Abstract
Background and aims
Nitrogen deposition and altered precipitation regime are likely to change plant growth, biomass allocation and community structure, which may influence susceptibility of ecosystem functions (i.e. ecosystem carbon exchange) to extreme climatic events, such as drought.
Methods
In a meadow steppe, we deployed a drought treatment on a long-term water and nitrogen addition experiment to investigate resource abundance changes induced variation in the sensitivity of ecosystem carbon exchange to extreme drought.
Results
Compared to the control plots, long-term water and nitrogen addition caused a strong increase in biomass, and a reduction in diversity and root/shoot ratio. Net ecosystem CO2 exchange (NEE) in water and nitrogen addition plots were more sensitive to drought stress than the control plots. The enhanced NEE drought sensitivity (SNEE) in nitrogen fertilization habitat is associated with changes in aboveground biomass and root/shoot ratio, rather than variation in species diversity, while SNEE in the unfertilized plots was controlled by root/shoot ratio. Compared to the water and nitrogen addition plots, the control plots had the highest percentage recovery of ecosystem carbon exchange (RNEE) during the rehydration period. RNEE is likely determined by aboveground biomass and level of damage in the photosynthetic organ.
Conclusion
These findings suggest that long-term changes in precipitation regimes and nitrogen deposition may significant alter the susceptibility of key ecosystem processes to drought stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ongoing global climate changes are likely to increase the occurrence of extreme climatic events (drought etc.) on global and regional scales in the near future (Hansen et al. 2012; IPCC 2007; Jiang et al. 2012b). Simultaneously, alterations in global precipitation regimes and nitrogen deposition were forecasted and observed by many studies over the past few decades (Christensen et al. 2004; Galloway et al. 2008; Wang et al. 2015a). These long-term alteration in precipitation and nitrogen deposition will not only affect plant growth, biomass allocation and community structure (Bai et al. 2008; Xu et al. 2014; Yang et al. 2012), but also influence the resistance and resilience of ecosystem processes (such as ecosystem carbon cycling) to extreme climatic events such as drought (Friedrich et al. 2012; Smith et al. 2016). Recent studies have focused on the impacts of either long-term global change factors or short-term climate extremes on temporal variability of ecosystem functions, for instance productivity, stability and nutrient cycling, in various types of grassland (Knapp et al. 2002; Niu et al. 2010; Smith 2011; Wang et al. 2015b). Nevertheless, there is a clear need to understand how severe drought will impact the ecosystem, which has been altered in species composition, biomass allocation by long-term and chronic global change factors, such as increase in precipitation and nitrogen deposition (Friedrich et al. 2012; Xu et al. 2014).
The responses of ecosystem processes to drought, especially ecosystem carbon (C) fluxes, are an important topic of global climate change research given that altered ecosystem carbon balance may have feedback effects to climate change (Ciais et al. 2005). Due to lack of deep roots and water storage organs, grassland plants are very dependent on abundant natural precipitation and suitable topsoil moisture (Cherwin and Knapp 2012). A short-term extreme drought event has the potential to induce significant, long-term and disproportionate ecological impacts (Knapp et al. 2017; Tilman and Elhaddi 1992). The results of previous studies on the responses of ecosystem carbon exchange to severe drought suggest that a severe drought will cause a great reduction in gross primary productivity with ecosystem respiration decreasing concurrently (Ciais et al. 2005; Hoover et al. 2014; Shi et al. 2018; Shi et al. 2014). Drought affects grassland ecosystem CO2 exchange primarily through decreasing in soil water content and breaking the balance of water supply-demand for a given plant community, which cause CO2 starvation and untimely senescence or mortality of photosynthetic tissues (Bréda et al. 2006; Frank et al. 2015; Hoover et al. 2017; Mirzaei et al. 2008). In general, the grassland drought resistance is determined by (1) higher plants diversity and more redundant structure (Yachi and Loreau 1999); (2) greater water use efficiency (De Boeck et al. 2006) and (3) better water acquisition and conservation ability (Wang et al. 2007). However, the impact of mechanisms (1) and (2) on resistance may mainly be reflected in prolonged and/or frequent drought climate, and the third mechanism may be more valuable in shorter isolated extreme drought events due to more direct mode of action. Overall, it is very difficult to assess and predict exact effects of changes of environmental factors (such as nitrogen and water addition) on drought susceptibility of ecosystem carbon exchange with a short period extreme drought because of unknown complex interrelations between environmental driving factors and plant community status, which are varying simultaneously (Knapp et al. 2008; Pfisterer and Schmid 2002).
Generally, most grassland ecosystems were limited by the availability of nitrogen and water, which can influence plant growth, biomass allocation and community composition (Knapp et al. 2008; Niu et al. 2009; Reynolds and D'Antonio 1996; Wang et al. 2015a; Xu and Zhou 2005). To date, most of studies have found that either water or nitrogen addition all could enhance biomass production (Brueck et al. 2010; Jiang et al. 2012a; Yan et al. 2011), and reduce plant diversity and root-shoot ratio (Gonzalez-Dugo et al. 2010; McConnaughay and Coleman 1999). Many studies have suggested that N and water addition could make grassland ecosystems more sensitive to extreme drought events (Xu et al. 2014), because of the changing of biomass and biomass allocation, which are associated with water acquisition and conservation ability of plant communities (Cherwin and Knapp 2012; Frank et al. 2015; Wang et al. 2007). The results of previous studies have shown a negative correlation between drought resistance and aboveground biomass, which presumably because less aboveground biomass is associated with reduced leaf area and water consumption (Huston 1997; Mirzaei et al. 2008). In moist subtropical grassland, Wang et al. (2007) found the resistant to drought stress benefits more from lower biomass than from higher plant diversity. In addition to biomass, biomass allocation may also have profound impacts on ecosystem drought resistance and recuperability by affecting the coupling of water supply and demand (Tilman et al. 2006). Previous drought studies focused more on changes in biomass and biomass allocation. Not much information are available on drought response and recovery of ecosystem carbon exchange, especially at fine timescale. Ecosystem carbon exchange measurements at high resolution are useful for the understanding of interactive effects of chronic global change factors and short-term climate extremes on key ecosystem functions (carbon cycling etc.).
