Abstract
Aims
Changes in root functional traits reveal important nutrient acquisition strategies, with well documented patterns in root trait expression within complex communities or along gradients of singular nutrients. In this field study, we investigate intra-root functional trait expression with six soil macro- and micro-nutrients in Theobroma cacao agroforestry systems.
Methods
Using image, chemical, and spatial analysis, the fine root distribution, architecture, and morphology of T. cacao were compared to localized soil nutrients on two-dimensional soil profiles with conspecific and heterospecific neighbours.
Results
Fine-scale variation in soil nutrients was observed within the range of T. cacao root systems. Higher NH4+ and Ca2+ was associated with greater root length and biomass densities, coupled with greater investment to individual roots, expressed as increased fine root tissue density and diameter and lower specific root length. Conversely, NO3− had the opposite effect. Overall, roots tended towards higher acquisitive trait values when next to a shade tree.
Conclusions
Plants generally employ several concomitant and at times opposing strategies for nutrient acquisition in heterogeneous soils. We show that fine-scale root plasticity is highly linked to localized nutrient-specific and neighbour-specific effects, driving patterns of nutrient acquisition in agroforestry systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agroforestry is a prime example of applied ecology: species combinations are, in principle, chosen to enhance niche complementarity and/or facilitation, and thus improve nutrient cycles. The success of these interactions largely depends on differences in plant root functions and/or spatial distributions that, when compared to monocultures, permit more complete acquisition of soil nutrients (Brooker et al. 2015; Cardinael et al. 2015a). However, plant nutrient acquisition patterns are not static, and phenotypic plasticity in root systems, from whole plant to lateral root scales, can transform belowground interactions with neighbouring plants (Li et al. 2006; Cahill et al. 2010). The extent of root scale phenotypic plasticity and subsequent root foraging success is highly species-specific (Blair and Perfecto 2004; Malamy 2005; Chen et al. 2018). Yet, in humid tropical agroforestry systems, where plant nutrient demand is high and supply is constrained, little is known on root system foraging patterns under extremely complex conditions.
Plants can benefit from localized areas of high nutrient availability in soil, i.e. soil nutrient hotspots (Chen et al. 2018), by modifying their root systems via signalling mechanisms in roots that encounter elevated concentrations of nutrients (Forde and Lorenzo 2001; Malamy 2005). Although typically at the plant scale there is relatively higher allocation of biomass to roots in nutrient-poor environments (Wright et al. 2011), in heterogeneous but nutrient-limited soil environments there is generally greater allocation of root biomass to locations in soil where nutrients are more abundant (Drew 1975; Hutchings and de Kroon 1994; Hodge 2004). Additionally, studies on root morphological traits across soil nutrient gradients indicate higher investment to fine root organs given increased soil nutrients, with construction of longer-lived roots characterized by thicker diameter (D), higher root tissue density (RTD), and lower specific root length (SRL) (Ostonen et al. 2007). Alternatively, the reverse has been observed where roots grow more rapidly with higher turnover to exploit nutrient-rich soil and, thus, show increased absorptive area per unit of biomass (e.g., higher SRL), while in nutrient-poor soil roots develop morphologies that limit nutrient losses (e.g., thicker D) (Fort et al. 2016). In sum, there is evidence that plants generally employ several concomitant and at times opposing strategies to increase the nutrient acquisition in heterogeneous soils by altering root initiation and growth and patterns of root morphology. Plastic responses can be nutrient specific, presumably influenced by the mobility of the nutrient in the soil matrix, the signalling and uptake pathways employed by roots, and the capacity to translocate the nutrient within the plant (Drew 1975; Mou et al. 1995; López-Bucio et al. 2003; Hodge 2004), and are further contingent on the overall nutrient status of the plant and localized distribution of nutrients within the range of the root system (López-Bucio et al. 2003; de Kroon et al. 2009).
In agroforestry, trees that are retained from previous forest or are later planted in agroecosystems can strongly influence the overall nutrient status of soil and crops. Organic deposits from aboveground sources (e.g., leaf litter) (Xia et al. 2015) and belowground sources (e.g., root turnover and exudation, and microbial activity) (Mommer et al. 2016) can modify soil nutrient availability at a range of scales (Jackson and Caldwell 1993; Xia et al. 2015). At the same time, roots from neighbouring plants generally deplete nutrients in localized areas, and root development patterns are expected to reflect integrated responses to soil nutrient levels and competition with neighbours (Cahill et al. 2010; Mommer et al. 2012). Numerous studies that manipulate soil conditions and neighbour interactions under controlled conditions show dramatic plasticity of root growth and placement in response to soil nutrients and competitors within localized patches (Mahall and Callaway 1992; Cahill et al. 2010; Semchenko et al. 2014). However, little is known on how root traits vary in relation to multiple co-limiting nutrients, nor on how this variation is expressed within a plant’s root system in naturally heterogeneous soil. Indeed, there is a general lack of empirical evidence for modular plasticity within root systems of individual plants in field conditions.
