Abstract
Background and aims
Phosphorus (P) availability is crucial for forest ecosystem productivity and soil organic matter (SOM) is an important source for P. This study was conducted to reveal carbon (C), nitrogen (N) and P distributions in functional SOM fractions. We hypothesised that (1) most of the organic P (Porg) is part of the particulate SOM, (2) particulate SOM stores increasing share of P with decreasing soil P content and (3) the C:Porg ratio of mineral-associated SOM is smaller than that of particulate SOM.
Methods
We analysed soil samples from five temperate forest sites (Fagus sylvatica) under different geological parent material with a wide range of total P concentrations. Density fractionation was used to separate free light fraction (fLF), particulate SOM occluded within soil aggregates (occluded light fraction; oLF), and mineral associated SOM (heavy fraction; HF). We determined the mass balance of P in these fractions, in addition to the C and N concentrations. Additionally, the P speciation of the topsoil was analysed by X-ray absorption near edge structure (XANES) spectroscopy at the P K-edge.
Results
The fLF contained 18–54% and the oLF 1–15% of total P (Ptot). High percentage of P in these light fractions was associated to soil minerals. Phosphorous in particulate SOM within aggregates tend to increase with decreasing soil P. The HF containing mineral-associated OM, comprised 38–71% of Ptot and their C:Porg ratios were consistently lower than those of the fLF irrespective of the P status of the soil.
Conclusions
We show that all three functional SOM fractions contain variable amount of both organic and inorganic P species. The free light fraction shows no response to changing P stocks of soils.. Despite physically protected particulate SOM, oLF, becomes increasingly relevant as P cache in soils with declining P status.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil organic matter (SOM) is heterogeneously distributed in soils on an aggregate-scale (Lehmann et al. 2007). According to a widely accepted conceptual model developed by Jastrow and Miller (1997) there are three SOM fractions in soils that differ strongly in their stage of decomposition and availability. The first SOM fraction, termed free light fraction (fLF), is located between solid mineral particles and aggregates as mineral-free particulate material of low-density. The turnover of the fLF is relatively rapid and the stage of decomposition is low. A second particulate SOM fraction stabilised within aggregates, termed occluded light fraction (oLF) is physically protected against microbial decomposition due to spatial separation. The third fraction is a high-density fraction, strongly bound to the surface of mineral particles and considered as the heavy fraction (HF). The HF is the most microbially decomposed and stable SOM fraction (Stewart and Tiessen 1987; Gunina and Kuzyakov 2014). Light fractions are particulate organic matter, while the heavy fraction is mainly mineral matter with organic matter tightly absorbed to mineral surfaces.
It is well known that SOM is an important source for N and P in soils (Ilg et al. 2009; Lambers et al. 2008). Soil aggregation leads to a heterogeneity of the distribution of SOM and hence plays an important role in determining the amounts of nutrients that are available for microbial, mycorrhizal and plant uptake (Marschner and Rengel 2012). Especially in forest ecosystems, SOM is a crucial source for P (Kaiser et al. 2002; Niederberger et al. 2015), where normally no mineral fertilizer is applied and the delivery of P from weathering is slow (Walker and Syers 1976). In most forest soils of central Europe, the low pH and the association of SOM compounds with reactive minerals like iron or aluminium hydrous oxides (Mikutta et al. 2006) limits the P mobility. However, P in soil is not only present in inorganic forms (Pinorg, e.g., orthophosphate, polyphosphates) but also as organic P (Porg, e.g., P mono- and diesters), which is introduced by plants litter and microorganisms or the decomposition of organic material (Attiwil and Adams 1993). Once introduced into the biogeochemical cycle, continuous recycling of P is crucial for ecosystem nutrition. More efficient recycling of P sources is especially assumed for P-poor soils in order to limit soil P losses (Stewart and Tiessen 1987; Lang et al. 2016). Such recycling could mean that in P-poor soils, P might be primarily contained in the fLF or oLF of SOM since sorption to minerals would render part of the P hardly accessible for plants and microorganisms. Phosphorus in bulk soil, plant biomass, soil microbial biomass and the sorption of P from the soil solution on SOM is relatively well-studied with a few open questions (McGroddy et al. 2004, Guppy et al. 2005, Cleveland and Liptzin 2007, Turner et al. 2013). However, to the best of our knowledge, studies on the distribution of P among SOM density fractions are completely missing. Even though the importance of physically protected P in particulate SOM was mentioned early (Stewart and Tiessen 1987), this issue has only be addressed in a small number of publications: Sinaj et al. (1997) found indications that up to 30% of the acid-extractable P of soils may be physically protected within water stable aggregates. Analysing P within the fLF (here, density ρ <1.0 g cm−3), O’Hara et al. (2006) observed that this fraction contributed much to the P nutrition of plants. These studies give important hints, that aggregates and particulate organic matter could be highly relevant in the P supply of forest ecosystems.
At present, however, the standard Hedley fractionation to quantify different P fractions in soils (Hedley et al. 1982) does not account for physical stabilisation of organic P sources in aggregates. Element ratios, like C:P and C:N, are general proxies for the transformation of SOM during decomposition and for potential nutrient limitations (Bimüller et al. 2014). Until now, the decomposition process in terms of P availability is not fully understood (Richardson and Simpson 2011). The interactions with various plant, mycorrhizal fungi, phosphatase enzymes and microorganisms are highly dynamic. Generally, C:N ratios decrease in the order fLF > oLF > bulk soil > HF because of the preferential loss of C relative to N during decomposition. However, the stoichiometry of P and Porg relative to C and N in specific SOM fractions, and the consequences for SOM turnover remain unclear (Turner et al. 2013). For example, a C:P ratio of 200–550 in SOM is considered as threshold, indicating whether P will be mineralised or immobilized during decomposition (Saggar et al. 1998).
Given the limited knowledge about P in functional SOM fractions our central research questions were: How is P distributed in the three functional SOM fractions? What is the composition of the P present within SOM fractions? Therefore, we analysed the distributions of three SOM fractions (fLF, oLF, HF) in five temperate forest soils under Fagus sylvatica along a P geogradient developed under different parent material and established mass balances of P, C, and N in the SOM fractions. The SOM fractions were isolated and the abundance of inorganic and organic P species in those density fractions was analysed. We specifically hypothesize that (1) the light fractions, fLF and oLF, contain P mainly in organic forms, while the HF reveals a significant higher contribution of inorganic P forms, that (2) the share of P stored in the light fractions, fLF and oLF, increases with decreasing P status of the soils, and that (3) the C:Porg ratios in the HF are smaller than in the respective light fractions.
