa in full sun when subjected to sub-optimal or extreme climate because of the excessive consumption of soil water in agroforestry. Hence, understanding the effects of different rainfall years on agroforestry systems is necessary for developing sustainable plantations in dryland environments. However, most previous studies mainly focus on the soil physical properties in a permanent grass-vegetated orchard, while little is known about the effects of economic crops in orchards and the soil physical properties and soil water content dynamics in years with varying rainfall in semiarid areas.
The Loess Plateau is one of the main dryland regions of China, on which jujube orchards deliver key economic, social, and ecological functions (Zhao et al. 2009). Due to the complicated topography (i.e., hills and gullies) of the Loess Plateau and high irrigation costs, most jujube orchards are rain-fed and managed under clean tillage conditions (Gao et al. 2016). Unfortunately, rainfall is not synchronized with the critical stages of the jujube production cycle. Moreover, sites, where clean tillage is practiced are prone to soil erosion and land degradation, as this method is characterized by low rainfall infiltration and utilization during intensive precipitation and higher post-rain evaporation (Pan et al. 2017; Bai et al. 2018). Intercropping (grass) in orchards can modify the distribution of soil water content as it influences both soil physical properties (e.g., infiltration, water holding capacity) and the soil water loss rate (Shaxson and Barber 2003; Yang et al. 2016). This study seeks to investigate how an agroforestry system affects soil physical properties and soil water content in a rain-fed jujube orchard with two economic crops over four years and if they could be explained by meteorological and root data. An annual plant, the fodder species canola (Brassica napus L.), and a perennial plant, daylily (Hemerocallis fulva L.), were chosen to examine the role of crops in a jujube orchard. To assist farmers in northwest China, it is important to determine which crop and/or soil management approaches are the most suitable for jujube agroforestry in the context of scarce water resources in a semiarid environment. The results presented also contribute to a better understanding of changes in soil physical properties and water allocation within specific agroforestry systems in the semiarid area.
Materials and methods
Study site and experimental design
A jujube orchard in Qingjian County, Shaanxi Province, located in the north of the Loess Plateau, was chosen for this study. The site has typical loess hill and gully terrain (37°14′N, 110°21′E), and a temperate continental monsoon climate. The annual mean temperature is 8.6 °C, with the lowest mean monthly temperature (−6.5 °C) in January and the highest mean monthly temperature (28 °C) in July. Annual mean precipitation amounts to 505 mm, of which 70% generally occurs between July and September. Field capacity at the site is about 25% (volumetric water content) while wilting occurs at about 7% (Table 1).
Jujube is the main economic crop grown in the area. A 6-year-old pear-jujube (Ziziphus jujuba Mill.) orchard was chosen for the experiment. This jujube orchard was mainly planted on the slopes of a hilly loess area (more details can be found in Ling et al. 2017). Each local small-scale farmer has their jujube orchard, with Daylily (Hemerocallis fulva L.) planted as a perennial species. The fodder species canola (Brassica napus L.) is an annual species, with farmers planting this crop in May and harvesting it at the end of October every year. More information is presented in Table 2.
There were three treatments in this study: the jujube/canola system (JFCS, Rotary tilling before planting canola and then clean tillage after), the jujube/daylily system (JDLS, Clean tillage) and the jujube conventional system (JCS, clean tillage) as the control. In a single original orchard, we randomly selected three plots as experimental areas, and each plot was divided into three treatments using random splits. The plot used had an area of 45 m2 (5 m × 9 m), and each treatment included three replicate plots, resulting in a total of nine study plots. More details can be found in Table 2. In the JFCS treatment, the rotary tilling was carried out before planting (annually in May) the canola and clean tillage repeated every year (since 2008). In the JDLS treatment, as the daylily is a perennial crop, clean tillage was maintained as for the JCS treatment after 2008 when the daylily was planted in the orchard. In the JCS treatment, clean tillage was employed because this is the most popular tillage system for weed removal practiced by the small farmers in the region.
Fine root collection
Fine root (≤2 mm) samples were collected at 20 cm increments to a depth of 180 cm under jujube tree (50 cm from the tree trunk) and between crop row (9 Oct. 2014, 6 Oct. 2015, 29 Sep. 2016, and 24 Sep. 2017). In every plot, three sample sites were selected under the tree and between rows, respectively. Roots of jujube trees and crops are easily distinguishable by color as the tree roots are brown, the daylily’s is light yellow, and the canola’s is white. The soil samples were collected with root auger, which internal diameter of 6 cm. Soil samples of root and soil were sealed in sample bags. The samples were gently washed in a 1 mm sieve, and the roots retained on the sieve were removed with tweezers. Dead roots were identified based on color (dead roots are dark) and mechanics (dead roots are little elastic) and discarded, despite subjectivity. Finally, using a Vernier caliper with a precision of 1 × 10−2 mm, the coarse roots (>2 mm) were separated from the fine roots (≤2 mm). The fine roots were scanned with a scanner at 300 dpi, and the files were saved in the TIFF format. The fine root lengths were calculated using DElLTA-T SCAN® image analysis software (Delta-T scan, Delta-T Devices Company, UK) based on the images of the fine roots. After the measurements were collected, the fine root samples in each layer were oven-dried at 70 °C and weighed to determine the root dry weight.
The root was described by the FRLD in vertical distribution (at 0–60 cm and 60–180 cm soil depths) and expressed the steepness of the root density decreased with soil depth by the β value of the regression equation:
Where y is the cumulative root biomass fraction in g cm−3 and d is the soil depth in cm (Gale and Grigal 1987). High β values indicate a large proportion of root biomass concentrates in deeper soil depths, whereas low values stand for a large proportion of root mass concentrates near the soil surface.
Soil sampling
On four dates covering the experimental period (7 Oct. 2014, 4 Oct. 2015, 27 Sep. 2016, and 26 Sep. 2017) soil was sampled at four depths: (0–5 cm, 5–10 cm, 10–20 cm, and 20–40 cm). The sampling position was beneath jujube trees and in the middle of the row, near the soil water content monitoring site. In every plot, four replicates under trees and two replicates between the rows were collected, totaling 54 soil samples in nine plots. A stack of the ring was pushed vertically into the soil, and when the top ring was nearly full, an empty ring was placed on top stack slightly below the soil surface. The stack was then carefully dug out, excess soil removed, and a metal cover with filter paper affixed to top and bottom to protect the soil during transit and storage.