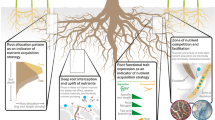
Plasticity of root systems in agroforestry systems can increase crop access to heterogeneous nutrient availability in soil but can also mitigate competitive effects from neighbouring trees (McGrath et al. 2001; Li et al. 2006; Isaac et al. 2014, 2017; Cardinael et al. 2015b). This is particularly important when there are few external nutrient inputs, which is generally the case for the tropical tree crop Theobroma cacao L. – the focal species in our study – that is commonly grown under the canopy of larger heterospecific neighbour trees (i.e., shade trees) on smallholder farms. While trees with more complementary root distributions can be preferentially planted with crops (i.e., tree species with deeper rooting profiles), typically there will be overlap of root systems in upper soil layers where nutrients are most abundant (Isaac et al. 2014; Borden et al. 2017b). To this end, we sought to capture two-dimensional distributions of cocoa root systems (rather than vertical zonation only) to account for more nuanced root allocation patterns (e.g., Sudmeyer et al. (2004), Li et al. (2006), and Laclau et al. (2013)).
In this study, we examined fine root distribution and functional trait expression of T. cacao in relation to soil nutrients and neighbour roots. We used two-dimensional vertical soil interfaces situated in three species combinations: at the interface with conspecific neighbours in monoculture and with two heterospecific neighbouring shade trees of distinctive growth strategies (early vs. late successional). We hypothesized that within these soil interfaces (i) localized areas of higher nutrient availability (characterized by six soil macro- and micro-nutrients) will have higher fine root length and biomass density, and (ii) these roots will express functional traits associated with root longevity. Additionally, (iii) systematic root trait variation with soil nutrients will be moderated by heterospecific neighbours based on differences in nutrient dynamics among species combinations.
Materials and methods
Study site and species combinations
The study was carried out in South Formangso, Ashanti Region, Ghana (6°36ˊ N, 0°58ˊ W) at a cocoa research station managed by the Forestry Research Institute of Ghana. The 2-ha site is situated on previously secondary forest that was cleared for cultivation and was left to fallow until the cocoa agroforestry system was established in 2001. T. cacao hybrid planting stock from the Cocoa Research Institute of Ghana was planted at a spacing of 3 × 3 m and, in agroforestry treatments, shade trees were planted in replacement of T. cacao at 12 × 12 m spacing. No fertilizer had been applied to the research site prior to the study. Soils are Acrisols with bulk density of 1.22 ± 0.02 g cm−3 and soil pH ranging from 6.2 ± 0.1 near the soil surface to 4.9 ± 0.0 near 60 cm depth. The site is in a moist semi-deciduous forest zone with mean annual rainfall of 1528 mm and mean annual temperature of 26.2 °C. Sampling was completed in the on-set of the rainy season, during T. cacao flowering and cocoa pod production and, thus, when nutrient demands were high (van Vliet and Giller 2017).
Study T. cacao trees (DBH = 14.6 ± 1.1 cm; mean ± SE) were selected from pre-established blocks of species combinations at the site, providing three replications of each species combination. The two shade tree species used in this study, Terminalia ivorensis Chev. (DBH = 58.8 ± 3.8 cm) and Entandrophragma angolense (Welw.) C. DC. (DBH = 19.9 ± 1.4 cm), are commonly used in this region to provide upper canopy shade (< 25% shade) for T. cacao cultivation. T. ivorensis is a fast-growing, early successional tree species and was the larger of the two heterospecific neighbour species. This species is characterized by many shallow lateral roots and has been shown to affect fine root length density (FRLD) of Coffea arabica L. (van Kanten et al. 2005) and was assumed to have strong belowground competitive effects due to high SRL (34.7 ± 9.3 m g−1; n = 30). Slower-growing, late-successional E. angolense is perceived by farmers to be deeper rooted and had lower SRL (29.7 ± 6.2 m g−1; n = 30; measured from the study site; data not shown).
T. cacao and neighbour soil interfaces: sampling on soil trenches
Nine soil trenches 1 m wide and at least 60 cm deep were manually excavated (three trenches per species combination). The exposed soil ‘interfaces’ in the trenches were perpendicular to transects connecting T. cacao with another T. cacao, or T. cacao with a shade tree, and located halfway between the trees’ stems (i.e., 1.5 m from each stem) (Fig. 1). The location and size of the soil interfaces were selected to represent an area occupied by an individual T. cacao root system and with limited root system interactions from non-study T. cacao trees (Isaac and Anglaaere 2013; Borden et al. 2017a), while sampling scale and intensity was first assessed from preliminary soil profiles that were tested for soil nutrients (data not shown; Soils Institute of Ghana, Kumasi, Ghana). In each of the present study’s soil interfaces, 40 soil cores (5 cm diameter; 100 cm3 volume) were taken horizontally and in a stratified random sampling scheme. Samples were taken such that the soil core was centred at 2.5, 7.5, 15, 27.5 cm depths (i.e., y direction) to capture the dominant rooting zone of T. cacao (i.e., to 30 cm) and centred at 57.5 cm depth to capture root strategies in deeper soils. This vertical sampling scheme was repeated every 20 cm intervals (at 0, 20, 40, 60, 80, 100 cm) across the length of the trench (i.e., x direction) followed by 10 additional samples taken at random, non-sampled locations in the soil interface, recorded using an x, y coordinate systems. Thus, in sum, samples were taken from five depths at six horizontal locations and an additional 10 randomly located on each of the nine interfaces for a total of 360 samples. In the lab, samples were gently homogenized by hand and then divided into two approximately equal volumes of soil, with half of each sample (~50 cm3) used for fine root analysis and the other half used for soil chemical analysis. Samples were stored in polyethylene bags and frozen until further processing.