Materials and methods
Site selection and sampling
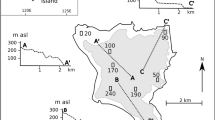
Soil sampling according to Ad-hoc-AG Boden (2005) took place on five acid forest stands dominated by Fagus sylvatica of approximately the same age across Germany in October and November 2013. According to Lang et al. (2016) the investigated sites represent a geogradient of decreasing availability in mineral-bound P in the parent materials following the order: Bad Brückenau (BBR) > Mitterfels (MIT) > Vessertal (VES) > Conventwald (CON) > Luess (LUE). Detailed information is given in Table 1. We took samples from the forest floor and at soil pits from the first mineral horizon (0–13 cm) and the lowermost 5 cm of the developed subsoil (73–106 cm); in total 24 samples. The samples were air-dried at 35 °C and sieved <2 mm after careful removal of plant debris and roots.
The acidic mineral topsoils cover a large range of P contents (quantified by hydrofluoric acid digestion) in the topsoils showing a gradient from 2966 mg kg−1 P at the best-supplied site BBR to 196 mg P kg−1 at the poorest site LUE. In the subsoils, the range of P concentrations is between 1844 and 175 mg P kg−1, respectively.
The gradient is furthermore represented in P solved by weaker extraction agents. The humus form and humus quality is associated with the geogradient, going from a favourable and relatively thin forest floor (8 cm, mull-like moder) to an unfavourable and thicker forest floor (13 cm, mor-like moder). Mineral soil had a range between 2.9 to 4.2 pH (KCl) and is not related to the P geogradient. For all five study sites tree height, standing volumes, diameters at breast height were comparable (Lang et al. 2017). In contrast to Turner et al. (2013) soil organic matter and microbial ratios increased with decreasing P contents of the soil (Lang et al. 2017).
Density fractionation
Density fractionation combined with sonication in up to quadruplicates was performed by a modified procedure according to Golchin et al. (1994) and Graf-Rosenfellner et al. (2016). Sodium polytungstate solution (SPT; Na6[H2W12O40]; Tungsten Compounds, Grub am Forst/Germany) with a density of 1.6 g cm−3 was used to separate the fractions of SOM. The SPT used was free of C, N, and P impurities. Possible C- or P-mobilization by SPT density solution or washing solution was tested in preliminary experiments. In all washing solutions, used and fresh SPT, PO4 content was measured according to Murphy and Riley (1962) using an UV/Vis Spectrophotometer. The absorbance in all washing solutions, used as well as fresh SPT were was lower than absorbance of the lowest standard concentration 0.1 mg PO4/l. The conservative assumption of a detection limit of 0.1 mg PO4/l leads to a maximum loss of 0.04 mg Ptot g−1 per fraction (cf. Table 7).
Dissolved organic C was measured with Multi N/C 2100 s (Analytik Jena®). Average loss of C by fractionating and washing for all fractions was 2.9 ± 2.0% (all values are given as ± standard deviation unless indicated otherwise). This indicates that C and P mobilization during fractionation was negligible.
After applying SPT to the soil samples (solid-to-solution ratio 1:5) and one hour of wetting, the suspension was centrifuged at 2600 rcf x ɡ for 26 min to separate the fLF from the oLF and HF. Floating material is inter-aggregate particulate organic matter, defined as the free light fraction (fLF), was recovered on glass microfiber filters (particle retention >1.5 μm), washed with deionized water and freeze-dried. The remaining material was treated with ultrasound (400 J ml−1) for disaggregation in order to release the oLF. Sonication was done with Sonics VCX 500 (Sonics & Materials, Newtown CT/USA) at a vibration frequency of 20 kHz, a cylindrical probe (13 mm) and an immersion depth of 1.5 cm as recommended by Amelung and Zech (1999). The output energy of 400 J ml−1 was determined calorimetrically (North 1976). After sonication, the released oLF floated on the surface of the SPT solution and was filtered, washed and freeze-dried. To remove the SPT, the remaining HF was shaken with deionized water and centrifuged at 2600 ɡ for 26 min until the solution reached a conductivity <200 μS cm−1. Afterwards, HF samples were freeze-dried and homogenized. The mass of all obtained SOM fractions as well as for the whole sample was determined to establish a mass balance.
The average recovery of dry topsoil mass after density fractionation was 115 ± 24% (min: 94, max:171). For C, N, and Ptot, the element recoveries were for carbon 90 ± 25% (min:67, max:171), for N 96 ± 26% (min: 69, max: 150) and for Ptot 68 ± 15% (min: 48, max: 91), respectively, and are within the range of published studies (Cerli et al. 2012; Graf-Rosenfellner et al. 2016).
Carbon and nitrogen
Carbon and N were measured in milled and water-free samples by dry combustion with a CNS elemental analyser (Vario EL III Elemental Analyser, Elementar®). Measured C contents are equal to organic C because carbonates were absent at the study sites (pH <4.2).
Phosphorus
Phosphorus was determined in the three SOM fractions and in bulk topsoil samples according to Saunders and Williams (1955). Prior analysis samples were milled, dried, and ignited by 550 °C. Phosphorus was extracted from triplicate density fractions using 0.5 M H2SO4 and measured using UV/VIS spectroscopy according to molybdenum-blue method (Murphy and Riley 1962).
Determination of total element composition
Additionally, total concentrations of P, Ca, Mg, Na, K, Al, Fe, and Mn were analysed after digestion with a mixture of concentrated HNO3/HClO4/HF and subsequent determination of the element concentrations in the digests by ICP-OES (Varian VISTA pro).