To examine the individual and combined effects of long-term changes in both precipitation and nitrogen deposition on drought sensitivity of ecosystem carbon exchange in grassland ecosystems, we experimentally simulated an extreme drought event in a native meadow steppe in northeast China, which have been treated with water and/or nitrogen addition for 3 years. We investigated the response of ecosystem C fluxes to drought and rehydration under the four experimental treatments (control, N addition, water addition, N and water addition in combination). We hypothesized that (1) ecosystem C exchanges will be inhibited by the manipulated drought treatment; (2) drought sensitivity of ecosystem C exchanges is likely to be enhanced in the nitrogen and water addition plots; (3) the plant water supply - demand balance may play important roles in regulating the response of ecosystem carbon exchange to drought in the studied meadow steppe.
Materials and methods
Study site
This experiment was carried out in Changling Horse Breeding Farm (44°30′-44°45′N, 123°31′-123°56′E), which is located in Western Jilin Province, Northeast China. For the studied area, mean annual temperature (1950–2004) is 6.4 °C and mean annual precipitation (1950–2004) is 471 mm with 80% rainfall occurring during the growing season (May to September). The main soil type is chernozem soil with soil organic carbon content of 2.0% and total nitrogen content of 0.15%. In the studied area, vegetation is dominated by perennial grass Leymus chinensis, other perennial plants, such as Phragmites australis, and Kalimeris integrifolia and annual plants, such as Chloris virgata are also abundant (Brad et al. 2010; Qu and Guo 2003). For the present study, plants were classified into three functional groups: annuals or biennial herbage (AH, i.e. C. virigata and Setaria viridis), perennial grasses (PG, i.e. L. chinensis, P. australis, K. integrifolia and Hemarthria altissima) and perennial forbs (PF, i.e. Lespedeza davurica and Artemisia mongolica). The studied meadow has been historically used for hay production. In 2010, we fenced an area of 100 m × 100 m experimental grassland, which has similar vegetation composition compare to the rest of the meadow. Grazing and mowing were excluded for the fenced grassland.
Experimental design
In May 2012, 6 blocks (each 20 m × 20 m, 10 m apart between each blocks at least) with similar vegetation composition were established within the experimental site. We divided each block into 4 plots (each plot has an area of 10 m × 10 m), and assigned one of the four treatments randomly (control, CK; nitrogen addition, N; water addition, W; nitrogen and water addition, NW). Water addition was applied manually as 15 mm of precipitation biweekly from May to September. In total, 120 mm water (about 25% of annual mean precipitation) was supplied throughout the growing season from 2012 to 2014. Groundwater (120 m deep) was used for the irrigation treatment. Compared to rainwater, groundwater has less nitrogen content and high pH value. Nitrogen addition plots were fertilized with urea at a rate of 10 g N m−2 yr.−1 (5 g N m−2 each for May and July), which was the maximum saturation nitrogen application for most grassland (Bai et al. 2010; Shi et al. 2018).
The extreme drought experiment was carried out in 2014 on the each aforementioned CK, N, W and NW plots, in total, with 6 replications. The extreme drought experiment consisted of a 45 d drought phase (16-Jun to 31-July). In each plot, a 3 m × 3 m sub-plot was established. The 2 mm corrosion resistant plate was placed around the sub-plot, with 20 cm aboveground and 30 cm underground, to prevent water from the overland runoff and belowground lateral soil infiltration. The extreme drought event was simulated by installing rainout shelters (3.5 m × 3.5 m) with transparent acrylic roof (> 90% light permeability), and the rainfall intercepted by the rainout shelters was collected with buckets and removed from the experiment blocks. At the end of the drought phase, the rainout shelters were removed and each subplot received a 30 mm precipitation at once in one day (manually irrigated with 120 m deep phreatic water).
Vegetation sampling
Vegetation survey was conducted on June 15th (before the drought treatment), July 31st (after the drought treatment) and August 30th (after the recovery period), respectively. In each subplot, we surveyed number of plant species, accounted individual number of each species and estimated percentage cover of each species in 4 randomly placed quadrats (0.5 m × 0.5 m). Plant community diversity was calculated as the Shannon-Wiener index:
Where SD is the Shannon-Wiener index, Pi is the proportion of the individual number for ith species over the individual number of all species, and S is the number of species.
For the 4 vegetation survey quadrats, we randomly picked one quadrat and harvested aboveground plant materials for the measurements of aboveground biomass (AGB) following the vegetation survey. Belowground biomass (BGB) was determined by washing the roots out of a soil core with a diameter of 10 cm and a depth of 15 cm. The harvested plant materials were oven-dried at 70 °C to a constant weight. The total biomass (TB) was calculated as the sum of AGB and BGB. The root/shoot ratio was calculated as BGB/AGB.