Measurements of soil physical properties
Several soil parameters were evaluated to determine the impact of the different treatments on soil physical properties. Soil bulk density (BD) and soil saturated hydraulic conductivity of the 0–5 cm, 5–10 cm, 10–20 cm, and 20–40 cm soil layers were determined using the core method (length 5 cm, diameter 5 cm). The core samples were immediately weighed, and then dried at 105 °C for 24 h to a constant weight and reweighed to calculate the soil bulk density. The soil samples were kept saturated under a constant head (5 cm) and recorded the water flow per unit time to calculate the saturated hydraulic conductivity. Field capacity (FC) was determined using a core of undisturbed soil (Wilcox method, Duan et al. 2010). A piece of filter paper was placed on the bottom of the core, fixed it with a mesh bag and rubber band, and filter paper was also placed on the top. The core was submerged for 12 h. After this, another core of dry soil was placed on top of the original soil core. After a further 8 h to allow water absorption, about 20 g of wet soil was taken from the middle of the original core and dried to calculate the field capacity. It is noteworthy that field- and lab- determined soil hydraulic properties (e.g., field capacity and soil hydraulic conductivity) could be noticeably different (Ferrer et al. 2008; Duan et al. 2010). For instances, Ferrer et al. (2008) found the field capacity measured by pressure plates (-33 kPa) was higher than by field method. Duan et al. (2010) suggested that if the soil structure was destroyed in the laboratory, the measured field capacity could be significantly higher. Also, soil hydraulic conductivity obtained from by laboratory-produced larger than in field, which affected by the size of the soil core (Bagarello and Provenzano 1996). In this study, all the soil physical properties in the different treatments were sampled and measured using the same method and at the same time, so the results could directly comparable between treatments (Bao 2007).
Total soil porosity (TP) was calculated using Eq. 2, based on the relationship between the soil bulk density and soil particle density. Soil Particle density (PD) is approximately 2.65 g cm−3 for minerals soils, as widely used in studies of the Loess Plateau (Wang et al. 2016; Zhang et al. 2019). Since the study area soil had relatively low organic matter, we used the 2.65 g cm−3 value. Capillary porosity (CP) was calculated on the basis of the relationship between field capacity and soil bulk density (Eq. 3 and S1). Non-capillary porosity (NCP) was calculated as the difference between total porosity and capillary porosity (Eq. 4). The equations used were as follows:
Soil water content dynamics
A portable Time Domain Reflectometry (TDR) system (TIME-PICO IPH / T3; IMKO, Ettlingen, Germany) was used to take volumetric measurements. The tubes for measurements were installed under the trees (30 cm away from the tree trunk) and crops (in the middle between the tree rows). Four replicates under trees and two under crops were collected in every plot. A total of 54 soil water content monitoring tubes were installed across all nine plots. Each TDR tube reached a depth of 180 cm, with 20 cm increments. Soil water content was measured every two weeks between mid-May to Mid-October, with additional monitoring after rainfall. In 2016, soil water content could only be measured until September because of bad weather conditions. The winter months, influenced by the arctic air from Siberia, are cold and dry, and precipitation is rare. This period lasts from November to March. Soil water content was, therefore, measured from March to April, to represent the jujube dormant period (2014–2015, 2015–2016, 2016–2017). A total of 67 measurements were taken, with each sampling session completed in 2 h. The TRIME-TDR system was calibrated against volumetric water content under field conditions. Based on jujube growth characteristics (Jin et al. 2018), there are two periods every year: a dormant period (DP, March to April) and a growth period (GP, May–October, DOY). The jujube growth period can be further classified into four stages: leaf emergence (Early-May–Mid-June); blossoming and young fruit (Mid-June-Mid-July); fruit swelling (Mid-July–Mid-September); and fruit maturation (Mid-September–Mid-October).
An automatic weather station (Rainroot Scientific Limited, Beijing, China) was installed 50 m from the study area. Sensors were installed at a height of 2.0 m and CR100 (Campbell Scientific Inc.; Edmonton, Canada) dataloggers collected data every 30 min from May–October during 2014–2017. The system recorded rainfall, air temperature, relative humidity, solar radiation, and wind speed. Reference evapotranspiration (ET0, mm d−1) was calculated based on the FAO Penman-Monteith equation (Allen et al. 1998) using data from the weather station in every year, as follows:
Where Rn is net radiation (MJ m−2 d−1); Gis the soil heat flux (MJ m−2 d−1); Δis the vapor pressure curve slope (kPa °C−1); γ is a psychrometric constant (kPa °C−1); T is the mean air temperature (°C); u2 is wind speed at 2 m height (m s−1); and es − ea is saturation vapor pressure deficit (kPa). The equation uses standard climatological records of solar radiation, air temperature, humidity and wind speed. The calculation process is shown in the supplementary (Eq. S2).
Statistical analysis
The temporal and spatial variations in soil water content under trees and between rows during the dormant and growth periods were plotted on a contour map derived by applying kriging interpolation.
A linear mixed model (LMM) was used to analyze the soil water content and soil physical properties data because the data were hierarchical and clustered among plots within the study site. Observations were collected repeatedly over the course of four years from the same plots. The data sets were analyzed in R, with the data file split into observations representing the four distinct years. In order to examine the soil water content change with soil depth in each month, we considered soil water content in the 0–60 cm and 60–180 cm layers. The LMM was fitted to soil water content in the four years (normal year 2014, drought year 2015, normal year 2016, normal year 2017). The LMMs include fixed effects associated with the treatments as well as random effects that are associated with the plants sampled for each treatment, thus capturing within-plant correlations. We considered treatment × month as fixed effects, to evaluate the change in soil water content in different treatments in different months during the four years. When analyzing changes in soil physical properties using the LMM, we considered the treatments and soil depths to be the fixed effects. The changes in soil physical properties with time were not analyzed. Random sampling position was treated as a random effect. The proportion of variance in the measurements can be attributed to random plant (position) effects and was estimated using random intercepts in the LMM (Gueorguieva and Krystal 2004).
