Abstract
Aim
We evaluated the impact of retaining dead standing biomass (marcescence) on subsequent litter decomposition in the soil organic layer.
Methods
Litter of plants that naturally keep dead standing biomass in various extents, Calamagrostis epigeios (keeps most of its dead biomass standing), Quercus robur (keeps some dead leaves on the tree), and Alnus glutinosa (all litter falls to the ground after senescence), were either exposed to environmental climate (ambient) conditions for one year or kept in a dry dark place. After a year, both litter treatments were placed in the soil organic layer for another year. We monitored the mass loss and chemical changes during decomposition.
Results
Changes in the chemical composition of aromatic components in C. epigeios litter and decreasing amounts of aromatic compounds in Q. robur and C. epigeios litter during exposure to ambient conditions indicate an effect of photodegradation on these compounds. The litter of Q. robur also exhibited accelerated subsequent litter decomposition in the soil organic layer. In contrast, an increase of aliphatic and aromatic compounds and a decrease of carbohydrates in A. glutinosa litter during exposure to ambient conditions rather points to leaching or microbial decay of labile compounds than an effect of photodegradation. Moreover, the subsequent decomposition of A. glutinosa litter in the soil organic layer was decelerated as compared to the unexposed litter.
Conclusions
Our results suggest that litter with comparably low quality (Q. robur and C. epigeios), as compared to litter with a high quality (A. glutinosa), is prone to photodegradation. This process facilitates subsequent decomposition in soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Leaves of most of non-evergreen plant species senesce and litter completely falls every autumn. However, some species keep marcescent leaves in various extents. Marcescence is characteristic for trees such as beech (Fagus L.) and oak (Quercus L.) but to date, the ecological consequence of keeping dead biomass aboveground is not well understood. Otto and Nilsson (1981) suggested that retention of dead leaves could close a nutrient cycle for the trees. The retention of marcescent leaves may prolong the time trees are able to take up nutrients from them before they are finally shed. This hypothesis, though, was challenged by e.g., Abadía et al. (1996) and Dunberg (1982). Abadía et al. (1996) proposed that marcescent leaves result from long photosynthetically active leaves that suddenly die off at the very end of the season. Other hypotheses were related to the ecological advantages of marcescence, such as a protection against herbivores (Mingo and Oesterheld 2009). However, a consensus on the reason for the existences of marcescence is still lacking.
In the present study, we hypothesize that marcescence may have a relevant facilitating effect on litter decomposition in the soil organic (litter) layer (OL) after the leaves have finally fallen off the tree. This assumption emerges mainly from the fact that the factors operating in early decomposition stages of marcescent litter and senesced litter that has fallen off the tree markedly differ. Soil biota is largely excluded from the decomposition of dead standing biomass and abiotic factors, such as leaching of water soluble organic compounds (Michalzik et al. 2001), physical fracturing (Yanni et al. 2015) and photodegradation of organic compounds susceptible to solar radiation (Austin et al. 2016) play a major role. These processes may accelerate the subsequent decomposition of marcescent litter in soil. In this regard, the impact of litter exposure to environmental climate (ambient) conditions on its decomposition would be related to the extensiveness of marcescence that varies between different species. The strategy of keeping dead biomass may be relevant especially for litter with a low quality containing high amounts of lignin, which is reported as being photochemically reactive (e.g. Austin and Ballaré 2010). The photochemical degradation of litter, especially of phenolic compounds, may be caused by solar radiation and the ultraviolet (UV) spectrum in particular (e.g. Gehrke et al. 1995; Rozema et al. 1997; Austin and Vivanco 2006). This process has been found to play an important role in dry environments of arid and semi-arid regions (Austin and Vivanco 2006; Austin et al. 2009; Uselman et al. 2011), but an enhanced decomposition of litter due to solar radiation has also been observed in temperate ecosystems (Rozema et al. 1997; Frouz et al. 2011).
To test our hypotheses, we established a field experiment using litters of plants that keep dead biomass in various extents: alder Alnus glutinosa (where all litter falls to the ground during senescence), oak Quercus robus (which keeps some dead leaves on the tree) and the grass Calamagrostis epigeios (which keeps most of dead biomass standing). All litters were exposed to the same ambient conditions in the field and subsequently buried in the soil OL together with litter of the same species, which was previously kept in a dark, dry place. Such an experimental design provided the possibility to observe whether the exposure to ambient conditions, especially UV radiation and leaching, facilitates subsequent decomposition in the soil OL or not.