Soil interfaces (n = 9) used in this study. Left panel: Schematic depicting the location of a soil interface between a T. cacao tree and a heterospecific or conspecific neighbour tree. Right panel: An excavated soil interface situated between a T. cacao tree (foreground) and a shade tree Entandrophragma angolense (background)
Fine root analysis
Roots were removed using forceps from soil samples passed through sequential sieving with water. Collected roots were then placed in water to further loosen and remove soil from roots. Fine roots were separated by species through visual inspection using a stereoscopic microscope. T. cacao fine roots were distinctly reddish-brown, whereas the shade tree roots were lighter in colour. We removed dead roots, characterized by their lack of turgor, black colouring, and easy separation of stele from cortex. Fine roots (≤ 2 mm) were then scanned using a flatbed scanner (STD4800; Regent Instruments Inc., Canada) at 600 dpi. From these images, average fine root diameter, fine root length, and fine root volume (approximated as cylindrical roots) from each core sample were measured using WinRhizo (Reg. 2016a; Regent Instruments, Canada). Fine root dry weights were measured after 48 h of drying at 65 °C. These data were used to calculate six root traits that characterized the root density in each soil sample: fine root length density (FRLD; cm cm−3) and fine root biomass density (FRBD; mg cm−3) of an individual T. cacao, and the morphology of the roots in each 100 cm3 sample: specific root length (SRL; m g−1), root tissue density (RTD = [dry root mass/ fresh root volume]; mg cm−3), and average root diameter (D; mm). We also estimated the ratio of the length of absorptive fine roots in relation to the length of fine transport roots (A:T) that were in each 100 cm3 sampling unit. A:T captures the relative amount of fine root length that is predominantly responsible for nutrient uptake and was calculated using a diameter cut off that captured the majority of the first three orders based on T. cacao root data from this site: fine roots of T. cacao below a cut-off of 0.50 mm did not exhibit secondary growth and represented 85.2 ± 0.07% (± SD; n = 30; data not shown) of absorptive (root orders 1 to 3) length (Freschet and Roumet 2017). This diameter cut off for very fine roots (Roumet et al. 2016) was used as root samples are challenging to identify by root order when root topology is lost from sampling a small soil volume. A correction factor of 0.5 for FRLD and FRBD was used for T. cacao in monoculture to adjust for assumed presence of two T. cacao root systems. Dry weight biomass of shade tree fine roots was used to calculate fine root biomass density of neighbouring shade trees (FRBDshade; mg cm−3) in each sample.
Soil chemical analysis
From each soil sample, available NO3− an NH4+ were extracted from field moist soils in KCl solution, filtered through Fisher P8 filter paper, and measured using a spectrophotometer flow injection analyzer (QuikChem 8500, Lachat Instruments, USA). The remaining soils from each sample were air-dried for 2 weeks and sieved through 2 mm mesh. From these soils, available PO4− was extracted in a 1:10 soil to Bray’s 1 solution, filtered through Fisher P5 filter paper, and measured using a spectrophotometer. Air-dried soil was further ground in a ball mill (Retsch Ltd., Germany). From these soils, exchangeable K+, Mg2+, and Ca2+ were extracted with ammonium acetate (NH4OAc), filtered through Fisher P8 filter paper, and analyzed using an atomic absorption spectrometer (AAnalyst 200, PerkinElmer, USA). Soil chemical analyses were carried out at the University of Toronto Scarborough, Toronto, Canada.
Statistical analysis
All statistical analyses were completed in R (version 3.2.4). We quantified and compared the in situ nutrient conditions within the scale of individual T. cacao root systems. The amount of variation in soil nutrients encountered by individual T. cacao root systems in the soil interfaces was assessed by the range and coefficient of variation (CV) of each soil nutrient. Overall soil nutrient levels in each species combination were described using the mean values calculated within 10 cm depth intervals on each interface, and differences of soil nutrient levels among treatments were tested using ANOVA and when significant this was followed by Tukey HSD. Next, we compared intra-root system variation of T. cacao with different neighbour species. Systematic variation in the vertical distribution, with data pooled into 10-cm intervals, of T. cacao fine root densities and morphology by species combination were assessed using ANOVA. Two-dimensional visual interpretations of root and soil variables in each 100-cm wide × 60-cm deep soil interface were produced using inverse distance weighting on a grid with cells of 5 × 5 cm, approximating soil core diameter, in the ‘gstat’ package and examples of these interfaces (one per species combination) were visualized using the ‘rasterVis’ package.