To describe the variation in soil characteristics between treatments, we used R (R Core Team 2016) and ImerTEST (Kuznetsova et al. 2016) to perform a linear mixed-effects analysis. The Chi-square (Chisq) test was used to examine treatment differences, a p value of 0.05 was the threshold for statistical significance. Pairwise comparisons were performed using the HSD-Tukey test at the 95% probability level (p < 0.05). Principle Components Analysis (PCA) and Pearson analysis were conducted in R to analyze interrelations between soil physical properties (0–40 cm), fine root length density (0–60 cm and 60–180 cm), and soil water content under tree and inter-row (0–60 cm and 60–180 cm). Data analysis was carried out using Microsoft Excel 2013 (Microsoft, Redmond, CA), IBM SPSS Statistics 23 (IBM, Stanford, USA), Origin 2016 Pro software (Origin Lab, Northampton, MA), and Surfer 12.0 (Golden Software, Golden, USA).
Results
Meteorological variability
The annual rainfall during the study years amounted to 383.4 mm, 254.6 mm, 319.4 mm, and 398.6 mm, respectively (Fig. 1). Based on the Qingjian County precipitation records from the years 1956–2006, years 2014, 2016 and 2017 could be considered normal, while 2015 could be considered a drought year. Effective rainfall and frequency are crucial factors in determining any increase in soil water content. A single rainfall event delivering less than 5 mm is considered ineffective. Rainfall occurred 60 times in 2014, with 31.6% effective; 55 times in 2015, with 25.5% effective; 60 times in 2016, with 23% effective; and 68 times in 2017, with 39% effective. The average temperature during each month in the period 2015–2017 was slightly higher than that recorded in 2014 (Fig. 1). The ET0 changes from month to month reveal that the study site dries quickly during early jujube growth stages (May–July) (Fig. 1), corresponding to the time of seasonal drought in the region.
Vertical change of tree and crop fine root
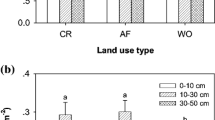
The jujube FRLD was significantly higher in JFCS-Tree and JDLS-Tree than in JCS-Tree at 0–60 cm (Fig. 2a). At the inter-row, the FRLD of tree in JFCS-Inter-row and JDLS-Inter-row were significantly higher and significantly lower than in the JCS-Inter-row at 0–60 cm, respectively (Fig. 2c). Furthermore, The FRLD of tree in JDLS-Inter-row was significantly higher than the JCS-Inter-row at 60–180 cm (Fig. 2d). The β value indicated that both the JFCS-Tree and JDLS-Tree tended to allocate more fine root biomass at shallower depths than the JCS-Tree (Table 3). At the inter-row, the β value of the tree in JFCS-Inter-row was lower than in JCS-Inter-row showed a concentration of fine root biomass at upper soil layers. Conversely, the β value of the tree in JDLS-Inter-row tended to allocate more fine root biomass at deeper soil layers compared to JCS-Inter-row (Table 3).
Fine root length density of trees and crops in three treatments under tree and inter-row at 0–60 cm and 60–180 cm. Lower case Latin letters indicate statistically significant differences between the different treatments. The root sampling point under the tree is 50 cm away from the trunk. Inter-row root sampling points in the middle of rows
The canola FRLD was significantly higher than daylily at 0–60 cm but significantly lower than daylily at 60–180 cm (Fig. 2e–f). Also, the daylily FRLD β value suggested a higher proportion of fine root biomass concentrated at deeper soil layers than the canola (Table 3).
Soil physical properties
The JFCS-Tree and JDLS-Tree soil bulk density, soil saturated hydraulic conductivity, total porosity, and non-capillary porosity were not significantly different from the equivalent values under the JCS-Tree treatment, but did decrease significantly with soil depths (0–5 cm > 5–10 cm > 10–20 cm > 20–40 cm) (Tables 4, 5 and S1). The field capacity under JFCS-Tree and JDLS-Tree were 1.6%–5.2% and 0.6%–2.9% higher than JCS-Tree (Table 4 and S1), respectively. However, there were no significant differences with soil depths. The soil capillary porosity was significantly higher under JFCS-Tree was 0.15%–5.1% higher than JCS-Tree (Table 5 and S1) but not significantly different with soil depths. In contrast, soil capillary porosity was not significantly different between JDLS-Tree and JCS-Tree, but there were significantly decreased with soil depths (0–5 cm > 5–10 cm > 10–20 cm > 20–40 cm).
Compared to the JCS-Inter-row, the JFCS-Inter-row and JDLS-Inter-row both significantly decreased the soil bulk density and increased the soil field capacity (Table 6 and S2). In addition, the soil saturated hydraulic conductivity decreased significantly with soil depths (0–5 cm > 5–10 cm > 10–20 cm > 20–40 cm). The soil bulk density decreased in the JFCS-Inter-row and JDLS-Inter-row, varying by 4.69%–6.28% and 1.9%–3.9%; the field capacity increased, by 10.7%–13.1% and 8.4%–10.4%; the soil saturated hydraulic conductivity increased, by 21.88%–36.24% and 23.89%–30.57%, respectively, over the four years.
Table 7 and S2 showed that, compared with JCS-Inter-row, the JFCS-Inter-row treatment exhibited significantly increased soil total porosity (5.1%–6.9%), capillary porosity (4.6%–6.8%), and non-capillary porosity (1.7%–11%) over the four years with significant decreased between soil depths (0–5 cm > 5–10 cm > 10–20 cm > 20–40 cm). Compared to the JCS-Inter-row, the JDLS-Inter-row treatment exhibited significantly increased total soil porosity (2.1%–4.4%) and capillary porosity (5.1%–6.8%) with significant decreased between soil depths (0–5 cm > 5–10 cm > 10–20 cm > 20–40 cm), but there was no significant effect on the non-capillary porosity during the four years.