Ecosystem carbon flux measurement
Ecosystem CO2 exchange was measured with a transparent chamber (width and depth of 0.5 m and height of 1 m) attached to an infrared gas analyzer (IRGA; LI-6400, LiCor, Lincoln, NE, USA). Within the chamber, 4 small electric fans (about 30 cm above the plants) ran continuously to promote air mixing during the ecosystem carbon exchange measurement. An iron frame (0.5 m × 0.5 m) was inserted into the soil to a depth of 5 cm in each subplot before the start of the drought experiment (May 2014). The iron frames were used to mount the ecosystem CO2 exchange chamber. For each ecosystem carbon exchange measurement, air inside the chamber was allowed to equilibrium for 30 s after the chamber was mounted on top of the iron frame. Concentrations of CO2 (μmol mol−1) and H2O (mmol mol−1) were consecutive logged at 10 s intervals during a 120 s measurement period. The net ecosystem CO2 exchange (NEE) and evapotranspiration (ET) were calculated from changes of the CO2 and H2O concentrations. After the measurement of NEE, the chamber was vented with ambient air for 30 s; CO2 concentration was measured again using the chamber covered by an opaque cloth in order to calculate ecosystem respiration (ER). Details about the measurements of NEE and ER have been described in a previous study (Jiang et al. 2012a). The gross ecosystem production (GEP) was calculated as: NEE + ER. On sunny days between 9:00 and 11:00 h, we conducted the NEE measurements, which represent the maximum photosynthetic capacity of the studied steppe ecosystem in the nature environment during the day. Ecosystem CO2 exchange measurements were conducted 6 times (Jun 16th, Jun 25th, Jul 2nd, Jul 13th, Jul 22nd and Jul 29th) during the drought phase.
Measurements of precipitation, soil moisture, temperature and pH
Precipitation and air temperature data were obtained from an eddy tower roughly 15 km away from the experimental site. Soil moisture and soil temperature at 10 cm depth were recorded with the measurements of ecosystem CO2 fluxes. Soil water content was measured using an ECH2O soil moisture sensor (EC-5, Decagon Ltd., Pullman, WA, USA) and data were read with a ProCheck (Decagon Ltd., Pullman, WA, USA). Soil temperature was measured by a temperature probe (6000-09TC) coupled to an infrared gas analyzer (LI-6400, LiCor Inc., Lincoln, NE, USA). In each subplot, 3 soil cores (0–15 cm) were collected and mixed into a soil sample at the end of July. Roots and organic debris were removed using a 2 mm sieve. After being air-dried to a constant weight, the soils were suspended in DI water (soil: water = 1:5) and the pH value was determined by a pH meter (PHS-3E INESA Scientific Instrument Co., Ltd., Shanghai, P. R. China).
Statistical analysis
For the drought and recovery phase, we calculated average soil moisture, soil temperature, AGB, BGB, TB, and root/shoot ratio, respectively. For the drought phase, we calculated the linear dependence of the drought induced relative change in NEE (ΔNEEs) on drought duration and used the slope as the sensitivity of ecosystem carbon exchange to drought (SNEE). The ΔNEEs was calculated as:
where ΔNEEsi is the drought induced change in NEE at the ith day, NEEsi is the NEE at the ith day, NEE0 is the NEE at the beginning of the drought phase (Jun 16th). The recuperability of ecosystem carbon exchange (RNEE) during the recovery phase was determined as:
where NEEL is the NEE at the end of the rehydration period (Aug 31st), NEE0 is the NEE at the beginning of drought phase (Jun 16th). ANOVA were employed to analyze the effects of water addition, nitrogen addition and their interactions on the soil temperature, AGB, BGB, root/shoot ratio, water use efficiency (WUE = NEE/ET), SNEE during the drought phase and RNEE during the recovery phase. One-way ANOVA followed by Duncan’s post hoc tests was used to compare differences among the CK, W, N, and WN sub-plots in SNEE, RNEE, WUE, AGB, BGB and root/shoot ratio, respectively. Repeated measures analysis was performed to compare differences among the CK, W, N, and WN sub-plots in NEE, soil moisture and temperature during the drought phase or recovery phase. Pearson correlation was conducted among the explanatory variables (WUE, AGB, leaf water content, root/shoot ratio, community cover, soil total nitrogen content, species diversity, species richness, soil pH and soil conductivity) to examine the covariation among variables. The principal component analysis (PCA) was used to achieve the representative first principal component (PC1CN explained ~ 99% of variation) for the redundancy analysis (RDA). Multiple linear regression analysis was used to evaluate the dependence of SNEE on AGB and root/shoot ratio, and the dependence of RNEE on soil moisture and AGB. Univariate linear regression analysis was carried out to assess relationships between SNEE or RNEE and the measured variables (AGB, root/shoot ratio, soil temperature and soil moisture). PCA and RDA were conducted by CANOCO for Windows 5.0 (www.microcomp uterpower.com, trial version from Richard E. Furnas, New York, USA). Other statistical analyses were performed using SAS software (SAS 8.0, SAS Institute Inc., Cary, NC, USA).
Results
Environmental conditions
There were no significant variations in daily mean air temperature throughout the drought and recovery phases (Fig. 1a). During the drought period, the rainout shelter excluded about 60 mm precipitation which was 14.6% of the long-term average growing season precipitation (411 mm). During the drought phase, soil moisture (0 – 10 cm) reduced by 63%, 48%, 40 and 45% in the CK, W, N and WN sub-plots, respectively (P < 0.001), with the relative decrease of soil moisture in the CK sub-plots was greater than others (Fig. 1b). For each treatment, we detected substantial temporal dynamics in 0 – 10 cm soil temperature during the experimental period. The N and WN sub-plots had lower 0 – 10 cm soil temperature relative to the CK and W sub-plots (Fig. 1c). Two years of water and nitrogen addition had no significant impacts on soil pH and soil electrical conductivity (Table 1).