We examined the directional relationships between T. cacao fine root distribution, architectural, and morphological traits with localized soil nutrient availability, focusing on data within the dominant rooting zone of T. cacao (0 to 30 cm depth). To do so, linear mixed models (LMMs) for each root trait in each species combination were fit with sampling depth assigned as fixed variable and soil interface assigned as a random factor. As the fine roots of mature T. cacao grow as dense root mat in the top soil (Nygren et al. 2013), we assumed that soil cores taken within the top 30 cm of each interface were independent observations without spatial autocorrelation after depth was included as a fixed term. All measured soil nutrients were included in the LMMs as fixed variables to evaluate how a change in availability of each nutrient within 100 cm3 soil volumes is related to variation in root traits while accounting for variation of the other measured soil nutrients under field conditions. For T. cacao in mixture, FRBDshade was also included as a fixed variable. To estimate the amount of variation in T. cacao root traits explained by all fixed variables, the ‘fixed effects r2’ was calculated using the ‘r2beta’ function (with method ‘nsj’) in the ‘r2glmm’ package (Nakagawa and Schielzeth 2013). This procedure also allowed us to estimate partial r2 of each fixed variable. For parametric analyses, residuals were tested for normality using the Shapiro Wilk test. To meet parametric assumptions, root and soil data were log10 transformed. The level of significance was at p < 0.05.
Results
Soil nutrients: distribution and variation
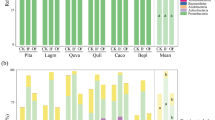
Within the dominant lateral rooting zone of an individual root system (i.e., to 30 cm depth) there was large variation in soil nutrients (Table 1). Soil NO3− and K+ could vary by two orders of magnitude, showing a large range and large CV, except for soil K+ in T. cacao-E. angolense mixture. There were some particularly high concentrations of soil NO3− in monoculture (max: 82.0 mg g−1), which was concentrated in surface soils (Fig. 2). Soil K+ was highest in monoculture, particularly when compared to the T. cacao-T. ivorensis mixture (Table 1; Fig. 2). Soil NH4+, Ca2+, and Mg2+ also showed high variability, while soil PO4− was the least variable with the lowest CV (6 to 47%) (Table 1). Overall, both mixtures had higher soil NH4+ than monoculture and soil PO4− was highest in T. cacao-T. ivorensis mixture (Fig. 2). Soil nutrients generally decreased with depth, although soil K+ was more evenly distributed vertically in the soil profiles (Fig. 2).
T. cacao fine roots: distribution and variation
As with soil nutrients, T. cacao vertical distributions of fine roots were concentrated near the soil surface and decreased with depth (Figs. 3 and 4). Over 90% of T. cacao fine roots were in the top 30 cm of soil regardless of neighbour species. T. cacao roots next to E. angolense tended to be concentrated in shallow soils with 71% of both fine root length and biomass located in the top 10 cm of soil (Fig. 4). For T. cacao with conspecifics, 67% of fine root length and 64% of fine root biomass were in surface soil (top 10 cm). When next to T. ivorensis, there was 70% of fine root length but only 59% of fine root biomass in the top 10 cm, with more vertically dispersed fine root biomass between 10 and 30 cm. Vertically, the two shade tree species also showed decreasing densities of roots within the top 60 cm of soil, but T. ivorensis showed a higher concentration of fine root biomass in surface soil (top 10 cm) compared to E. angolense that had more evenly distributed fine root biomass within the soil interfaces (Figs. 3 and 4).
Vertical distribution of fine root density (FRLD and FRBD), architecture (A:T), and morphology (SRL, D, RTD) of an individual T. cacao tree at a distance of 1.5 m from stems (mean ± SE, n = 3). Also shown is vertical distribution of fine root biomass shade trees (FRBDshade). Same letters are non-significant differences for individual T. cacao among treatments at same depth when there was a significant treatment effect (ANOVA)
Examples of interpolated interface maps depicting the distribution of soil nutrients (e.g., NH4+ and Ca2+), shade tree fine roots (FRBDshade), and T. cacao fine roots (e.g., FRLD and SRL) in three soil interfaces between two T. cacao (left column), T. cacao and E. angolense (middle column), and T. cacao and T. ivorensis (right column)
There were no significant differences in fine root densities for T. cacao on the vertical profile 1.5 m from a T. cacao stem (Fig. 3). However, notably in surface soils (0 to 10 cm), where root densities were highest, FRBD for T. cacao next to shade trees was 13 to 19% higher than for individual T. cacao in monoculture, though this was not significant: 1.23 ± 0.18 mg cm−3 and 1.30 ± 0.17 when next to T. ivorensis and E. angolense, respectively, and in monoculture: 1.09 ± 0.17 mg cm−3. Mean FRLD of individual T. cacao trees in surface soils was 1.85 ± 0.28 and 1.90 ± 0.29 cm cm−3 when next to E. angolense and T. ivorensis, respectively, which was 83 to 88% above that from a T. cacao tree in monoculture (1.01 ± 0.28 cm cm−3) (Fig. 3). Generally, T. cacao fine roots in monoculture expressed more conservative morphology with significantly lower RTD (p = 0.01) than when next to a shade tree (Fig. 3).
T. cacao fine root distribution and morphology in relation to soil nutrients and shade tree roots
Significant directional effects of each nutrient on root traits were consistent regardless of species combination (Table 2). Soil NH4+ and Ca2+ had a generally positive effect on T. cacao fine root densities (FRLD and FRBD) and investment at the root scale, expressed as positive coefficients for D and negative coefficients for SRL and A:T in LMMs (Table 2; Table S1). However, opposite trends were observed for soil NO3− and K+, particularly for T. cacao in monoculture and T. cacao next to T. ivorensis. Soil PO4− was limited as a predictor variable in root trait variation with the exception of A:T for T. cacao in mixture with E. angolense. Soil Mg2+ generally had a negative effect on localized investment to roots for T. cacao in mixture with T. ivorensis, with a significant negative D coefficient (p = 0.04) (Table 2; Table S1).