Soil water content variations under jujube trees
Compared to the jujube JCS-Tree, the values for JFCS-Tree and JDLS-Tree average soil water content (0–180 cm) were significantly higher during the GP and DP period (Fig. 3a–c and Fig. S1). The interannual variations in soil water content in 2014 (Fig. S1, i) was quite different from 2015 to 2017 for JCS-Tree (Fig. S1: j-l), but such difference was not strong, if one compared in 2014 (Fig. S1: a, e) and 2015–2017 (Fig. S1: b-d, f-h) of JFCS-Tree and JDLS-Tree. Compared to JCS-Tree, JFCS-Tree and JDLS-Tree significantly increased in terms of soil water content at a depth of 0–60 cm over the four study years (Fig. 4a–d, Table S3). In contrast, the soil water content measurements at a depth of 60–180 cm did not differ significantly over the four years (Fig. 4e–h). When analyzed on a monthly basis, soil water content at a depth of 0–60 cm in the JFCS-Tree and JDLS-Tree treatments also significantly higher than the JCS-Tree over the four years (Figure 4a1–d1). For the depth of 60–180 cm, the analysis only revealed significant month-to-month soil water content differences in 2015, which was a drought year (Figure 4e1–h1). In 2015, the soil water content significantly decreased between April and October in all three treatments. This change occurred at the same time during the drought and implied that this event influenced soil water content in the 60–180 cm layer.
Soil water content variations under inter-row crops
Compared to the jujube GP, the average soil water content in all treatments between rows was significantly higher in the DP in 2015 (Fig. 3d–f and Fig. S2). The average soil water content in the JDLS-Inter-row treatment was significantly lower than in the JCS-Inter-row in the four years. In addition, significantly lower values were recorded in JFCS-Inter-row compared to JCS-Inter-row in 2015 and 2016 in the GP and DP (Fig. 3d–f). There was a quite difference in 2015 (Fig. S2, b) from 2014, 2016, 2017 (Fig. S2: a, c, d) in JFCS-Inter-row, and in 2014 (Fig. S2: e) from 2015 to 2017 (Fig. S2: f-h) in JDLS-Inter-row, but not strong, when compared in JCS-Inter-row (Fig. S2: i-l). Compared to JCS-Inter-row, JFCS-Inter-row and JDLS-Inter-row soil water content from a depth of 0–60 cm exhibited significant differences among the three treatments in 2015–2017, but no significant between-treatment differences in 2014 (Fig. 5a–d, Table S4). In the 60–180 cm layer, the soil water content measurements revealed that JDLS-Inter-row significantly decreased compared to the clean cultivation plots during each of the four study years (Fig. 5e–f). At the same depth, the soil water content measured for plots intercropped with JFCS-Inter-row significantly decreased compared to the clean cultivation plots in 2015–2017, but not in 2014 (Fig. 5e–f). When analyzed month-by-month, there were significant between-treatment soil water content differences at the 0–60 cm depth over the four study years (Figure 5a1–d1), but no significant differences between intercropping and clean cultivation could be discerned in the 60–180 cm layer during the study period (Figure 5e1–h1).
Interrelations between soil physical properties, soil water content and fine root under tree
The under-tree FC and CP showed a close relationship with the FRLD of tree at 0–60 cm along the PC1 axis. Also, the soil physical properties (except the FC) showed a close association with soil water content at 0–60 cm and 60–180 cm along the PC2 axis (Fig. 6a; Table S5). From the Pearson analysis (Table 8), the FC, TP, CP, and soil water content under tree at 0–60 cm were positively correlated to the FRLD of tree at 0–60 cm. The BD showed a negative correlation with soil water content at both 0–60 cm and 60–180 cm, while the TP and NCP showed a positive correlation with soil water content at 60–180 cm. Moreover, HC and CP showed a positive correlation with soil water content at 0–60 cm and a negative correlation with soil water content at 60–180 cm.
Interrelations between soil physical properties, soil water content and fine root at inter-row
At the inter-row, The HC and CP showed a close relationship related to the FRLD of daylily and soil water content at 60–180 cm along the PC1 axis. All the soil physical properties and soil water content at 0–60 cm showed a close correlation with FRLD of tree at 0–60 cm and FRLD of canola along the PC2 axis (Fig. 6b; Table S6). According to the Pearson results (Table 9), the FRLD of trees at 0–60 cm showed a positive correlation with TP and NCP, and a negative correlation with BD. While FRLD of trees at 60–180 cm showed a positive correlation with HC and CP, and a negative correlation with soil water content at 60–180 cm. All the soil physical properties were positively related to the FRLD of canola at 0–60 cm and 60–180 cm, except for that BD was negatively associated with FRLD of canola. The FRLD of daylily at 0–60 cm and 60–180 cm showed a positive correlation with CP and a negative correlation with NCP and soil water content at 60–180 cm. Moreover, the HC and FC were only positively correlated to FRLD of daylily at 0–60 cm. A negative correlation was found between BD and soil water content at 0–60 cm, meantime, FC and CP were negatively correlated to soil water content at 60–180 cm. Besides, HC, FC, and NCP were positively correlated to soil water content at 0–60 cm.