Synchrotron-based P K-edge XANES spectroscopy
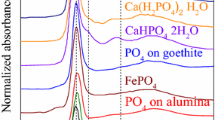
The XANES measurements were performed at the Synchrotron Light Research Institute (SLRI) in Nakhon Ratchasima, Thailand as described in Werner and Prietzel (2015) and Prietzel et al. (2016a, b). After energy calibration using elemental P powder (E = 2145.5 eV; precision ±0.11 eV), spectra were acquired in fluorescence-yield mode for 17 reference compounds (see below) and fine ground samples in the energy range 2110–2230 eV in 1.0 eV (2110 to 2130 eV; 2200 to 2230 eV) and 0.25 eV increments (2130 to 2200 eV). For each reference compound and soil sample, at least two spectra were acquired. We used an InSb (111) double crystal monochromator with an energy resolution (∆E/E) of 3 × 10−4 (i.e., 0.6 eV at the P K-edge), and a dwell time of 1 s for standard samples and between 1 and 5 s for soil samples, depending on their P content. Incident X-ray intensity was measured by a mixed N2/He-gas-filled ion chamber while the fluorescence emitted from the sample (irradiated area 1.8 × 0.12 cm2) was recorded with a 13-channel Ge detector. To minimize X-ray absorption by air, the sample compartment was purged continuously with helium gas. For all samples, the respective spectra were merged (n = 2–5; depending on P content), base-line-corrected, and edge-step-normalized as described in Werner and Prietzel (2015). The contribution of different P species to total P in each sample was calculated by linear combination fitting (LCF) in the energy range 2140 to 2200 eV. For LCF, we used a set of 17 reference compounds which are likely to be present in the investigated aggregates according to earlier studies (Prietzel et al. 2016b; Werner and Prietzel 2015): The reference compounds include X-ray amorphous AlPO4 and FePO4; orthophosphate adsorbed to ferrihydrite – representing Fe(III) oxyhydroxides, boehmite – representing Al oxyhydroxides, Al-saturated montmorillonite – representing high-activity clay minerals in acidic soils, and Al-saturated SOM (forest floor O layer Waldsassen, Germany, finely ground and saturated with Al by repeated shaking with Al(NO)3), respectively; Na inositol hexakisphosphate (IHP; “phytate”); IHP adsorbed to ferrihydrite, boehmite, Al-saturated montmorillonite, and Al-saturated SOM, respectively; Ca, Al, and Fe phytates; CaHPO4*2H2O, Ca(H2PO4)2*H2O, and hydroxy apatite. For all reference compounds, spectra and background information is presented in detail in Prietzel et al. (2016a). After calibration, spectra were acquired in energy ranges from 2045.5 eV to 2495.5 eV with a 2 s dwell time per energy step. Energy steps were chosen as follows: from 2045.5 eV to 2105.5 eV and from 2245.5 eV to 2495.5 eV: energy step of 5 eV; from 2105.5 eV to 2135.5 eV and from 2195.5 eV to 2245.5 eV: energy step of 1 eV; from 2135.5 eV to 2195.5 eV: energy step of 0.25 eV. A minimum of two spectra was acquired. The XANES spectra were analysed and interpreted using the standard protocol of Werner and Prietzel (2015): Data deconvolution and linear combination fitting initial spectrum deconvolution was performed with the program ATHENA of the software package DEMETER, version 0.9.25 (Ravel and Newville 2005). As a first step, E0 of all raw spectra was calibrated to the zero crossing of the second derivative of the absorption. Replicate spectra of a given sample were then examined visibly for glitches, drifts, noise, and general quality before merging them. The data was further processed using the newly established package LCF from the statistical software R (R Core Team 2015). The exported spectra were initially baseline-corrected from −36 to −15 eV (linear regression) and normalised and flattened to an absorption (edge-step) of 1 from +37 to +58 eV (linear regression) with respect to E0 Linear combination fitting, using quadratic programming, was conducted from −14 eV to +46 eV (with respect to E0) with the package LCF, version 1.6–6. We allowed the baseline and normalisation parameters of the samples to be modified, on the basis of the observation that minor modifications of these parameters can lead to considerable changes in the P speciation results (Werner and Prietzel 2015). Computerised combination of all possible baseline correction and edge-step normalisation parameters using R code resulted in over 60,000 baseline-corrected and edge-step-normalised sample spectra. Among these spectra, the lower energy level used for baseline correction varied between −43 and −30 eV (1 eV step), and the upper energy level varied between −19 and −9 eV (0.5 eV step) with respect to E0. The lower energy level used for edge-step normalisation varied between +34 and +40 eV (0.5 eV step), and the upper level varied between +50 and +65 eV (1 eV step) with respect to E0. All spectra were automatically fitted, using the initially baseline-corrected and edge-step-normalised standard spectra as predictor compounds. Phosphorus speciation shares below 5% of total P were excluded from the result list and LCF was repeated without the respective standards. The best fit was chosen, as selected by the lowest R-factor (∑ (data – fit)2/∑(data2)). The P forms identified by XANES were forced to add to 100%.
Results
Density fractionation
The share of C, N, and P associated with the fLF showed a broad range across the sites (Fig. 1, C: 25–61%, N: 19–55%, Ptot: 18–54%). At least 18% of the investigated elements were stored in the fLF (37 ± 17%). The sites with a better P supply (BBR and MIT) had a larger portion of C, N and P in the fLF than the poorer sites. The Ptot concentration in the fLF was from 33 to 593 μg Ptot g−1 soil (Table 2). At all sites, the fraction occluded within aggregates (oLF) had the least share of C, N, and P (except the share of C at LUE). With decreasing P status of the mineral soil and increasing thickness of the forest floor, respectively, the portion of C, N, and P in the oLF tended to increase (Mean for C, N and Ptot at BBR 3 ± 2% and at LUE 23 ± 15%) (Fig. 1). The better-supplied sites BBR and MIT had very little share of C (8 and 14%), N (5 and 10%) and Ptot (1 and 8%) within the aggregates (oLF).
Together, both light fractions (fLF and oLF) in the mineral topsoils were found to contain one third up to three-fourths of C, N and P: (C: 48–75%, N: 44–63%, Ptot:33–54%). For the fLF we observed several linear correlations between the relative abundance of C and N (N = 1.00C -4.22, r2 = 0.97), C and (Ptot = 0.59C +8.74, r2 = 0.54), C and Porg (Porg = 0.66C +3.53, r2 = 0.68), N and Ptot (Ptot = 0.59 N +11.03, r2 = 0.57), and finally N and Porg (Porg = 0.67 N +6.09, r2 = 0.72). In contrast, C and N were not related to Pinorg in the fLF (Pinorg = 0.23C +34.15, r2 = 0.03 and Pinorg = 0.12 N +39.05, r2 = 0.01). Regarding the oLF, only a relation between the relative abundance of C and N was found (N = 1.00C -4.22, r2 = 0.96).
At our study sites the HF contained a share of at least 23% of C, N, and Ptot (Mean for C, N and Ptot: 51 ± 15%). At most sites, the share of Ptot in the HF was the highest of all fractions (38–71%), except at MIT where the HF contained only 38% of Ptot and the fLF 54% (Fig. 1).
The amounts of the light fractions in the investigated forest subsoils (below 73 cm) were very small (Table 3). In subsoil samples the relative C and N distribution was largely dominated by HF (79–99% and 86–98% of total C and N, respectively, Table 3).
C:N:P stoichiometry of forest floor layers and topsoil SOM density fractions
In this study all element ratios are given in molar ratios.