Depth, soil nutrients, and FRBDshade together explained similar proportion of variation in FRLD and FRBD of T. cacao in monoculture and T. cacao in mixture with E. angolense (fixed effects r2 = 0.52 to 0.65) as well as FRLD for T. cacao in mixture with T. ivorensis (r2 = 0.61) (Table 2). However, these same variables were less effective in explaining variation in FRBD of T. cacao next to T. ivorensis (r2 = 0.29). In most cases, variation in root densities (FRLD and FRBD) was better explained by the fixed variables (depth, nutrients, FRBDshade) than was the variation in root architecture (A:T; r2 = 0.07 to 0.19) or morphology (SRL and D; r2 = 0.09 to 0.22), except for a notably high fixed effects r2 for RTD (r2 = 0.30 to 0.55). For T. cacao in mixture with E. angolense, variation in root traits was mainly explained by differences in depth (partial r2 = 0.08 to 0.21) while the effects of localized nutrient variation at similar depths were weakly related to variation in root traits. In contrast, variation in nutrients were just as, or more important than depth in explaining variation in root traits of T. cacao in monoculture and T. cacao in mixture with T. ivorensis (Table 2). We did not observe significant effects of FRBDshade on T. cacao fine root densities in localized soil volumes, which would indicate root avoidance, but only non-significant negative coefficients of FRBDshade of both shade tree species with T. cacao FRBD and FRLD. We did not observe localized impact of FRBDshade on the fine root morphology of T. cacao, but there was a marginally significant positive effect observed for SRL with FRBDshade of T. ivorensis (p = 0.07) and a marginally significant negative effect for RTD (p = 0.07) (Table 2; Table S1).
Discussion
Intra-root system foraging strategies for specific nutrients
In tropical ecosystems, tree roots are generally concentrated in the top 30 cm of soil (Jackson et al. 1996), reflecting rapid uptake of soil nutrients and nutrient deposition by leaf litter in this upper soil layer. Our study confirmed high densities of T. cacao fine roots in the uppermost mineral soil, which mirrored the vertical patterns in soil nutrient availability. We also found important soil nutrient variation that occurred laterally within the scale of individual root systems. Cumulatively, we show that fine roots of T. cacao were spatially coupled to heterogeneously distributed nutrients indicating active modular root development in the foraging of soil nutrients for this species.
Foraging strategies realized through root system architectural and morphological plasticity can be nutrient-specific (Drew 1975; Hodge 2004). For soil NH4+ and Ca2+, our first hypothesis was consistently supported: within the scale of individual root systems, locations with higher soil nutrient availability were associated with higher density of fine roots (i.e., higher FRLD and FRBD). This trend was coupled with greater investment to root tissue (expressed as lower SRL and higher D and RTD), which was in support of our second hypothesis. However, inconsistent and/or opposite effects were found for soil NO3−, and to a lesser extent K+, and Mg2+: patterns were generally neutral or, in some cases, higher localized concentrations of these nutrients in soil were associated with reduced density of roots (lower FRLD and FRBD) and ‘less expensive’ roots (higher A:T and SRL; lower D and RTD). In the case of the more mobile soil nutrients: NO3− and Mg2+ (Gransee and Führs 2013), it may be more economical for plants to increase uptake with short-lived, younger roots (Blair and Perfecto 2004). Additionally, however, these negative associations between fine root density and nutrients were found when there was distinctly higher availability of the nutrient compared to other species combinations. Thus, we speculate over-supply in nutrients favours reduced root allocation; this explanation seems likely for soil K+ in T. cacao monocultures.
The higher proportion of thinner absorptive root length that was associated with increased availability of PO4−, specifically for T. cacao when in mixture with E. angolense, would permit higher precision foraging for this relatively immobile nutrient (Hodge 2004; Hinsinger et al. 2011). Otherwise, however, root trait variation was generally unrelated to localized variation in soil PO4−. McGrath et al. (2001) reported increased proliferation of fine roots of T. grandifolium into soil cores that were artificially enriched with PO4−. However, PO4− gradients under natural conditions may occur predominantly at smaller scales (e.g., gradients of 1 mm or less within the rhizosphere) (Hinsinger et al. 2011). As rhizosphere soil was mixed in with bulk soil within 5 cm diameter soil cores, our sampling design likely limited our ability to detect foraging for this nutrient, a conclusion also supported by the relatively limited variation in PO4− in this present study. More importantly, other root characteristics, which were not measured in our study, may better capture acquisition strategies, such as root hair abundance or mycorrhizal associations (Hodge 2004; Chen et al. 2018). More generally, the use of a 2 mm diameter cut off for measuring fine root SRL, D, and RTD may have obscured some relationships between the fine roots predominantly responsible for nutrient acquisition and soil nutrients (Freschet and Roumet 2017). Increased precision in the delineation of absorptive roots, as well as the addition of root hairs and mycorrhizal associations into root functional trait research is critical for advancing assessment of root-soil patterns.