Discussion
Soil physical properties change under tree and inter-row
A favorable soil physical property is essential for a better orchard environment and high-quality production (Palese et al. 2014; Xu and Zhang 2004). At the inter-row, both agroforestry systems considered herein modified the inter-row bulk density, field capacity, saturated hydraulic conductivity, and soil porosity. This conclusion is consistent with Steele et al. (2012) and Schwab et al. (2015), who found that the long-term use of cover crops could change the orchard micro-surface environment, leading to benefits such as reduced soil bulk density and increased hydraulic conductivity (Sun et al. 2018). Higher soil porosity and field capacity would increase water infiltration, facilitate the faster downward movement of water, and enhance water-holding capacity (Liu et al. 2013; Huang et al. 2016b). Popova et al. (2016) suggested that root systems are able to grow in a heterogeneous soil environment to modify soil properties. In this study, the results suggested that the FRLD of canola at 0–60 cm and 60–180 cm and daylily at 0–60 cm are significant related to soil physical properties (Fig. 6b, Table 9), indicated the role of crop root to modify the soil physical properties (DuPont et al. 2014). The root can be targeted as a natural management tool for soil structural porosity to enhance water holding capacity as well as saturated hydraulic conductivity (Bodner et al. 2014). Dexter et al. (2001) and Jiang et al. (2018) suggested that roots were directly involved in the improvement of hydraulic behavior and bulk density in field. Compared to JCS-Inter-row, the non-capillary porosity was significantly higher in JFCS-Inter-row, but not in JDLS-Inter-row (Table 7). The modification degree of soil properties may depend on the root traits and quantity, lager and longer root and more concentrated root distribution would get better performance on soil properties (DuPont et al. 2014; Jiang et al. 2018). The FRLD of daylily and tree at inter-row was lower than the FRLD of canola and tree at 0–60 cm. Meantime, the average β value in the JFCS-Inter-row was smaller than in JDLS-Inter-row, suggesting more fine root biomass concentrated in topsoil in the JFCS-Inter-row, which facilitates the formation of more soil porosity (Scanlan 2009; Whalley et al. 2005). This was supported by the Bodner et al. (2014), who indicated species with dense of fine root systems induced heterogenization of the pore space and higher micropore volume. However, Cardinael et al. (2015) found that because of competition with crops, the tree roots tended to concentrate relatively more in deeper soil layers. This phenomenon was also found in JDLS-Inter-row of tree fine root distribution (Fig. 2), which may not favor the formation of more soil porosity at topsoil than JFCS-Inter-row.
In this study, the two agroforestry systems have limited effect on soil bulk density, hydraulic conductivity, total porosity, and non-capillary porosity under tree row, compared to inter-row. This is a difference from Cardinael et al. (2015), who worked on soil organic carbon and found agroforestry only has a noticeable effect on the tree row. This may highlight the importance of characterizing and taking into account spatial heterogeneity of the indicators in the assessment of ecosystem functioning and service provisioning for agroforestry systems. In our experiment, the field capacity under tree were both increased in each agroforestry system compare to JCS (Table 4). This may be because of greater amount of fine root aggregated in soil, which increased the soil porosity and field capacity (Bodner et al. 2014). In our study, The PCA and Pearson analysis suggested that the FRLD of tree at 0–60 cm was correlated with the field capacity and soil capillary porosity, which agree with Bodner et al. (2014)‘s result (Fig. 6a, Table 8). However, we found that the soil capillary porosity was only increased in JFCS, not in the JDLS, compared with JCS. This may due to the denser FRLD of tree in JFCS-Tree than JDLS-Tree (Table 3), which induced higher soil porosity (Scanlan 2009; Whalley et al. 2005). Scanlan (2009) suggested that the pore radius reduced via root ingrowth. Dense fine root systems provided intense root-soil contact space, which could enhance soil micropores formation in soil. The increase of soil porosity is conducive to the increase of water holding capacity in the field (Li et al. 2013). This reflected in our conclusion that the field capacity increased higher in JFCS-Tree, compared to JDLS-Tree (Table 4 and S1).
Soil water content response during jujube growth and dormant periods in the four years
The periods of blossoming and young fruit development (Mid-June–Mid-July) and fruit swelling (Mid-July–Mid-September) are the stages of the jujube production cycle that require the most water (Cui et al. 2008; Li et al. 2011; Feng et al. 2017; Han et al. 2012a, b). Favorable soil bulk density and saturated hydraulic conductivity can enhance the rainfall into the soil layers (Steele et al. 2012; Schwab et al. 2015); Subsequently, the higher field capacity and soil porosity could keep more soil water in soil layers (Li et al. 2013; Bilek 2007; Pan et al. 2017). Hence, the tested intercropping systems could be pivotal to alleviating tree water stress during the growth period in semiarid areas (Basche et al. 2016). In this study, most of the rainfall in the Loess Hilly region was concentrated in July to September (About 70% of annual rainfall), with less rainfall from March to June (Fig. 1). There is great potential, during this stage, to store soil water content in the orchard (Palese et al. 2014). The benefit to soil physical properties was found better in the agroforestry systems than the control (Palese et al. 2014; Schwab et al. 2015), and more soil water could be stored in soil (Li et al. 2013; Li et al. 2007). In our study, soil water content in the JDLS-Tree and JFCS-Tree plots during the jujube dormant period was noticeably higher than that in JCS-Tree (Fig. 3a–c). This indicates that significant carryover effects in two agroforestry than control, from the previous year, had a role in determining the soil water content at the start of the growing season (Fig. 3 and S1). This is an important water source for jujube trees, particularly during the early growth period when precipitation is scarce (May-to-June, Fig. 1). Other studies have also shown how the soil water content left over from the previous dormant period is pivotal in determining the growth conditions for the next year’s crop (Enloe et al. 2004; Yimam et al. 2014; Palese et al. 2014; Jin et al. 2018). Moreover, soil water content remaining from a normal year could be used in a drought year (Fig. S1-S2).
Testing intercropping species for effective agroforestry systems
In this study, jujube intercropped with two plants, canola and daylily, resulted in significantly more soil water content in the 0–60 cm layer compared to the JCS-Inter-row control in four years, but not in 2014 both in two agroforestry inter-row and 2015 in JDLS-Inter-row (Fig. 5 and Table S4). This result may be because, in 2014, effective rainfall distribution was more uniform and the average temperature was the lowest among four years, resulting in lower soil evaporation in all treatments (Bai et al. 2018). During the 2015–2017 period, effective rainfall was less, and temperatures were higher. The soil water content from JCS-Inter-row declined more quickly than JFCS-Inter-row and JDLS-Inter-row due to lower infiltration and higher soil evaporation. Since both of these mechanisms were affected by soil physical properties and vegetation cover (de Almeida et al. 2018; Bai et al. 2018), significant differences in soil water content were observed in 2015–2017 between the two agroforestry systems and the control. Islam et al. (2006) suggested that vegetation cover may consume more soil water by crop root water uptake. Perennial and semi-perennial crops are characterized by proportionally more water capture in deep layers than annual crops (Ferchaud et al. 2015), contributed by its deeper rooting in soil depths (Fig. 2 and Table 3). The Pearson analysis also suggested that the FRLD of daylily at 0–60 cm and 60–180 cm and FRLD of tree at 60–180 cm have a negative relationship with soil water content at 0–60 cm and 60–180 cm (Table 9). This result implies that an agroforestry system that includes intercropping with the perennial daylily consumes more water than a system including intercropping with the annual canola (Ferchaud et al. 2015).