Information on (a) daily precipitation (bars, mm) and daily mean air temperature (lines, °C) of the field site during the experimental period. Patterns on (b) soil moisture (V/V%) and (c) soil temperature (°C) at 0 – 10 cm depth in the CK, W, N, and WN sub-plots during the drought and recovery phase. Insets in panel (b) and (c) are the means of drought-induced changes in soil moisture and soil temperature during drought phase, respectively. Different lowercase letters indicate significant differences (P < 0.05) among the treatments (Duncan’s test). CK: control; W: water addition; N: nitrogen addition; WN: water and nitrogen added in combination. Data are reported as mean ± 1 SD (n = 6). Arrows on bottom of panel (b) and panel (c) indicate date that the rainout shelters were removed and the rehydration treatments were applied
Vegetation and biomass
Plant community density in the N and WN plots was significantly higher than in the control plots; whereas water addition had no significant impacts on plant community density (Table 1). Both water and nitrogen addition reduced plant species diversity and richness; however, the magnitude of decrease was greater in the N plots (Table 1). Plant functional group responded differently to nitrogen and water treatments, with perennial grasses and forbs increased in aboveground biomass (AGB) in all fertilized plots, but no apparent change in water addition plots (Table 1). For annuals or biennial herbage, we found a stimulating effect of the water addition treatment on their AGB (Table 1). In all plots, the AGB was dominated by perennial grasses.
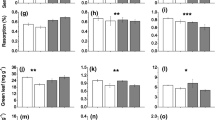
For all treatments, the AGB and belowground biomass (BGB) increased during the drought phase. The BGB increased, whereas AGB decreased during the recovery phase. Compared to the control treatment, the average values of AGB and BGB were greater in the nitrogen addition sub-plots during both drought and recovery phases (Fig. 2). For the drought period, the average values of BGB were lower in the water addition subplots relative to the control subplots; whereas no differences were detected in AGB between the W and CK sub-plots (Fig. 2). For the recovery phase, there were no differences in average AGB and BGB between the W and CK treatments (Fig. 2). After 2 years of water and nitrogen addition, root/shoot ratio was significantly reduced in the N, W and WN sub-plots relative to the control sub-plots (Fig. 2). There was no significant difference among the CK, W and WN sub-plots in root/shoot ratio; whereas root/shoot ratio in the N sub-plots was lower than in the CK sub-plots during the recovery phase (Fig. 2).
Patterns of (a) total biomass (TB, g m−2), (b) aboveground biomass (AGB, g m−2), (c) belowground biomass (BGB, g m−2) and (d) root/shoot ratio during the drought and recovery phase. The inset bar figures are the means of TB, AGB, BGB and root/shoot ratio for the drought (left) and recovery (right) phase. Different lowercase letters on top of the bars indicate significant differences (P < 0.05) among the treatments (Duncan’s test). CK: control; W: water addition; N: nitrogen addition; WN: water and nitrogen added in combination. Data are reported as mean ± 1 SD (n = 6)
Ecosystem CO2 exchange
With the intensification of the drought stress, net ecosystem CO2 exchange (NEE) and gross ecosystem production (GEP) showed patterns of reducing for all the sub-plots (Fig. 3a, b). However, the magnitude of decrease was more significant in the N and WN sub-plots. Compared to the CK and W sub-plots, nitrogen addition sub-plots had faster recovery rates in NEE and GEP (Fig. 3a). During the rehydration period, NEE increased by 18.6%, 73.6%, 107.5 and 93.5% in the CK, W, N and WN sub-plots, respectively. Ecosystem respiration (ER) showed no apparent variation for both drought and recovery phases (Fig. 3c). N addition significantly enhanced the values of evapotranspiration (ET) at early period of the drought treatment (Jun 16th, Jun 25th, Jul 2nd; P < 0.01, Fig. 3d). Decreasing patterns in ET during the drought period were detected in the fertilized plots but not in the unfertilized plots. There were positive correlations between NEE and SM in all treatments, however the relationships between NEE and soil temperature were insignificant (Fig. S1).
Variation of (a) net ecosystem CO2 exchange (NEE, μmol CO2 m−2 s−1), (b) gross ecosystem production (GEP, µmol CO2 m−2 s−1), (C) ecosystem respiration (ER, µmol CO2 m−2 s−1) and (D) ecosystem transpiration (ET, mmol H2O m−2 s−1) in the CK, W, N, and WN sub-plots during the drought and recovery phase. CK: control; W: water addition; N: nitrogen addition; WN: water and nitrogen added in combination. Arrows on bottom of each panel indicate date that the rainout shelters were removed and the rehydration treatments were applied. Data are reported as mean ± 1 SD (n = 6)
Drought susceptibility of NEE
For the drought phase, strong positive correlations were detected between NEE and soil water content (P < 0.05). There were significant differences in SNEE among the treatments (Fig. 4a) with SNEE values in the water and nitrogen addition subplots were greater than it in the CK sub-plots (Fig. 4a). There were significant interactive effects between nitrogen and water addition on SNEE (Tables 2 and 3).
Differences in (above) the drought sensitivity of NEE (SNEE, % d−1) and (below) the recovery of net ecosystem CO2 exchange (RNEE, %) among the treatments. CK: control; W: water addition; N: nitrogen addition; WN: water and nitrogen added in combination. Data are reported as mean ± 1 SD (n = 6). Different letters above the bars denote significant differences among the treatments (P < 0.05)
Redundancy analysis (RDA) was performed to discern possible linkages between community SNEE and vegetation or environment parameters (Fig. 5). Nine significant parameters were included in the RDA. Samples were grouped together on the basis of experimental treatments, which was consistent with the clustering result of the community SNEE. The first axis was correlated with WUE, AGB, root/shoot ratio, community cover and soil total nitrogen content, which were mainly caused by N fertilization. The second axis was negatively correlated with species diversity, species richness and root/shoot ratio. On the basis of the relationship between the variables and community stability, the AGB, root/shoot ratio and species richness was the major factors influencing the community SNEE.