How is root foraging modified by shade trees?
Relationships between soil nutrients and fine root densities and morphology of T. cacao differed among species combinations. Such responses of fine roots to localized sources of nutrients are expected to be driven by differential nutrient demands of the plant (Forde and Lorenzo 2001). Previous research has shown the nutrient status of T. cacao to be modified by interactions with neighbouring shade trees (Isaac et al. 2007). We found that significant trends between root traits and soil nutrients were most pronounced for T. cacao in monoculture, and specifically for N (both NO3− and NH4+) and Ca, suggesting these may be co-limiting nutrients in the sole-cropping system. Patterns differed markedly in soil interfaces near shade trees. No dominant nutrient emerged for T. cacao next to E. angolense, while available NH4+ best explained root patterns for T. cacao next to T. ivorensis, suggesting a T. cacao response to N limitation within this species combination.
Differential tree root distribution and activity can contribute to belowground complementarity in tree-based agroecosystems (Brooker et al. 2015; Borden et al. 2017b). While fine roots of T. cacao below 60 cm can contribute to improved complementarity and total soil resource acquisition (Abou Rajab et al. 2018), we focus on the extensive lateral roots of T. cacao that are at highest concentration in the top 30 cm of soil (Nygren et al. 2013; Isaac et al. 2014; Borden et al. 2017a). Within this dominant rooting zone, previous studies have shown vertical stratification in T. cacao root distribution and activity with neighbouring shade trees (Moser et al. 2010; Isaac et al. 2014; Abou Rajab et al. 2018). The present study found some evidence that the species of shade tree controls developmental plasticity in T. cacao. We found that T. cacao next to T. ivorensis had more evenly distributed fine roots in the upper 30 cm of soil suggesting greater complementarity belowground, while T. cacao roots next to E. angolense were more concentrated near the surface and variation in root traits showed a stronger vertical trend. Significant effects of FRBDshade on localized distribution, architecture, and morphology of T. cacao roots were not detected in this study, but it is intriguing and worth noting the marginally significant directional trends of T. cacao root traits in relation to fine root density of the fast-growing T. ivorensis, that would suggest T. cacao fine roots have more acquisitive root morphology when in localized competition with roots of T. ivorensis. However, to elucidate root-root responses, more empirical evidence is needed of root trait response when there are higher densities of neighbouring tree roots to presumably increase the effects of neighbour root activity. Root-root interactions between conspecific and heterospecific neighbouring plants can be complex (Mommer et al. 2016) and we speculate that the strength of competitive (e.g., resource depletion) and facilitative effects (e.g., organic deposits) from root activity at localized scales is likely to depend on species combination and merits further investigation.
In low-input agroforests, nutrient cycling is a significant component of nutrient delivery and shade tree leaves can constitute a substantial proportion of litterfall in shaded cocoa agroecosystems (perhaps a third to a half of total litter inputs (van Vliet and Giller 2017)). Leaf litter from fast-growing species such as T. ivorensis is commonly associated with higher rates of decomposition (Cornwell et al. 2008) and, along with its extensive canopy, is likely an important determinant of nutrient dynamics and distribution. Belowground, variation in root traits, such as higher SRL and lower RTD, has been associated with shorter root lifespan and faster root turnover and decomposition (Freschet and Roumet 2017). In the present study, T. cacao fine roots had lower RTD when in mixture with a shade tree compared to when in monoculture, suggesting more rapid nutrient cycling. More acquisitive root traits in mixture compared to monoculture have been reported in other tree-based ecosystems (e.g., Bolte and Villanueva (2006) and Duan et al. (2017)) and in T. cacao specifically (Abou Rajab et al. 2018). The impacts of farm- and ecosystem-scale processes on the plastic responses of root morphology in agroforests deserve further research attention, particularly as this will be critical to the precision of nutrient management on farms.
Conclusions
Our results support the conclusion that soil nutrient heterogeneity occurs at scales relevant to individual trees in a tropical low-input agroforest. We carried out one of the first studies on multiple soil macro- and micro-nutrient effects on root functional trait expression within a species in naturally heterogeneous soils. Root system phenotypic plasticity was expressed as variation in the distribution of fine roots (FRLD and FRBD), fine root architecture (A:T), and morphology (SRL, D, and RTD). By relating root traits to soil nutrient availability on two-dimensional soil interfaces, we found that fine root trait expression had nutrient-specific relationships at localized scales (100 cm3) within the dominant rooting zone of individual T. cacao. At the plant scale, intraspecific root traits shifted towards nutrient-acquiring morphology when next to a shade tree. Taken together, these results indicate that modelling of the fine root system architecture and nutrient acquisition patterns in agroforests must consider species interactions to capture the full scope of root trait expression. Measuring drivers of this root trait variability is critical to improve our understanding of the root-soil continuum in agroforestry systems and for the development of ecologically-informed agricultural practices.