Carbon and nitrogen
Along the gradient in declining soil P the C:N ratios in the light fractions, fLF and oLF increased (Table 4). This was most recognizable in the oLF where we observed an increase in C:N ratio with decreasing P supply from 16 to 52. The C:N ratios in the HF differed only marginally at all sites. In the mineral topsoil the following pattern was found for C:N ratios at most sites: oLF > fLF > bulk soil > HF. The well-supplied site BBR with the pattern: fLF > bulk soil > oLF ≥ HF, was exceptional. The smallest (11 in the HF) and the largest C:N ratio was found at LUE (54 in Oi).
Carbon and organic phosphorus
In the forest floor C:Porg ratios increased with decreasing P availability of the site (Table 5). At LUE the ratio in the Oa horizon was very high (3558) compared to the other Oa horizons. The C:Porg ratio at each site decreased from the organic layers to the mineral topsoil. In the mineral soil, the oLF had generally the largest C:Porg ratio, with smaller ratios at the better-supplied sites BBR, MIT, and VES (954 to 1210) and larger ratios at the P-poor sites CON and LUE (3094 and 4863). With few exceptions, all C:Porg ratios in the organic layers, bulk soil, and SOM density fractions increased along the P. In the SOM density fractions all C:Porg ratios revealed the pattern: HF < fLF < oLF with the C:Porg ratios of bulk soil being transitional between the HF and the fLF.
Carbon, nitrogen and total phosphorus
Comparing the sites, in general the molar C:N:P ratios increased with decreasing P supply. Specifically, in the forest floor the C:N:P ratios were smaller at sites with better P supply than at poor-supplied sites (Table 6). In the SOM fractions of the mineral soil, the oLF had the largest ratio, while the HF the smallest at every site. While the fLF and HF covered a relatively small range (304:15:1 to 818:29:1 and 85:6:1 to 532:25:1), the C:N:P ratios of the oLF (577:37:1 to 4606:89:1) were much larger, especially at the two poor sites CON and LUE (Table 6).
In the mineral soil the C:N:P ratios of the fLF at the three well-supplied sites were larger than the ratios of the corresponding HF. In contrast, at the sites CON and LUE the C:N:P ratios of fLF were the smaller than the C:N:P ratio of the HF.
XANES analyses of SOM density fractions
The signal-to-noise-ratio of the P K-edge XANES spectra of the different samples (Fig. 2) depended on their P concentration (fLF > oLF > HF; BBR > MIT > CON > VES > LUE, Tables 7 and 8). The quality of most spectra was excellent or at least satisfactory (HF at VES); however, for the oLF and the HF at LUE, which had particular small P concentrations (<0.3 mg g−1), no spectra of satisfactory quality could be acquired. According to the LCF results, Porg representing 50–60% of Ptot slightly exceeded Pinorg in most samples (Table 8). For the different SOM density fractions, no systematic change of their Porg:Pinorg ratio could be noticed. Most P in the samples was bound to Fe oxyhydroxides (primarily organic P) and clay minerals (Porg and Pinorg).
Soil P was mostly bound to Fe (oxyhydroxides) in the BBR samples, to Al (on clay mineral surfaces) in the VES samples, and about equally bound to Al and Fe in the MIT and CON samples. The P speciation as quantified by XANES mirrored the different Fe and Al content ratios of the studied soils (BBR > > MIT, CON > > VES; Table 2) and is well in line with the P speciation results as quantified for bulk soil samples (Fe-bound P/Al-bound P: BBR > MIT > CON; Prietzel et al. 2016b). Generally, the XANES results show systematic differences in the P speciation between the study sites, but not between the density fractions at one particular site. The fLF tends to be an exception, which contained preferentially Ca phytate compared to the other density fractions (Table 8).
According to the LCF results, free Porg, which is neither bound to Al, Fe, or Ca, appeared entirely absent in all samples – as the corresponding reference compound (free inositol hexakisphosphate” was always below detection limit.
Discussion
C, N and P distribution among functional SOM fractions in mineral topsoils
According to Fig. 1, the fLF showed a higher share of C and N at the better-supplied sites BBR and MIT. This might be explained by the higher biological activity at these sites (Yang et al. 2016) also documented by thinner forest floor layer compared to the P poor sites CON and LUE (Lang et al. 2017). We observed much lower as well as much higher Ptot concentrations in the fLF than O’Hara et al. (2006). At our study sites the range of Ptot in fLF is from 33 to 1066 μg g−1 and O’Hara et al. (2006) found between 313 to 698 μg g−1. This might due to different vegetation and climate conditions. Interestingly, O’Hara et al. (2006) found P concentrations in bulk soil were very similar to the concentrations measured in the fLF.
The percentage contribution of of C in the oLF to total soil C at all our sites (21 ± 14%) correspond to the findings of Graf-Rosenfellner et al. (2016) and Stutz et al. (2017) for forest topsoils (12–36% and 12–16%, respectively). Faster aggregate turnover at the better-supplied sites characterised by a higher biological activity might explain the observation of very low portions of C, N, and P in the oLF. The differences of C, N and P abundances between the two light fractions thus might arise from the different origin or stabilisation processes of OM or both (Wagai et al. 2009).
The relevance of the HF was generally higher for P compared to C and N, because orthophosphate is associated to the mineral surface and P will consequently be enriched in this fraction. The mass distribution and the C content of the HF depends strongly on the texture, especially on the clay content as shown by Christensen (2001) and Graf-Rosenfellner et al. (2016). Furthermore, the element distribution in the HF could be explained with the thickness of the forest floor, which increases from BBR to LUE. The thicker the forest floor, the more dissolved organic matter (DOM) or decomposition products of OM can be leached into in the soil (Kaiser and Guggenberger 2000). Therefore, C in the HF should be relatively more abundant with increasing thickness of the forest floor, due to the high affinity of DOM to minerals. This process is not reflected in our data and the share of C in the HF does not seem to be related to the thickness of the forest floor. Yet, it should be considered that the amount of accessible mineral surfaces further controls the amount of C in the HF. Quality and quantity of minerals clearly differed in the soils at our study sites (Lang et al. 2017), which may result in the poor correlation between the forest floor thickness and C in the HF. In contrast, Ptot in the HF was decreasing from 1.2 g Ptot kg −1 at BBR to 0.2 g Ptot kg −1 at LUE with decreasing thickness of the forest floor and P supply at the study sites (thickness of the forest floor in cm = −0.002 Ptot + 13.15, r2 = 0.96, Table 2,). This suggests a tight recycling of P within the light fractions, without “loosing” P to the P fixing heavy fraction of soils.
The content of C and N decreased from the topsoil to the deeper horizons due to the lack of litter input from the forest floor (Kaiser et al. 2002). In the depth below 73 cm, hardly any light fractions were recognized (Table 3). Consequently, P in the light fractions will not significantly contribute to P nutrition in subsoils.