Redundancy analysis (RDA) with SNEE in all treatments as response variable and nine variables constrained by plant and soil properties as explanatory variables. The abbreviations of the explanatory variables are WUE, water use efficiency; AGB, aboveground biomass; R/S, root/shoot ratio; C, community cover; SN, soil total nitrogen content; SD, species diversity; SR, species richness; pH, soil pH; SC, soil conductivity. The values of Axis 1 and 2 are percentages of total variations that can be attributed to the corresponding axis
After data from the 4 treatments were pooled together, the SNEE values were divided into the fertilized group and unfertilized group. SNEE was negatively correlated with root/shoot ratio (Fig. 6b), and positive related to AGB in both the fertilized and unfertilized plots (Fig. 6a). We also detected a significant dependence of SNEE on AGB and root/shoot ratio in the unfertilized (SNEE = − 0.204 Root/shoot ratio + 1.424, R2 = 0.852, P < 0.001) and fertilized (SNEE = 5.785 AGB - 0.204 Root/shoot ratio - 0.070, R2 = 0.722, P = 0.003) plots.
Response of the drought sensitivity of net ecosystem carbon exchange (SNEE) to (a) aboveground biomass (AGB, g m−2), (b) root/shoot ratio. Dependence of the recovery of net ecosystem CO2 exchange during the rehydration period (RNEE) on (c) root/shoot ratio, (d) mean soil moisture (V/V, %). *** represents significant relationships at the P < 0.001 level; ** represents significant relationships at the P < 0.01 level; and * represents significant relationships at the P < 0.05 level
Effects of water and nitrogen addition on the recovery of NEE
After the rainout shelters were removed and plants were rehydrated, NEE gradually increased in all treatments; however there were significant differences in the recuperability of ecosystem carbon exchange (RNEE) among the treatments (Fig. 4b). The CK subplots had greater RNEE values relative to the W, N and WN subplots (Fig. 4b), meanwhile the CK subplots also reached the peak value faster (Fig. 3a). There were no interactive effects between nitrogen addition and water addition on RNEE (Table 2). For the recovery phase, we found RNEE was positively correlated with AGB in both fertilized and unfertilized plots (Fig. 6c), and uncorrelated with soil water content (Fig. 6d).
Discussion
Effects of drought on ecosystem C fluxes
The ecological importance of more frequent and severe drought in the future for terrestrial ecosystem is widely recognized (Ciais et al. 2005; Hoover et al. 2014; Reichstein et al. 2002). Our results revealed that the simulated extreme drought caused sharp reduction in surface soil moisture (Fig. 1) and significant decrease in NEE in all experiment treatments (CK, W, N and WN). Similar results have also been reported in other grassland ecosystems (Jongen et al. 2011; Knapp et al. 2002; Shi et al. 2014). These results suggest that soil water availability, but not temperature (fluctuated randomly throughout the experimental period, Fig. 1), play a dominating role in regulating impact of drought events on C uptake or release in terrestrial ecosystems (Niu et al. 2008; Wang et al. 2015a). Indeed, we found that NEE were more tightly associated with changes in soil moisture (P < 0.05) than the soil temperature (P > 0.05) during the drought phase (Fig. S1). The observed water-dependent of ecosystem CO2 fluxes may be partially attributed to the lack of deep roots and water storage organs for the dominant plant community in the Songnen meadow steppe.
In this study, the drought treatment had no apparent impacts on ER; therefore the observed reduction in NEE are likely primarily caused by decrease in GEP (Fig. 2). Differential drought susceptibility between carbon assimilation and respiration were detected previously in other grassland ecosystems (Ciais et al. 2005; Jentsch et al. 2011; Schwalm et al. 2010). Our results, along with other studies (Bloor and Bardgett 2012; Shi et al. 2014), suggest that most grassland plants, especially the dominant perennial grasses, tend to maintain basic biological activities of the existing biomass than to give up some existing tissues to keep carbon fixation under the conditions of water stress. This more cautious tactic, akin to isohydric plants, avoids the potentially fatal risks related to an excessive reduction in water potential, and have higher photosynthetic recovery potential after the drought (Kursar et al. 2009; McDowell et al. 2008). In both unfertilized and fertilized subplots, we found that RNEE in the recovery phase was positively correlated with the AGB (Fig. 6d), which is tightly associated with leaf area index (LAI) and photosynthetic units. The isohydric tactic undoubtedly benefits grassland plants to adapt to capricious climate with frequent and transitory drought events. However, the benefits associated with isohydric tactic may diminish under conditions of severe and prolonged drought stress because of abiding carbon starvation (McDowell et al. 2008).
Factors affecting SNEE
In line with our expectation, we found that nitrogen addition (with or without water addition) had strong impacts on SNEE in the studied meadow steppe (Fig. 5c; Table 2). Recently, Hautier et al. (2014) reported that N enrichment decreases grassland stability through a combination of diversity-dependent effects on temporal variation of productivity and species asynchrony. We detected a potential positive trend, although it’s not continuous, between SNEE and AGB when data from the 4 treatments were pooled together (Fig. 6a). The results of the RDA also showed that AGB and its potentially related variables contributed the most (38.0%) to SNEE variation (Fig. 5), indicating a significant impact of the AGB or related factors (water demand or water transpiration etc.) on drought sensitivity of NEE (Fan et al. 2008; Müller et al. 2000; Seagle and McNaughton 1993). In fact, we found that the N and WN subplots had greater ET values relative to the CK and W subplots at early period of the drought treatment (Jun 16th, Jun 25th, Jul 2nd; P < 0.01, Fig. 3d). It is reasonable that greater AGB is associated with greater resource requirements to maintain plant physiological activities and growth, which will ultimately lead to a larger risk that drought event make water and/or carbon supply unable to meet plant optimal transpiration and/or respiration demand and eventually cause more damage on plant (Brooks and Coulombe 2009; van Heerwaarden et al. 2005). In a previous study, Wang et al. (2007) also suggested that the communities with lower biomass production were more resistant to drought stress than productive communities. Together, these results highlight the controlling effects of aboveground biomass on SNEE.