In any successful agroforestry system, complementarity in the use of above- and below-ground resources between the tree and the crop must outweigh between-species competition (Cannell et al. 1996). In semiarid regions, soil water content becomes a more crucial factor than light and nutrients, as limited water resources severely affecting crop growth. Planting crops between rows of trees increases surface vegetation cover and creates a new “soil-vegetation-atmosphere” environment that differs from the “soil-atmosphere” environment from a clean cultivation orchard. Moreover, Li et al. (2007) revealed that the ecological suitability of orchard grass is a problem worthy of attention in the context of orchards in northern China. They found that grass should be used cautiously when managing orchards in the Loess Plateau, where annual precipitation can be less than 550 mm. Due to the imbalance between precipitation and the growth and development of vegetation, soil water content variations in orchards depend on grass type and rainfall patterns. Padovan et al. (2018) found the evergreen shadow tree Simarouba glauca to be more suitable than deciduous Tabebuia rosea for intercropping in a coffee plantation due to lower water consumption. The selection of appropriate crop species is essential for the success of an agroforestry system. As mentioned before, grass has a positive effect on soil water content under tree during normal year and drought year (Fig. 3a–c; Fig. S1). While, there is lower soil water content under intercropping area than monoculture, especially in JDLS-Inter-row (Fig. 3d–f; Fig. S2). It is worth reminding that there is a clear trade-off between tree and inter-row species: while jujube gains more soil water content, inter-row species may induce water deficit. This implies agroforestry could be “a sword with two blades” in terms of water use (Li et al. 2007; Padovan et al. 2018).
Conclusions
Soil physical properties, soil water content, and fine root data were analyzed at two agroforestry systems (JFCS and JDLS) and control (JCS) over four rainfall years. This study demonstrated the two agroforestry systems examined influence most of the inter-row soil physical properties (e.g., the soil bulk density, saturated hydraulic conductivity, field capacity, total porosity, capillary porosity and non-capillary porosity (only significant in JFCS-Inter-row)), which attributed to the role of crops fine root. Meantime, the under-tree field capacity and capillary porosity (only significant in JFCS-Tree) were modified. Furthermore, they led to increased soil water content at 0–60 cm under tree, which ultimately reduced the risk of tree damage due to water stress. The JFCS-Inter-row showed a different water absorption strategy compared to JDLS-Inter-row, characterized by the soil water content and fine root distribution between inter-rows. These revealed that the JFCS treatment was more beneficial for improving soil water content than JDLS treatment. Agroforestry is an easy and effective risk avoidance strategy for farmers who may suffer from increased climatic stress and food insecurity. Although agroforestry systems are potentially favorable to reform soil physical properties and water conditions in the semiarid orchard, it needs to pay attention to the excessive consumption of resources (e.g., soil water content) at the crop-tree overlapping area. This implies the importance of selecting relevant cover crops with particular traits when designing the semiarid agroforestry system.
References
Abdulai I, Vaast P, Hoffmann MP, Asare R, Jassogne L, Van Asten P, Graefe S (2018) Cocoa agroforestry is less resilient to sub-optimal and extreme climate than cocoa in full sun. Glob Chang Biol 24:273–286
Allen RG, Pereira LS, Raes D, Smith M (1998) Crop evapotranspiration-Guidelines for computing crop water requirements-FAO Irrigation and drainage paper 56. FAO, Rome. 300, D5109
Altieri MA (2002) Agroecology: the science of natural resource management for poor farmers in marginal environments. Agr Ecosyst Enivron 93:1–24
Altieri MA, Nicholls C (2017) The adaptation and mitigation potential of traditional agriculture in a changing climate. Clim Chang 140:33–45
Bagarello V, Provenzano G (1996) Factors affecting field and laboratory measurement of saturated hydraulic conductivity. T ASABE 39:153–159
Bai GS, Zou CY, Du SN (2018) Effects of self-sown grass on soil moisture and tree growth in apple orchard on Weibei dry plateau. Trans Chin Soc Agric Eng 34:151–158 in Chinese with English abstract
Bao S (2007) Soil and agricultural chemistry analysis. China Agricultural Press, Beijing in Chinese with English abstract
Basche AD, Kaspar TC, Archontoulis SV, Jaynes DB, Sauer TJ, Parkin TB, Miguez FE (2016) Soil water improvements with the long-term use of a winter rye cover crop. Agri Water Manage 172:40–50
Bilek MK (2007) Winter annual rye cover crops in no-till grain crop rotations: impacts on soil physical properties and organic matter. In: M.S. Thesis. University of Maryland, College Park, MD
Bodner G, Leitner D, Kaul HP (2014) Coarse and fine root plants affect pore size distributions differently. Plant Soil 380:133–151
Bradford JB, Schlaepfer DR, Lauenroth WK, Yackulic CB, Duniway M, Hall S, Jia S, Jamiyansharav K, Munson SM, Wilson SD, Tietjen B (2017) Future soil moisture and temperature extremes imply expanding suitability for rainfed agriculture in temperate drylands. SCI REP UK 7:12923
Cannell M, Van Noordwijk M, Ong CK (1996) The central agroforestry hypothesis: the trees must acquire resources that the crop would not otherwise acquire. Agrofor Syst 34:27–31