In summary, the data obtained confirmed the relevance of the oLF for P in forest soils. Specifically, we observed an increasing portion of P stored in the oLF with decreasing P supply, which confirms partly our second hypothesis. Soils with low P supply from the mineral parent material tend to store more P within the aggregates (Fig. 1).
C:N:P stoichiometry
Molar C:N ratios
Comparing the C:N ratio of the SOM density fractions with the C:N ratio of SOM of the Oi horizon, which is one of the origins of SOM in the mineral soil, provides information on the decomposition of SOM. While fresh organic residues present in Oi horizons typically exhibt large C:N ratios, increasing residence time and microbial degradation result in smaler C:N ratios due to the loss of C and the integration of N in the biomass. As a result the C:N ratio of SOM usually also decreases with increasing soil depth (Rumpel and Kögel-Knabner 2011). These traits are in line with our findings at our study sites (Table 4): At all sites the C:N ratios decreased in both light fractions compared to the fresh litter of the Oi horizon. Only at LUE the C:N ratio of the oLF is the same as in the Oi horizon. At the best-supplied site BBR the C:N ratios decreased in both light fractions much stronger compared to the other sites, thus mirroring the faster turnover of SOM at BBR.
According to Golchin et al. (1994) the fLF should be less decomposed than the oLF reflected by a smaller C:N ratio. However, the oLF showed larger C:N ratios than the fLF for most analysed samples ((except BBR, Table 4). Therefore, the oLF is less decomposed than the fLF. Gordon and Jackson (2000) reviewed 56 studies and found an average C:N ratio of 43 in average for fine roots. Therefore, it is assumed that the oLF is made out of relatively fresh root litter and is not derived directly from the forest floor. Furthermore, there are indications that the oLF is a mixture between more labile and stable SOM components (Wagai et al. 2009). For example, the oLF can be stabilised in a less transformed state than the organic matter bound at mineral surfaces. Sincethere is hardly any bioturbation at the poor-supplied sites. Therefore, it is not possible that fresh litter from the relatively thick forest floor becomes occluded in mineral topsoil aggregates very quickly. Here, the aggregates are formed around the roots as a “nucleus” (Angers and Caron 1998) reflected by the very high C:N ratios of the oLF at the poor-supplied sites. In general, the fLF is the very youngest matter, while the HF contains the most decomposed and oldest C (Kögel-Knabner et al. 2008; Paul 2016). The small C:N ratios of the HF confirm this (Table 4). Accordingly, Gunina and Kuzyakov (2014) measured 13C values in the fLF and the oLF, which were nearly the same as in fresh litter. The authors found that more transformed products were in the HF as well as in a “dense oLF”. Schrumpf and Kaiser (2015) confirm with 14C radiocarbon measurement fast turnover times for the light SOM fractions and a slower turnover of organic matter in the HF.
In soils under deciduous forest, Grüneberg et al. (2013) found larger C:N ratios in the fLF than in the oLF. This is not in line with our data from deciduous forests which shows an order of HF < fLF < oLF, but in line with the findings in a conifer forest of Rasmussen et al. (2005). The variety of C:N ratios in density fractions is apparently not caused by a different litter supply from the vegetation.
Molar C:Porg ratios
Ratios of C to organic P compounds in the organic matter were presented (Table 5). The surprisingly high C:Porg ratio at LUE in the Oa could be well explained by the intense rooting of this horizon. The C to Porg ratio is considered an overall indicator for the nutritional status of the soils (Condron et al. 2005). According to the conceptual model, the fLF and the oLF are effective to provide more available P than the HF, where P is tightly bound to the mineral surfaces. Studies have shown that microorganism are able to incorporate up to one third of the P of the fresh litter within one week (McLaughlin et al. 1988). Comparing the fLF, which is assumed the less decomposed material in mineral soils, with the HF, the most decomposed material, gives a hint how litter is decomposed in soil. In an ecosystem where P is not limited the C:Porg ratio should narrow from the fLF to the HF as we hypothesized. Indeed, the C:Porg ratios decrease comparing the fLF to the HF at all five study sites. So, independently from the P status of the soils, SOM decomposition proceed to a similar outcome regarding the C:Porg element ratios.
C:N:P ratios
Published results provide evidence that element ratios can also be used to characterise the nutritional status of ecosystems and the transformation of SOM (Elser et al. 2000; Cleveland and Liptzin 2007). The C:N:P concept was first introduced by Redfield (1958) showing that for marine planktonic biomass there was no nutrient limitation at a molar ratio of 106:16:1. The C:N:P ratio is commonly used for explaining biotic feedbacks and the exchange of nutrients between microorganisms with their environment. Cleveland and Liptzin (2007) reported in their review a ratio of 186:13:1 for bulk soil and 60:7:1 for soil microbial biomass terrestrial ecosystems. In this study, soil microbial biomass was not measured directly, but it is known that microbial biomass as part of the SOM becomes increasingly important which soil development (Turner et al. 2013). At the study sites microbial biomass was analysed by Bergkemper et al. (2016). The authors found an adaption of the bacterial community to a P limitation in soils. In the P-rich soil at BBR the microbial potential for inorganic P solubilisation was significantly higher than in the P-depleted soil at LUE.
In contrast to tropical forests, data for temperate forests are rare. Chen et al. (2000) reported a ratio of 443:18:1 for a bulk soil under forest in New Zealand, whereas for soils in the Bohemian forest Šantrůčková et al. (2004) determined ratios of 342:16:1 and 679:21. The C:N:P ratios at the sites BBR and LUE extend this data range (85:6:1 and 367:32:1), thus confirming that our study sites represent a very broad gradient in P supply.
Temperate forest soils usually have higher C:N:P ratios than, for example, grassland soils because the organic layers in forests tend to be much thicker. In the organic layers at our study sites, C:N:P ratios declined from Oi to Oe and Oa horizons (Table 6) because of the progressive organic matter decomposition at the study sites. Exception is LUE where the C:N:P ratio in the Oa horizon is larger than in the intense-rooted Oe horizon. The differences in the C:N:P ratios in the forest floor at the sites are due to a certain feedback at the given site. The assumption is, that the fresh litter, which comes into the system correspond to the soil C:N:P with an already adapted ratio.
When it comes to soil nutrition cycling, driven by litter fall, soil water flow, and atmospheric deposition and exchange, C and N can gain or lose, whereas P is relatively stable in the system (Stewart and Tiessen 1987; McGroddy et al. 2004). Thus, Walker and Syers (1976) reported that the ecosystem status is strongly affected by the P availability. This can be confirmed by the C:N:P ratios obtained at our study sites. At the P-rich site BBR we found far smaller ratios than at the P-poor site LUE. Our data reveal that the C:N:P ratios differ not very much at the intermediate sites MIT, VES and CON, which again suggests that the P status of the study soils has to diverge widely to observe contrasts in the C:N:P ratios.