Abbreviations
- A:T:
-
Ratio of absorptive to transport fine root length
- D:
-
Average root diameter
- FRLD:
-
Fine root length density
- FRBD:
-
Fine root biomass density
- RTD:
-
Fine root tissue density
- SRL:
-
Specific root length
References
Abou Rajab Y, Hölscher D, Leuschner C, Barus H, Tjoa A, Hertel D (2018) Effects of shade tree cover and diversity on root system structure and dynamics in cacao agroforests: the role of root competition and space partitioning. Plant Soil 422:349–369
Blair BC, Perfecto I (2004) Successional status and root foraging for phosphorus in seven tropical tree species. Can J For Res 34:1128–1135
Bolte A, Villanueva I (2006) Interspecific competition impacts on the morphology and distribution of fine roots in European beech (Fagus sylvatica L.) and Norway spruce (Picea abies (L.) Karst.). Eur J For Res 125:15–26
Borden KA, Anglaaere LCN, Adu-Bredu S, Isaac ME (2017a) Root biomass variation of cocoa and implications for carbon stocks in agroforestry systems. Agrofor Syst. https://doi.org/10.1007/s10457-017-0122-5
Borden KA, Thomas SC, Isaac ME (2017b) Interspecific variation of tree root architecture in a temperate agroforestry system characterized using ground-penetrating radar. Plant Soil 410:323–334
Brooker RW, Bennett AE, Cong W, Daniell TJ, George TS, Hallett PD, Hawes C, Iannetta PPM, Jones HG, Karley AJ, Li L, McKenzie BM, Pakeman RJ, Paterson E, Schöb C, Shen J, Squire G, Watson CA, Zhang C, Zhang F, Zhang J, White PJ (2015) Improving intercropping: a synthesis of research in agronomy, plant physiology and ecology. New Phytol 206:107–117
Cahill JF, Mcnickle GG, Haag JJ, Lamb EG, Nyanumba SM, Cassady CSC (2010) Plants integrate information about nutrients and neighbors. Science 328:1657
Cardinael R, Chevallier T, Barthès BG, Saby NPA, Parent T, Dupraz C, Bernoux M, Chenu C (2015a) Impact of alley cropping agroforestry on stocks, forms and spatial distribution of soil organic carbon - a case study in a Mediterranean context. Geoderma 259–260:288–299
Cardinael R, Mao Z, Prieto I, Stokes A, Dupraz C, Kim JH, Jourdan C (2015b) Competition with winter crops induces deeper rooting of walnut trees in a Mediterranean alley cropping agroforestry system. Plant Soil 391:219–235
Chen W, Koide RT, Eissenstat DM, Field K (2018) Nutrient foraging by mycorrhizas: from species functional traits to ecosystem processes. Funct Ecol 32(4):858–869
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Perez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, van Bodegom P, Brovkin V, Chatain A, Callaghan TV, Díaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Victoria Vaieretti M, Westoby M (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071
de Kroon H, Visser EJW, Huber H, Mommer L, Hutchings MJ (2009) A modular concept of plant foraging behaviour: the interplay between local responses and systemic control. Plant Cell Environ 32:704–712
Drew MC (1975) Comparison of the effects of a localized supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytol 75:479–490
Duan ZP, Gan YW, Wang BJ, Hao XD, Xu WL, Zhang W, Li LH (2017) Interspecific interaction alters root morphology in young walnut/wheat agroforestry systems in Northwest China. Agrofor Syst. https://doi.org/10.1007/s10457-017-0133-2
Forde B, Lorenzo H (2001) The nutritional control of root development. Plant Soil 232:51–68
Fort F, Cruz P, Lecloux E, Bittencourt de Oliveira L, Stroia C, Theau JP, Jouany C (2016) Grassland root functional parameters vary according to a community-level resource acquisition-conservation trade-off. J Veg Sci 27:749–758
Freschet G, Roumet C (2017) Sampling roots to capture plant and soil functions. Funct Ecol 31:1506–1518
Gransee A, Führs H (2013) Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 368:5–21
Hinsinger P, Betencourt E, Bernard BA, Plassard C, Shen J, Tang X, Zhang F (2011) P for two, sharing a scarce resource: soil phosphorus acquisition in the rhizosphere of intercropped species. Plant Physiol 156:1078–1086
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162:9–24
Hutchings M, de Kroon H (1994) Foraging in plants: the role of morphological plasticity in resource acquisition. Adv Ecol Res 25:159–238
Isaac ME, Anglaaere LCN (2013) An in situ approach to detect tree root ecology: linking ground-penetrating radar imaging to isotope-derived water acquisition zones. Ecol Evol 3(5):1330–1339
Isaac ME, Ulzen-Appiah F, Timmer VR, Quashie-Sam SJ (2007) Early growth and nutritional response to resource competition in cocoa-shade intercropped systems. Plant Soil 298:243–254
Isaac ME, Anglaaere LCN, Borden K, Adu-Bredu S (2014) Intraspecific root plasticity in agroforestry systems across edaphic conditions. Agric Ecosyst Environ 185:16–23
Isaac ME, Martin AR, de Melo Virginio Filho E, Rapidel B, Roupsard O, Van den Meersche K (2017) Intraspecific trait variation and coordination: root and leaf economics spectra in coffee across environmental gradients. Front Plant Sci 8:1–13