The addition of limiting resources (such as nitrogen and water) not only enhance biomass production, but also reduce root/shoot ratios, which have been observed in our experiment and many other studies (Friedrich et al. 2012; Wang et al. 2015a; Yan et al. 2011). In contrast to the strong reduction in soil moisture, the manipulated drought treatment had limited impacts on aboveground biomass and root/shoot ratios, which was largely due to the irreversibility of plant growth (Fig. 2). In this study, we observed positive effects of water and nitrogen addition on SNEE (Fig. 4), which may partially have resulted from differences in biomass allocation on account of the long-term adaptation of local plants to altered environment resource level (Tilman et al. 2006). In resource-rich habitats, the assimilated carbon is primarily allocated to the growth of the aerial portion to compete for light rather than below-ground resource acquisition as a result of the existing roots were enough to absorb water and nutrients for plant growth (Tilman 1990). However, lower root/shoot ratio is often associated with greater water dissipation and/or lesser water absorption ability, which may make the balance of water supply and demand more fragile and reduce drought tolerance of plant community. Indeed, significant negative correlations between SNEE and root/shoot ratio were detected in both fertilized and unfertilized subplots (Fig. 6b), which suggest that root/shoot ratio may play a crucial role in regulating SNEE. Moreover, in line with the results of many drought resistance studies, we also observed that SNEE was negatively correlated with diversity (R2 = 0.752, P < 0.001) which was altered by the water and nitrogen addition. Nonetheless, the observed relationships between diversity and resistance may be only a statistical artifact, which was caused by potential link between biomass and diversity. Moreover, over 95% of biomass in the experimental ecosystem was contributed by a single perennial grass species (L. chinensis), which also makes the impacts of diversity likely ignorable.
Factors affecting RNEE
Although existing NEE and carbon sequestration potential was maintained at a higher level in resources rich environment (Fig. 3), the water and/or nitrogen addition strongly reduced the recuperability of ecosystem carbon exchange during the recovery period (Fig. 4). This may essentially just be on account of the more drought damage on plant photosynthesis organ with high AGB during the drought period, rather than AGB itself during the recovery period (Fig. 6c). There is no doubt that the more severe imbalance of water supply and demand results in the dehydration of plant leaves and the damage of photosynthetic enzyme in the fertilized subplots. Compared to the stomatal limitation, enzymatic limitation requires longer time to recover (Brad et al. 2010; Hu et al. 2010; Taylor et al. 2011), which may responsible for the slower recovery of NEE in the fertilized subplots during the rehydration period. Moreover, the effects of nitrogen and water addition on recovery of NEE may also depend on drought intensity and/or duration (Greaver et al. 2016).
Conclusions
In Songnen meadow steppe, 3 - year manipulated changes in precipitation (water addition) and nitrogen deposition (nitrogen addition) altered primary productivity biomass allocation and community composition. With the intensification of the drought stress, NEE showed decreasing patterns in all subplots; however, the sensitivity of ecosystem carbon exchange to drought in the nitrogen and water addition plots was greater than in the control plots. Meanwhile, NEE in both nitrogen and water subplots also had lower recuperability during the rehydration period than in the control subplots. Aboveground biomass and root/shoot ratio played critical roles in regulating ecosystem C exchange and its response to drought and rehydration in the studied meadow steppe, but they had different degrees of importance depend on the level of N fertilization. Our observations suggest the complexity and importance of the interactive effects of global climate change factors on ecosystem C fluxes in grassland ecosystem. The ecosystems carbon sequestration could be benefitted from the stimulation of plant growth by water and nitrogen addition, despite they may also enhance ecosystem drought susceptibility and reduce recuperability.
References
Bai Y, Wu J, Xing Q, Pan Q, Huang J, Yang D, Han X (2008) Primary production and rain use efficiency across a precipitation gradient on the Mongolia plateau. Ecology 89:2140–2153
Bai Y et al (2010) Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: evidence from inner Mongolia grasslands. Glob Chang Biol 16:358–372
Bloor JMG, Bardgett RD (2012) Stability of above-ground and below-ground processes to extreme drought in model grassland ecosystems: interactions with plant species diversity and soil nitrogen availability. Perspectives in Plant Ecology Evolution and Systematics 14:193–204
Brad R, Kristen F, Matthew G (2010) Differences in drought sensitivities and photosynthetic limitations between co-occurring C3 and C4 (NADP-ME) Panicoid grasses. Ann Bot 105:493–503
Bréda N, Huc R, Granier A, Dreyer E (2006) Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Ann For Sci 63:625–644
Brooks JR, Coulombe R (2009) Physiological responses to fertilization recorded in tree rings: isotopic lessons from a long-term fertilization trial. Ecol Appl 19:1044–1060
Brueck H, Erdle K, Gao Y, Giese M, Zhao Y, Peth S, Lin S (2010) Effects of N and water supply on water use-efficiency of a semiarid grassland in Inner Mongolia. Plant Soil 328:495–505