Organisms with low C:P or N:P ratios tend to grow fast (Elser et al. 2000). If there are low C:P or N:P ratios in SOM, one can assume that there are fast growing microbial communities or at least favourable conditions for them. At least at BBR the C:N:P ratios indicate that the conditions obtaining for microbial growth are optimal, which is line with the findings at BBR of Bergkemper et al. (2016).
Speciation of P in SOM density fractions
Our findings, that the portion of Ca phytate in the fLF is relatively high compared to the other fractions (Table 4) are in line with the observation that the fLF of soils mainly represents organic material of relatively low decomposition state (Paul 2016). Thus, Ca phytate introduced into soils with plant litter was still present only in this fraction and the initial Ca phytate P was already completely transformed and associated with Fe/Al–hydroxide species in the other – supposedly older - SOM fractions.
At first glance, this finding as well as the large contribution of Pinorg to the total P content of the fLF and the oLF (in most samples 40–50%)seems surprising, and may be attributed to methodological errors in the differentiation between Pinorg and Porg by XANES. However, the spectral differences of orthophosphate and organic P (IHP) adsorbed to the most relevant soil minerals are sufficiently large to permit a reliable differentiation of Porg and Pinorg (Prietzel et al. 2016a), and even a cautious quantification. A more scrutinizing look at the soil chemistry data supports our XANES result of a dominance of (i) Al-, Fe- or Ca-bound rather than free Porg and of (ii) a significant contribution of Pinorg to total P not only in the HF, but also the light fractions. The comparably low C concentrations in the light fractions (200–300 mg g−1 in the fLF and 240–420 mg g−1 in the oLF; Table 4) showed that particularly the fLF but also the oLF are characterised by a significant contribution of mineral matter (fLF: 30–50 mass percent according to the C concentrations). Large total Fe and Al concentrations in the fLF and the oLF (20–40 mg Fe g−1 and 15–50 mg Al g−1) support this assumption. Primary minerals can be supposed to have mostly disappeared due to long and intensive weathering in the strongly acidic topsoils of the investigated soils. Therefore, most of the Al and Fe in our SOM density fractions can be assigned to secondary pedogenic minerals, such as ferrihydrite and Al-saturated clay minerals, which have been identified as most relevant P-bearing compounds by XANES (Table 5). The cut-off value of 1.6 g cm−3 of the SPT solution in our density fractionation procedure allows mineral-SOM associations consisting of 30 mass percent Fe(III) oxyhydroxides (accepted density: 4 g cm−3) or 50 mass percent Al hydroxide or Al-clay minerals (accepted densities: 2.6 g cm−3) to float in the SPT solution with 70 or 50 mass percent OM respectively (accepted density: 0.5 g cm−3). Thus it is reasonable to assume that most P in our light SOM fractions is likewise bound to these mineral phases or Fe, Al (poly)hydroxy colloids or both rather than representing free orthophosphate or inositol hexakis phosphate. In addition, also the existence of mixed SOM-Fe, Al-(oxy)hydroxy-P-phases could explain our results. Morris and Hesterberg (2012) provided evidence for the presence of polynuclear Fe-hydroxide species within peat soil samples, which might also be accessible for phosphate or phytate sorption. In summary, the XANES analyses falsify our hypothesis that the light fractions contain mainly organic forms of P. However, the results were consistent with current literature on element speciation in organic-rich soils.
Conclusions
We analysed P in different SOM density fractions of soils along a P geogradient. All fractions (fLF, oLF, HF) accumulated P to variable extents. The expectation that P contained in the light fractions, fLF and oLF, is mainly organically bound was wrong. Nearly half of the P in the light fractions was present in inorganic forms and the heavy fraction (HF) contained a significantly amount of organic P compounds. Our assumptions that the light fractions represent indicator for recycling is no longer applicable, because the share of C, N and P stored in the light fractions did not increased with decreasing P status of the soils. Only the storage of P in the fraction within soil aggregates, which is assumed to be physically protected, increased in P-poor soils. This underscores the importance of physically protected fraction, oLF, for P accumulation in soils and possibly as “cache” for available P. We found transformation of SOM independent from the P status, while C:Porg ratios decrease from fLF to HF to a similar extend in P-rich and P-poor soils.
The XANES results and total element data obtained for the different SOM density fractions in our study suggest that mineral-organic associations are relevant constituents not only for the HF, but also for the light fractions of the investigated topsoils. In contrast to original litter P, the P in all SOM density fractions is characterised by intensive P recycling mirrored by decreasing C:Porg ratios, partial conversion into orthophosphate, and P associations with pedogenic Fe or Al minerals.