Jackson RB, Caldwell MM (1993) The scale of nutrient heterogeneity around individual plants and its quantification with geostatistics. Ecology 74(2):612–614
Jackson RB, Canadell J, Ehleringer JR, Mooney HA, Sala OE, Schulze ED (1996) A global analysis of root distributions for terrestrial biomes. Oecologia 108(3):389–411
Laclau J-P, da Silva EA, Lambais GR, Bernoux M, le Maire G, Stape JL, Bouillet J-P, de Moraes Gonçalves JL, Jourdan C, Nouvellon Y (2013) Dynamics of soil exploration by fine roots down to a depth of 10 m throughout the entire rotation in Eucalyptus grandis plantations. Front Plant Sci 4:12
Li L, Sun J, Zhang F, Guo T, Bao X, Smith F, Smith S (2006) Root distribution and interactions between intercropped species. Oecologia 147:280–290
López-Bucio J, Cruz-Ramírez A, Herrera-Estrella L (2003) The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol 6:280–287
Mahall BE, Callaway RM (1992) Root communication mechanisms and intracommunity distributions of two Mojave desert shrubs. Ecology 73:2145–2151
Malamy JE (2005) Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ 28:67–77
McGrath D, Duryea M, Cropper W (2001) Soil phosphorus availability and fine root proliferation in Amazonian agroforests 6 years following forest conversion. Agric Ecosyst Environ 83:271–284
Mommer L, van Ruijven J, Jansen C, van de Steeg HM, de Kroon H (2012) Interactive effects of nutrient heterogeneity and competition: implications for root foraging theory? Funct Ecol 26:66–73
Mommer L, Kirkegaard J, van Ruijven J (2016) Root-root interactions: towards a rhizosphere framework. Trends Plant Sci 21:209–217
Moser G, Leuschner C, Hertel D, Hölscher D, Köhler M, Leitner D, Michalzik B, Prihastanti E, Tjitrosemito S, Schwendenmann L (2010) Response of cocoa trees (Theobroma cacao) to a 13-month desiccation period in Sulawesi, Indonesia. Agrofor Syst 79:171–187
Mou P, Jones R, Mitchell R, Zutter B (1995) Spatial distribution of roots in sweetgum and loblolly pine monocultures and relations with above-ground biomass and soil nutrients. Funct Ecol 9:689–699
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142
Nygren P, Leblanc HA, Lu M, Gomez Luciano CA (2013) Distribution of coarse and fine roots of Theobroma cacao and shade tree Inga edulis in a cocoa plantation. Ann For Sci 70:229–239
Ostonen I, Püttsepp Ü, Biel C, Alberton O, Bakker MR, Lõhmus K, Majdi H, Metcalfe D, Olsthoorn AFM, Pronk A, Vanguelova E, Weih M, Brunner I (2007) Specific root length as an indicator of environmental change. Plant Biosyst 141:426–442
Roumet C, Birouste M, Picon-Cochard C, Ghestem M, Osman N, Vrignon-Brenas S, K-f C, Stokes A (2016) Root structure-function relationships in 74 species: evidence of a root economics spectrum related to carbon economy. New Phytol 210:815–826
Semchenko M, Saar S, Lepik A (2014) Plant root exudates mediate neighbour recognition and trigger complex behavioural changes. New Phytol 204:631–637
Sudmeyer RA, Speijers J, Nicholas BD (2004) Root distribution of Pinus pinaster, P. radiata, Eucalyptus globulus and E. kochii and associated soil chemistry in agricultural land adjacent to tree lines. Tree Physiol 24:1333–1346
van Kanten R, Schroth G, Beer J, Jiménez F (2005) Fine-root dynamics of coffee in association with two shade trees in Costa Rica. Agrofor Syst 63:247–261
van Vliet JA, Giller KE (2017) Mineral nutrition of cocoa: a review. Adv Agron 141:185–270
Wright SJ, Yavitt JB, Wurzburger N, Turner BL, Tanner EVJ, Sayer EJ, Santiago LS, Kaspari M, Hedin LO, Harms KE, Garcia MN, Corre MD (2011) Potassium, phosphorus, or nitrogen limit root allocation, tree growth, or litter production in a lowland tropical forest. Ecology 92:1616–1625
Xia S-W, Chen J, Schaefer D, Detto M (2015) Scale-dependent soil macronutrient heterogeneity reveals effects of litterfall in a tropical rainforest. Plant Soil 391:51–61
Acknowledgements
We would like to thank Luke Anglaaere at the Forestry Research Institute of Ghana as well as Agyeman Kofi and community members of South Formangso for assistance in the field. We thank Stephanie Gagliardi, Serra Buchanan, and Luzianne Reid at University of Toronto Scarborough for assistance with laboratory work. We thank the editors and three anonymous reviewers whose valuable comments and suggestions greatly enhanced the quality of the manuscript. We are grateful for funding support from the Canada Research Chairs program, Natural Sciences and Engineering Research Council of Canada and the Department of Geography & Planning, University of Toronto.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Zhun Mao.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 69 kb)
Rights and permissions
About this article
Cite this article
Borden, K.A., Thomas, S.C. & Isaac, M.E. Variation in fine root traits reveals nutrient-specific acquisition strategies in agroforestry systems. Plant Soil 453, 139–151 (2020). https://doi.org/10.1007/s11104-019-04003-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04003-2