Cardinael R, Mao Z, Prieto I, Stokes A, Dupraz C, Kim JH, Jourdan C (2015) Competition with winter crops induces deeper rooting of walnut trees in a Mediterranean alley cropping agroforestry system. Plant Soil 391:219–235
Cui N, Du T, Kang S, Li F, Zhang J, Wang M, Li Z (2008) Regulated deficit irrigation improved fruit quality and water use efficiency of pear-jujube trees. Agr Water Manage 95:489–497
de Almeida WS, Panachuki E, de Oliveira PTS, da Silva MR, Sobrinho TA, de Carvalho DF (2018) Effect of soil tillage and vegetal cover on soil water infiltration. Soil Tillage Res 175:130–138
Dexter AR, Czyż EA, Niedzwiecki J, Maćkowiak C (2001) Water retention and hydraulic conductivity of a loamy sand soil as influenced by crop rotation and fertilization. Arch Agron Soil Sci 46:123–133
Doran JW, Parkin TB (1994) Defining and assessing soil quality. In: Doran JW, Coleman DC, Bezdicek DF, Stewart BA (eds) Defining soil quality for a sustainable environment. Soil Science Society of America Special Publication, Madison, pp 3–21
Duan X, Xie Y, Liu G, Gao X, Lu H (2010) Field capacity in black soil region, Northeast China. Chin Geogr Sci 20:406–413
DuPont ST, Beniston J, Glover JD, Hodson A, Culman SW, Lal R, Ferris H (2014) Root traits and soil properties in harvested perennial grassland, annual wheat, and never-tilled annual wheat. Plant Soil 381:405–420
Enloe SF, DiTomaso JM, Orloff SB, Drake DJ (2004) Soil water dynamics differ among rangeland plant communities dominated by yellow starthistle (Centaurea solstitialis), annual grasses, or perennial grasses. Weed Sci 52:929–935
Feng Y, Cui N, Du T, Gong D, Hu X, Zhao L (2017) Response of sap flux and evapotranspiration to deficit irrigation of greenhouse pear-jujube trees in semiarid Northwest China. Agr Water Manage 194:1–12
Ferchaud F, Vitte G, Bornet F, Strullu L, Mary B (2015) Soil water uptake and root distribution of different perennial and annual bioenergy crops. Plant Soil 388:307–322
Ferrer F, Pla I, Fonseca DH, Villar JM (2008) Combining field and laboratory methods to calculate soil water content at field capacity and permanent wilting point. In 10th Congress of the European Society for Agronomy. Bologna-Italy. Rivista di Agronomia 3:279–280
Gale MR, Grigal DF (1987) Vertical root distribution of northern tree species in relation to successional status. Can J For Res 17:829–834
Gao X, Zhao X, Wu P, Brocca L, Zhang B (2016) Effects of large gullies on catchment-scale soil moisture spatial behaviors: a case study on the Loess Plateau of China. Geoderma. 261:1–10
Gueorguieva R, Krystal JH (2004) Move over ANOVA: Progress in analyzing repeated-measures data and its reflection in papers published in the archives of general psychiatry. Arch Gen Psychiatry 61:310–317
Han LX, Wang YK, Zhang LL (2012a) Response of pear jujube trees on fruit development period to different soil water potential levels. Acta Ecol Sin 32:2004–2011 in Chinese with English abstract
Han LX, Wang YK, Zhang LL (2012b) Response of pear-jujube to different soil water potentials during budding and flowering stages. Chin J Eco-Agri 20:454–458 in Chinese with English abstract
Huang J, Yu H, Guan X, Wang G, Guo R (2016a) Accelerated dryland expansion under climate change. Nat Clim Chang 6:166–171
Huang J, Wang J, Zhao X, Li H, Jing Z, Gao X, Chen X, Wu P (2016b) Simulation study of the impact of permanent groundcover on soil and water changes in jujube orchards on sloping ground. Land Degrad Dev 27:946–954
Huang J, Yu H, Dai A, Wei Y, Kang L (2017) Drylands face potential threat under 2°C global warming target. Nat Clim Chang 7:417–422
Islam N, Wallender WW, Mitchell J, Wicks S, Howitt RE (2006) A comprehensive experimental study with mathematical modeling to investigate the affects of cropping practices on water balance variables. Agr Water Manage 82:129–147
Jiang P, Wang H, Fu X, Dai X, Kou L, Wang J (2018) Elaborate differences between trees and understory plants in the deployment of fine roots. Plant Soil 431:433–447
Jin S, Wang Y, Shi L, Guo X, Zhang J (2018) Effects of pruning and mulching measures on annual soil moisture, yield, and water use efficiency in jujube (Ziziphus jujube Mill.) plantations. Glob. Ecol Conserv 15:e00406
King AP, Berry AM (2005) Vineyard δ15N, nitrogen and water status in perennial clover and bunch grass cover crop systems of California's central valley. Agr Ecosyst Enivron 109:262–272
Klik A, Rosner J, Loiskandl W (1998) Effects of temporary and permanent soilcover on grape yield and soil chemical and physical properties. J Soil Water Conserv 53:249–253
Kuznetsova A, Brockhoff PB, Christensen RHB (2016) LmerTest: Tests in linear mixed effects models. (R package version 2.0-33)[Computer software]. Retrieved from https://cran.r-project.org/web/packages/lmerTest/index.html
Li HK, Zhang GJ, Zhao ZY, Li KR (2007) Effects of interplanted herbage on soil properties of non-irrigated apple orchards in the Loess Plateau. Acta Agrestia Sin 1:76–81 in Chinese with English abstract
Li XB, Wang YK, Zhao CH, Wang Y, Zhang YY, Wang X, Zhang JG (2011) Effect of regulated irrigation on input-output benefits of pear jujube. Chin J Eco Agri 19:818–822 in Chinese with English abstract
Ling Q, Gao X, Zhao X, Huang J, Li H, Li L, Wu P (2017) Soil water effects of agroforestry in rainfed jujube (Ziziphus jujube Mill.) orchards on loess hillslopes in Northwest China. Agr. Ecosyst. Environ 247:343–351
Liu Y, Gao M, Wu W, Tanveer SK, Wen X, Liao Y (2013) The effects of conservation tillage practices on the soil water-holding capacity of a non-irrigated apple orchard in the Loess Plateau. China Soil Till Res 130:7–12