Abbreviations
- DOM:
-
Dissolved organic matter
- fLF:
-
Free light fraction
- HF:
-
Heavy fraction
- ICP-OES:
-
Inductively coupled plasma optical emission spectrometry
- LCF:
-
Linear combination fitting
- oLF:
-
Occluded light fraction
- OM:
-
Organic matter
- SOM:
-
Soil organic matter
- SPT:
-
Sodium polytungstate
- UV/VIS:
-
Ultraviolet–visible spectroscopy
- XANES:
-
X-ray absorption near edge structure
References
Ad-hoc-AG Boden (2005) Bodenkundliche Kartieranleitung (KA 5), Hannover, 5th edn. E. Schweizerbart'sche Verlagsbuchhandlung, Stuttgart
Amelung W, Zech W (1999) Minimisation of organic matter disruption during particle-size fractionation of grassland epipedons. Geoderma 92:73–85. http://dx.doi.org/10.1016/S0016-7061(99)00023-3
Angers DA, Caron J (1998) Plant-induced changes in soil structure: processes and feedbacks. Biogeochemistry 42:55–72. https://doi.org/10.1023/A:1005944025343
Attiwil PM, Adams MA (1993) Nutrient cycling in forests. New Phytol 124:561–582. https://doi.org/10.1111/j.1469-8137.1993.tb03847.x
Bergkemper F, Schöler A, Engel M, Lang F, Krüger J, Schloter M, Schulz S (2016) Phosphorus depletion in forest soils shapes bacterial communities towards phosphorus recycling systems. Environ Microbiol 18:1988–2000. https://doi.org/10.1111/1462-2920.13188
Bimüller C, Mueller CW, Lützow M v, Kreyling O, Kölbl A, Haug S, Schloter M, Kögel-Knabner I (2014) Decoupled carbon and nitrogen mineralization in soil particle size fractions of a forest topsoil. Soil Biol Biochem 78:263–273. https://doi.org/10.1016/j.soilbio.2014.08.001
Cerli C, Celi L, Kalbitz K, Guggenberger G, Kaiser K (2012) Separation of light and heavy organic matter fractions in soil testing for proper density cut-off and dispersion level. Geoderma 170:403–416
Chen GC, He ZL, Huang CY (2000) Microbial biomass phosphorus and its significance in predicting phosphorus availability in red soils. Commun Soil Sci Plant Anal 31:655–667. https://doi.org/10.1080/00103620009370467
Christensen BT (2001) Physical fractionation of soil and structural and functional complexity in organic matter turnover. Eur J Soil Sci 52:345–353. https://doi.org/10.1046/j.1365-2389.2001.00417.x
Cleveland CC, Liptzin D (2007) C:N:P stoichiometry in soil: is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 85:235–252. https://doi.org/10.1007/s10533-007-9132-0
Condron LM, Turner BL, Cade-Menun BJ (2005) Chemistry and dynamics of soil organic phosphorus. In: Sims JT, Sharpley AN (eds) Phosphorus: agriculture and the environment, agronomy monograph 46. ASA, CSSA, and SSSA, Madison, p 87–121. https://doi.org/10.2134/agronmonogr46.c4
Elser JJ, Sterner RW, Gorokhova E, Fagan WF, Markow TA, Cotner JB, Harrison JF, Hobbie SE, Odell GM, Weider LW (2000) Biological stoichiometry from genes to ecosystems. Ecol Lett 3:540–550. https://doi.org/10.1111/j.1461-0248.2000.00185.x
Golchin A, J. M. Oades, J. O. Skjemstad, P. Clarke (1994) Study of free and occluded particulate organic matter in soils by solid state C CP/MAS NMR spectroscopy and scanning electron microscopy. Aust J Soil Res 32:285–309.
Gordon WS, Jackson RB (2000) Nutrient concentrations in fine roots. Ecology 81:275–280
Graf-Rosenfellner M, Cierjacks A, Kleinschmit B, Lang F (2016) Soil formation and its implications for stabilization of soil organic matter in the riparian zone. Catena 139:9–18. https://doi.org/10.1016/j.catena.2015.11.010
Grüneberg E, Schöning I, Hessenmöller D, Schulze E-D, Weisser WW (2013) Organic layer and clay content control soil organic carbon stocks in density fractions of differently managed German beech forests. For Ecol Manag 303:1–10. https://doi.org/10.1016/j.foreco.2013.03.014
Gunina A, Kuzyakov Y (2014) Pathways of litter C by formation of aggregates and SOM density fractions: implications from 13C natural abundance. Soil Biol Biochem 71:95–104. http://dx.doi.org/10.1016/j.soilbio.2014.01.011
Guppy CN, Menzies NW, Moody PW, Blamey FPC (2005) Competitive sorption reactions between phosphorus and organic matter in soil: a review. Aust J Soil Res 43:189–202
Hedley MJ, Stewart JWB, Chauhan BS (1982) Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory Incubations1. Soil Sci Soc Am J 46:970–976. https://doi.org/10.2136/sssaj1982.03615995004600050017x
Ilg K, Wellbrock N, Lux W (2009) Phosphorus supply and cycling at long-term forest monitoring sites in Germany. Eur J For Res 128:483. https://doi.org/10.1007/s10342-009-0297-z
Jastrow JD, Miller RM (1997) Soil aggregate stabilization and carbon sequestration: feedbacks through organomineral associations. In: Lal R, Kimble JM, Follett RF, Stewart BA (eds) Soil processes and the carbon cycle. CRC Press, Boca Raton, p 207–223
Kaiser K, Guggenberger G (2000) The role of DOM sorption to mineral surfaces in the preservation of organic matter in soils. Org Geochem 31:711–725. https://doi.org/10.1016/S0146-6380(00)00046-2
Kaiser K, Eusterhues K, Rumpel C, Guggenberger G, Kögel-Knabner I (2002) Stabilization of organic matter by soil minerals — investigations of density and particle-size fractions from two acid forest soils. J Plant Nutr Soil Sci 165:451–459
Kögel-Knabner I, Guggenberger G, Kleber M, Kandeler E, Kalbitz K, Scheu S, Eusterhues K, Leinweber P (2008) Organo-mineral associations in temperate soils: integrating biology, mineralogy, and organic matter chemistry. J Plant Nutr Soil Sci 171:61–82. https://doi.org/10.1002/jpln.200700048
Lambers H, Raven JA, Shaver GR, Smith SE (2008) Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol 23:95–103. https://doi.org/10.1016/j.tree.2007.10.008
Lang F, Bauhus J, Frossard E, George E, Kaiser K, Kaupenjohann M, Krüger J, Matzner E, Polle A, Prietzel J, Rennenberg H, Wellbrock N (2016) Phosphorus in forest ecosystems: new insights from an ecosystem nutrition perspective. J Plant Nutr Soil Sci 179:129–135. https://doi.org/10.1002/jpln.201500541
Lang F, Krüger J, Amelung W, Willbold S, Frossard E, Bünemann E, Bauhus J, Nitschke R, Kandeler E, Marhan S, Schulz S, Bergkemper F, Schloter M, Luster J, Guggisberg F, Kaiser K, Mikutta R, Guggenberger G, Polle A, Pena R, Prietzel J, Rodionov A, Talkner U, Meesenburg H, von Wilpert K, Hölscher A, Dietrich HP, Chmara I (2017) Soil phosphorus supply controls P nutrition strategies of beech forest ecosystems in Central Europe. Biogeochemistry. https://doi.org/10.1007/s10533-017-0375-0
Lehmann J, Kinyangi J, Solomon D (2007) Organic matter stabilization in soil microaggregates: implications from spatial heterogeneity of organic carbon contents and carbon forms. Biogeochemistry 85:45–57. https://doi.org/10.1007/s10533-007-9105-3