Cherwin K, Knapp A (2012) Unexpected patterns of sensitivity to drought in three semi-arid grasslands. Oecologia 169:845–852
Christensen L, Coughenour MB, Ellis JE, Chen ZZ (2004) Vulnerability of the Asian typical steppe to grazing and climate change. Clim Chang 63:351–368
Ciais P, Reichstein M, Viovy N, Granier A, Ogée J, Allard V, Aubinet M, Buchmann N, Bernhofer C, Carrara A, Chevallier F, de Noblet N, Friend AD, Friedlingstein P, Grünwald T, Heinesch B, Keronen P, Knohl A, Krinner G, Loustau D, Manca G, Matteucci G, Miglietta F, Ourcival JM, Papale D, Pilegaard K, Rambal S, Seufert G, Soussana JF, Sanz MJ, Schulze ED, Vesala T, Valentini R (2005) Europewide reduction in primary productivity caused by the heat and drought in 2003. Nature 437:529–533
De Boeck H et al (2006) How do climate warming and plant species richness affect water use in experimental grasslands. Plant Soil 288:249–261
Fan JW et al (2008) Allocation of vegetation biomass across a climate-related gradient in the grasslands of Inner Mongolia. J Arid Environ 73:521–528
Frank D, Reichstein M, Bahn M, Thonicke K, Frank D, Mahecha MD, Smith P, van der Velde M, Vicca S, Babst F, Beer C, Buchmann N, Canadell JG, Ciais P, Cramer W, Ibrom A, Miglietta F, Poulter B, Rammig A, Seneviratne SI, Walz A, Wattenbach M, Zavala MA, Zscheischler J (2015) Effects of climate extremes on the terrestrial carbon cycle: concepts, processes and potential future impacts. Glob Chang Biol 21:2861–2880
Friedrich U, Oheimb GV, Kriebitzsch W-U, Schleßelmann K, Weber MS, Härdtle W (2012) Nitrogen deposition increases susceptibility to drought - experimental evidence with the perennial grass Molinia caerulea (L). Moench Plant and Soil 353:59–71
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892
Gonzalez-Dugo V, Durand JL, Gastal F (2010) Water deficit and nitrogen nutrition of crops. A review. Agron Sustain Dev 30:529–544
Greaver TL, Clark CM, Compton JE, Vallano D, Talhelm AF, Weaver CP (2016) Key ecological responses to nitrogen are altered by climate change. Nat Clim Chang 6:836–843
Hansen J, Sato M, Ruedy R (2012) Perception of climate change. Proceedings of the National Academy of Sciences USA 109:2415–2423
Hautier Y et al (2014) Eutrophication weakens stabilizing effects of diversity in natural grasslands Nature 508:521–525
Hoover DL, Knapp AK, Smith MD (2014) Contrasting sensitivities of two dominant C4 grasses to heat waves and drought. Plant Ecol 215:721–731
Hoover DL, Duniway MC, Belnap J (2017) Testing the apparent resistance of three dominant plants to chronic drought on the Colorado plateau. J Ecol 105:152–162
Hu L, Wang Z, Huang B (2010) Diffusion limitations and metabolic factors associated with inhibition and recovery of photosynthesis from drought stress in a C3 perennial grass species. Physiol Plant 139:93–106
Huston MA (1997) Hidden treatments in ecological experiments: re-evaluating the ecosystem function of biodiversity. Oecologia 110:449–460
IPCC (2007) Climate Change 2007: The Physical Science Basis: Summary for Policymakers IPCC WGI Fourth Assessment Report
Jentsch A, Kreyling J, Elmer M, Gellesch E, Glaser B, Grant K, Hein R, Lara M, Mirzae H, Nadler SE, Nagy L, Otieno D, Pritsch K, Rascher U, Schädler M, Schloter M, Singh BK, Stadler J, Walter J, Wellstein C, Wöllecke J, Beierkuhnlein C (2011) Climate extremes initiate ecosystem regulating functions while maintaining productivity. J Ecol 99:689–702
Jiang L, Guo R, Zhu T, Niu X, Guo J, Sun W (2012a) Water- and plant-mediated responses of ecosystem carbon fluxes to warming and nitrogen addition on the Songnen grassland in Northeast China. PLoS One 7:e45205
Jiang Z, Song J, Li L, Chen W, Wang Z, Wang J (2012b) Extreme climate events in China: IPCC-AR4 model evaluation and projection. Clim Chang 110:385–401
Jongen M, Pereira JS, Aires LMI, Pio CA (2011) The effects of drought and timing of precipitation on the inter-annual variation in ecosystem-atmosphere exchange in a Mediterranean grassland. Agric For Meteorol 151:595–606
Knapp AK, Fay PA, Blair JM, Collins SL, Smith MD, Carlisle JD, Harper CW, Danner BT, Lett MS, McCarron J (2002) Rainfall variability, carbon cycling, and plant species diversity in a Mesic grassland. Science 298:2202–2205
Knapp AK, Beier C, Briske DD, Classen AT, Luo Y, Reichstein M, Smith MD, Smith SD, Bell JE, Fay PA, Heisler JL, Leavitt SW, Sherry R, Smith B, Weng E (2008) Consequences of more extreme precipitation regimes for terrestrial ecosystems. BioScience 58:811–821
Knapp AK, Ciais P, Smith MD (2017) Reconciling inconsistencies in precipitation–productivity relationships: implications for climate change. New Phytol 214:40–47
Kursar TA, Engelbrecht BMJ, Burke A, Tyree MT, Omari BE, Giraldo JP (2009) Tolerance to low leaf water status of tropical tree seedlings is related to drought performance and distribution. Funct Ecol 23:93–102
McConnaughay KDM, Coleman JS (1999) Biomass allocation in plants: ontogeny or optimality? A test along three resource gradients. Ecology 80:2581–2593
McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, Yepez EA (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought. New Phytol 178:719–739
Mirzaei H, Kreyling J, Hussain MZ, Li Y, Tenhunen J, Beierkunlein C, Jentsch A (2008) A single drought event of 100-year recurrence enhances subsequent carbon uptake and changes carbon allocation in experimental grassland communities. J Plant Nutr Soil Sci 171:681–689
Müller I, Schmid B, Weiner J (2000) The effect of nutrient availability on biomass allocation patterns in 27 species of herbaceous plants. Perspectives in Plant Ecology Evolution and Systematics 3:115–127
Niu S, Wu M, Han Y, Xia J, Li L, Wan S (2008) Water-mediated responses of ecosystem carbon fluxes to climatic change in a temperate steppe. New Phytol 177:209–219