Merwin IA, Stiles WC, van Es HM (1994) Orchard groundcover management impacts on soil physical properties. J Am Soc Hortic Sci 119:216–222
Padovan MP, Brook RM, Barrios M, Cruz-Castillo JB, Vilchez-Mendoza SJ, Costa AN, Rapidel B (2018) Water loss by transpiration and soil evaporation in coffee shaded by Tabebuia rosea Bertol. and Simarouba glauca dc. compared to unshaded coffee in sub-optimal environmental conditions. Agr. Forest Meteorol 248:1–14
Palese AM, Vignozzi N, Celano G, Agnelli AE, Pagliai M, Xiloyannis C (2014) Influence of soil management on soil physical characteristics and water storage in a mature rainfed olive orchard. Soil Tillage Res 144:96–109
Pan D, Song Y, Dyck M, Gao X, Wu P, Zhao X (2017) Effect of plant cover type on soil water budget and tree photosynthesis in jujube orchards. Agr Water Manage 184:135–144
Popova L, van Dusschoten D, Nagel KA, Fiorani F, Mazzolai B (2016) Plant root tortuosity: an indicator of root path formation in soil with different composition and density. Ann Bot 118:685–698
Ramos ME, Benítez E, García PA, Robles AB (2010) Cover crops under different managements vs. frequent tillage in almond orchards in semiarid conditions: effects on soil quality. Appl Soil Ecol 44:6–14
R Core Team (2016) R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. https://www.R-project.org/
Scanlan CA (2009) Processes and effects of root-induced changes to soil hydraulic properties. Dissertation. University of Western Australia
Schwab N, Schickhoff U, Fischer E (2015) Transition to agroforestry significantly improves soil quality: a case study in the central mid-hills of Nepal. Agric Ecosyst Environ 205:57–69
Shaxson F, Barber R (2003) Optimizing soil moisture for plant production: the significance of soil porosity. FAO, Rome
Sida TS, Baudron F, Kim H, Giller KE (2018) Climate-smart agroforestry: Faidherbia albida trees buffer wheat against climatic extremes in the Central Rift Valley of Ethiopia. Agric For Meteorol 248:339–347
Siriri D, Tenywa MM, Raussen T, Zake JK (2005) Crop and soil variability on terraces in the highlands of SW Uganda. Land Degrad Dev 16:569–579
Slingo JM, Challinor AJ, Hoskins BJ, Wheeler TR (2005) Introduction: food crops in a changing climate. Philos Trans R Soc Lond Ser B Biol Sci 360:1983–1989
Steele MK, Coale FJ, Hill RL (2012) Winter annual cover crop impacts on no-till soil physical properties and organic matter. Soil Sci Soc Am J 76:2164–2173
Sun D, Yang H, Guan D, Yang M, Wu J, Yuan F, Jin C, Wang A, Zhang Y (2018) The effects of land use change on soil infiltration capacity in China: a meta-analysis. Sci Total Environ 626:1394–1401
Tahir M, Lv Y, Gao L, Hallett PD, Peng X (2016) Soil water dynamics and availability for citrus and peanut along a hillslope at the Sunjia red soil critical zone observatory (CZO). Soil Tillage Res 163:110–118
Verdin J, Funk C, Senay G, Choularton R (2005) Climate science and famine early warning. Philos Trans R Soc Lond Ser B Biol Sci 360:2155–2168
Wang R, Wang ZQ, Sun Q, Zhao M, Du LL, Wu DF, Li RJ, Gao X, Guo SL (2016) Effects of crop types and nitrogen fertilization on temperature sensitivity of soil respiration in the semi-arid Loess Plateau. Soil Tillage Res 163:1–9
Whalley WR, Riseley B, Leeds-Harrison PB, Bird NRA, Leech PK, Adderley WP (2005) Structural differences between bulk and rhizosphere soil. Eur J Soil Sci 56:353–360
Xu X, Zhang J (2004) Effect of sown grass and organism mulching on orchard soil fertility. J Sichuan Agric Univ 22:88–91 in Chinese with English abstract
Yang L, Ding X, Liu X, Li P, Eneji AE (2016) Impacts of long-term jujube tree/winter wheat–summer maize intercropping on soil fertility and economic efficiency - a case study in the lower North China Plain. Eur J Agron 75:105–117
Yimam YT, Ochsner TE, Kakani VG, Warren JG (2014) Soil water dynamics and evapotranspiration under annual and perennial bioenergy crops. Soil Sci Soc Am J 78:1584–1593
Zhang B, He C (2016) A modified water demand estimation method for drought identification over arid and semiarid regions. Agric For Meteorol 230:58–66
Zhang QY, Shao MA, Jia XX, Wei XR (2019) Changes in soil physical and chemical properties after short drought stress in semi-humid forests. Geoderma. 338:170–177
Zhao XN, Wu PT, Feng H, Wang YK, Shao HB (2009) Towards development of eco-agriculture of rainwater-harvesting for supplemental irrigation in the semi-arid Loess Plateau of China. J Agron Crop Sci 195:399–407
Acknowledgements
This work was jointly supported by the National Key Research and Development Program (2016YFC0400204), the National Natural Science Foundation of China (41571506, 41771316, 51579212), the Integrative Science-Technology Innovation Engineering Project of Shaanxi (No. 2016KTZDNY-01-03), the Shaanxi Innovative Research Team for Key Science and Technology (No. 2017KCT-15), the‘111’Project (No. B12007), and CAS “Youth Scholar of West China” Program (XAB2018A04). The authors would also like to thank the editor and anonymous reviewers for their valuable comments and suggestions, which substantially improved the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Zhun Mao.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1336 kb)
Rights and permissions
About this article
Cite this article
Ling, Q., Zhao, X., Wu, P. et al. Effect of the fodder species canola (Brassica napus L.) and daylily (Hemerocallis fulva L.) on soil physical properties and soil water content in a rainfed orchard on the semiarid Loess Plateau, China. Plant Soil 453, 209–228 (2020). https://doi.org/10.1007/s11104-019-04318-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04318-0