Marschner P, Rengel Z (2012) Nutrient availability in soils. In: Marschner P (ed) Marschner's mineral nutrition of higher plants, 3rd edn. Academic Press, San Diego, pp 315–330
McGroddy ME, Daufresne T, Hedin LO (2004) Scaling of C:N:P stoichiometry in forests worldwide: implications of terrestrial redfield-type ratios. Ecology 85:2390–2401. https://doi.org/10.1890/03-0351
Mclaughlin MJ, Alston A, Martin JK (1988) Phosphorus cycling in wheat pasture rotations .II. The role of the microbial biomass in phosphorus cycling. Soil Res 26:333–342
Mikutta R, Kleber M, Torn MS, Jahn R (2006) Stabilization of soil organic matter: association with minerals or chemical recalcitrance? Biogeochemistry 77:25–56. https://doi.org/10.1007/s10533-005-0712-6
Morris AJ, Hesterberg DL (2012) Iron(III) coordination and phosphate sorption in peat reacted with ferric or ferrous iron. Soil Sci Soc Am J 76:101. https://doi.org/10.2136/sssaj2011.0097
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Niederberger J, Todt B, Boča A, Nitschke R, Kohler M, Kühn P, Bauhus J (2015) Use of near-infrared spectroscopy to assess phosphorus fractions of different plant availability in forest soils. Biogeosciences 12:3415–3428. https://doi.org/10.5194/bg-12-3415-2015
North PF (1976) Towards an absolute measurement of soil structural stability using ultrasound. J Soil Sci 27:451–459. https://doi.org/10.1111/j.1365-2389.1976.tb02014.x
O’Hara CP, Bauhus J, Smethurst PJ (2006) Role of light fraction soil organic matter in the phosphorus nutrition of Eucalyptus Globulus seedlings. Plant Soil 280:127–134. https://doi.org/10.1007/s11104-005-2675-8
Paul EA (2016) The nature and dynamics of soil organic matter. Plant inputs, microbial transformations, and organic matter stabilization. Soil Biol Biochem 98:109–126. https://doi.org/10.1016/j.soilbio.2016.04.001
Prietzel J, Harrington G, Häusler W, Heister K, Werner F, Klysubun W (2016a) Reference spectra of important adsorbed organic and inorganic phosphate binding forms for soil P speciation using synchrotron-based K-edge XANES spectroscopy. J Synchrotron Radiat 23:532–544. https://doi.org/10.1107/S1600577515023085
Prietzel J, Klysubun W, Werner F (2016b) Speciation of phosphorus in temperate zone forest soils as assessed by combined wet-chemical fractionation and XANES spectroscopy. J Plant Nutr Soil Sci 179:168–185
Rasmussen C, Torn MS, Southard RJ (2005) Mineral assemblage and aggregates control carbon dynamics in a California conifer Forest. Soil Sci Soc Am J 69. https://doi.org/10.2136/sssaj2005.0040
Ravel B, Newville M (2005) Athena, artemis, hephaestus: data analysis for X-ray absorption spectroscopy using IFEFFIT. J Synchrotron Rad 12:537–541. https://doi.org/10.1107/S0909049505012719
R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Redfield AC (1958) The biological control of chemical factors in the environment. American scientist : the magazine of sigma XI, the scientific research. Society 46:205–221
Richardson AE, Simpson RJ (2011) Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol 156:989–996. https://doi.org/10.1104/pp.111.175448
Rumpel C, Kögel-Knabner I (2011) Deep soil organic matter—a key but poorly understood component of terrestrial C cycle. Plant Soil 338:143–158. https://doi.org/10.1007/s11104-010-0391-5
Saggar S, Parfitt RL, Salt G, Skinner MF (1998) Carbon and phosphorus transformations during decomposition of pine forest floor with different phosphorus status. Biol Fertil Soils 27:197–204. https://doi.org/10.1007/s003740050420
Šantrůčková H, Vrba J, Picek T, Kopáček J (2004) Soil biochemical activity and phosphorus transformations and losses from acidified forest soils. Soil Biol Biochem 36:1569–1576. https://doi.org/10.1016/j.soilbio.2004.07.015
Saunders WMH, Williams EG (1955) Observations on the determination of total organic phosphorus in soils. J Soil Sci 6:254–267. https://doi.org/10.1111/j.1365-2389.1955.tb00849.x
Schrumpf M, Kaiser K (2015) Large differences in estimates of soil organic carbon turnover in density fractions by using single and repeated radiocarbon inventories. Geoderma 239-240:168–178. https://doi.org/10.1016/j.geoderma.2014.09.025
Sinaj S, Frossard E, Fardeau JC (1997) Isotopically exchangeable phosphate in size fractionated and unfractionated soils. Soil Sci Soc Am J 61:1413. https://doi.org/10.2136/sssaj1997.03615995006100050019x
Stewart JWB, Tiessen H (1987) Dynamics of soil organic phosphorus. Biogeochemistry 4:41–60. https://doi.org/10.1007/BF02187361
Stutz KP, Dann D, Wambsganss J, Scherer-Lorenzen M, Lang F (2017) Phenolic matter from deadwood can impact forest soil properties. Special issue on developments in soil organic phosphorus cycling in natural and agricultural. Ecosystems 288:204–212. https://doi.org/10.1016/j.geoderma.2016.11.014
Turner BL, Lambers H, Condron LM, Cramer MD, Leake JR, Richardson AE, Smith SE (2013) Soil microbial biomass and the fate of phosphorus during long-term ecosystem development. Plant Soil 367:225–234. https://doi.org/10.1007/s11104-012-1493-z
Wagai R, Mayer LM, Kitayama K (2009) Nature of the “occluded” low-density fraction in soil organic matter studies: a critical review. Soil Sci Plant Nutr 55:13–25. https://doi.org/10.1111/j.1747-0765.2008.00356.x
Walker TW, Syers JK (1976) The fate of phosphorus during pedogenesis. Geoderma 15:1–19. https://doi.org/10.1016/0016-7061(76)90066-5
Werner F, Prietzel J (2015) Standard protocol and quality assessment of soil phosphorus speciation by P K-edge XANES spectroscopy. Environ Sci Technol 49:10521–10528. https://doi.org/10.1021/acs.est.5b03096
Yang N, Zavišić A, Pena R, Polle A (2016) Phenology, photosynthesis, and phosphorus in European beech ( Fagus sylvatica L.) in two forest soils with contrasting P contents. J Plant Nutr Soil Sci 179:151–158. https://doi.org/10.1002/jpln.201500539
Acknowledgements
We want to thank the German Research Foundation DFG for funding this study as part of the priority program SPP 1685 (Projects: LA 1398/12-1, MI 1377/7-1and PR 534/6-1) and Sigrid Hiesch for the carefully realisation of the HNO3/HClO4/HF digestion.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Phil Haygarth
Electronic supplementary material
ESM 1
(DOCX 112 kb)
Rights and permissions
About this article
Cite this article
Stahr, S., Graf-Rosenfellner, M., Klysubun, W. et al. Phosphorus speciation and C:N:P stoichiometry of functional organic matter fractions in temperate forest soils. Plant Soil 427, 53–69 (2018). https://doi.org/10.1007/s11104-017-3394-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3394-7