Niu S, Yang H, Zhang Z, Wu M, Lu Q, Li L, Han X, Wan S (2009) Non-additive effects of water and nitrogen addition on ecosystem carbon exchange in a temperate steppe. Ecosystems 12:915–926
Niu S, Wu M, Han Y, Xia J, Zhang Z, Yang H, Wan S (2010) Nitrogen effects on net ecosystem carbon exchange in a temperate steppe. Glob Chang Biol 16:144–155
Pfisterer AB, Schmid B (2002) Diversity-dependent production can decrease the stability of ecosystem functioning. Nature 416:84–86
Qu G, Guo J (2003) The relationship between different plant communities and soil characteristics in Songnen grassland Acta Pratacultural. Science 12:18–22
Reichstein M et al (2002) Severe drought effects on ecosystem CO2 and H2O fluxes at three Mediterranean evergreen sites: revision of current hypotheses? Glob Chang Biol 8:999–1017
Reynolds HL, D'Antonio C (1996) The ecological significance of plasticity in root weight ratio in response to nitrogen: opinion. Plant Soil 185:75–97
Schwalm CR et al (2010) Assimilation exceeds respiration sensitivity to drought: a FLUXNET synthesis. Glob Chang Biol 16:657–670
Seagle SW, McNaughton SJ (1993) Simulated effects of precipitation and nitrogen on Serengeti grassland productivity. Biogeochemistry 22:157–178
Shi Z, Thomey ML, Mowll W, Litvak M, Brunsell NA, Collins SL, Pockman WT, Smith MD, Knapp AK, Luo Y (2014) Differential effects of extreme drought on production and respiration: synthesis and modeling analysis. Biogeosciences 11:621–633
Shi B, Wang Y, Meng B, Zhong S, Sun W (2018) Effects of nitrogen addition on the drought susceptibility of theLeymus chinensisMeadow ecosystem vary with drought duration. Front Plant Sci 9:254
Smith MD (2011) An ecological perspective on extreme climatic events: a synthetic definition and framework to guide future research. J Ecol 99:656–663
Smith NG, Schuster MJ, Dukes JS (2016) Rainfall variability and nitrogen addition synergistically reduce plant diversity in a restored tall grass prairie. J Appl Ecol 53:579–586
Taylor SH, Ripley BS, Woodward FI, Osborne CP (2011) Drought limitation of photosynthesis differs between C3 and C4 grass species in a comparative experimentpce. Plant Cell Environ 34:65–75
Tilman D (1990) Constraints and tradeoffs: toward a predictive theory of competition and succession. Oikos 58:3–15
Tilman D, Elhaddi A (1992) Drought and biodiversity in grasslands. Oecologia 89:257–264
Tilman D, Reich PB, Knops JMH (2006) Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature 441:629–632
van Heerwaarden LM, Toet S, van Logtestijn RSP, Aerts R (2005) Internal nitrogen dynamics in the graminoid Molinia caerulea under higher N supply and elevated CO2 concentrations. Plant Soil 277:255–264
Wang Y, Yu S, Wang J (2007) Biomass-dependent susceptibility to drought in experimental grassland communities. Ecol Lett 10:401–410
Wang Y, Jiang Q, Yang Z, Sun W, Wang D (2015a) Effects of water and nitrogen addition on ecosystem carbon exchange in a meadow steppe. PLoS One 10:e0127695
Wang Z, Silva LCR, Sun G, Luo P, Mou C, Horwath WR (2015b) Quantifying the impact of drought on soil-plant interactions: a seasonal analysis of biotic and abiotic controls of carbon and nutrient dynamics in high-altitudinal grasslands. Plant Soil 389:59–71
Xu ZZ, Zhou GS (2005) Effects of water stress and nocturnal temperature on carbon allocation in the perennial grass, Leymus chinensis. Physiol Plant 123:272–280
Xu Z, Ren H, Cai J, Wang R, Li MH, Wan S, Han X, Lewis BJ, Jiang Y (2014) Effects of experimentally-enhanced precipitation and nitrogen on resistance, recovery and resilience of a semi-arid grassland after drought. Oecologia 176:1187–1197
Yachi S, Loreau M (1999) Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proceedings of the National Academy of Science USA 96:1463–1468
Yan L, Chen S, Huang J, Lin G (2011) Increasing water and nitrogen availability enhanced net ecosystem CO2 assimilation of a temperate semiarid steppe. Plant Soil 349:227–240
Yang HJ, Jiang L, Li LH, Li A, Wu MY, Wan SQ (2012) Diversity dependent stability under mowing and nutrient addition: evidence from a 7-year grassland experiment. Ecol Lett 15:619–626
Acknowledgements
Qi Jiang helped with the field experiment. Xia Yuan and Zhongnan Wang provided valuable comments and suggestions on early drafts. This study was financially supported by the National Key Basic Research Program of China (2015CB150800), National Natural Science Foundation of China (31570470, 41671207, 31660679), the 9th Thousand Talents Program of China (2013), Main Service Project of Characteristic Institute of Chinese Academy of Sciences (TSS-2015-014-FW-5-1), the Fundamental Research Funds for the Central Universities (2412018ZD010).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Harry Olde Venterink
Electronic supplementary material
Fig. S1
Response of the mean of net ecosystem carbon exchange (NEE) to (A) soil temperature (°C), (B) soil moisture (V/V, %) in the CK, W, N, and WN sub-plots during the drought and recovery phase. Data are reported as mean ± 1 SD (n = 6). Dependence of net ecosystem carbon exchange (NEE) on (C) soil temperature (°C), (D) soil moisture (V/V, %) in the CK, W, N, and WN sub-plots during the drought and recovery phase. *** represents significant relationships at the P < 0.001 level. (PNG 1055 kb)
ESM 1
(DOC 40 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Meng, B., Zhong, S. et al. Aboveground biomass and root/shoot ratio regulated drought susceptibility of ecosystem carbon exchange in a meadow steppe. Plant Soil 432, 259–272 (2018). https://doi.org/10.1007/s11104-018-3790-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-018-3790-7