Materials and methods
Study area and study sites
The field decomposition experiment was carried out on a spoil heap in the Sokolov brown-coal mining district, Czech Republic. The spoil heaps were formed during the last 40 years by the piling of tertiary clay overburden originating from brown-coal mines. The pH of the soil was about 8 (Helingerová et al. 2010). The mean annual precipitation was 650 mm, and the mean annual temperature was 6.8 °C.
The study area was covered by a mosaic of reclaimed forest sites with various dominating tree species of the same age of 40 years (Frouz et al. 2013). For this study, oak (Quercus robur) and alder (Alnus glutinosa) forest sites were chosen. Several parts of the post-mining area were unreclaimed and were colonized by spontaneous revegetation with predominance of the grass Calamagrostis epigeios. This grass was also examined in this study.
Experiment and measurements
For the decomposition experiment, senescent litter was collected during the time of litter fall using litter traps that were made from 0.5 mm nylon mesh mounted onto an iron frame (0.5 × 0.5 m). The litter traps were located 0.5 m above the soil surface and set up in triplicate. The grass C. epigeios was collected as standing dead biomass. All litter was air-dried, and mixed. Litter of C. epigeios was additionally cut into ~5 cm long pieces. One part of the litter material was placed in litterbags (10 g of dry litter per bag in five replications for each type of litter) and the other part stored in a dark, dry cabinet at room temperature. The nylon litterbags (Silk and Progress, Czech Republic) were 10 × 15 cm large with 0.2 mm mesh size, the open mesh area was 52% of the total area and the thickness of the mesh was 80 μm. An absorption spectrum obtained from measuring the litterbag-mesh prior to the experiment showed that the mesh absorbed approximately one third of incoming radiation with a maximum in the range of 190–240 nm. The litterbags were placed on a rope about 1 m above the soil surface for one year at the study area. After that year of exposure, the litter was removed from litterbags, air-dried and weighed. Subsequently, 3 g of both types of litter, i.e. stored and exposed litter, were placed in litterbags and buried within the soil OL (which mainly consisted of litter) at the study site about 4 cm underneath the surface. Both, stored and exposed litter of all studied species, were buried in five replications. The litterbags were collected from the soil OL after another year and the litter was air-dried and weighed. The litter of C. epigeios was overgrown by fungal hyphae, we therefore manually removed them. Because some litterbags were damaged after the incubation in the soil OL, three replications of every treatment were eventually used for further analyses. Ultimately, four types of litter were analysed: initial litter, litter exposed for one year to ambient conditions (Exposed), exposed litter buried for one year in the soil OL (Exposed decomposed) and initial litter buried for one year in the soil OL (Decomposed).
Because of the presence of dust and soil particles on initial litter and in the litterbags, about 1.5 g of initial litter and material from each litterbag was used for determination of organic matter (OM) contents by loss on ignition (at 550 °C for 6 h). The values were then used for calculating the ash-free dry remaining mass of litter during the experiment (mass loss expressed as % of initial mass remaining). The rest of the material and initial litter was milled to a fine powder and used for determination of litter composition. Total carbon (C) and nitrogen (N) contents were determined using an EA 1108 Elementar Analyser (Carlo Erba Instruments, Italy). Thermochemolysis-GC-MS was used for the determination of lignin-related structures: guaiacyl, syringyl and hydroxymethyl (Sampedro et al. 2009). The initial litter and both types of litter that were buried in the soil OL were treated with an excess of tetramethylammonium hydroxide (TMAH, 25% aqueous solution) in four replicates, placed on wolfram wire spirals and dried in a desiccator at room temperature. Pyrolysis was performed with a PYR-01 pyrolyzer (Labio, Czech Republic) directly in the injector of a GC-MS (Varian 3400/Finnigan ITS 40 ion trap detector). The precise description of TMAH-Py-GC-MS instrumentation is presented in Frouz et al. (2011). Pyrolysis products were identified both, by interpreting the fragmentation patterns and comparing mass spectra with the NIST02 library. The percentages of pyrolysis products were calculated from the relative areas of the peaks after recalculation according to the exact weight of samples. The final values were the means of triplicate runs.
Aliquots of each three replicate samples were pooled to yield one replication of every treatment and subjected to 13C cross polarization magic angle spinning (CP/MAS) nuclear magnetic resonance (NMR) spectroscopy. The NMR spectra were measured with a Bruker Avance III HD 500 WB/US NMR spectrometer (Karlsruhe, Germany) in a 4-mm ZrO2 rotor. The magic angle spinning (MAS) speed was 9–11 kHz in all cases, with a notation frequency of B 1 (1H) and B 1 (13C) fields for cross-polarization ω1/2π = 62.5 kHz. Repetition delay and number of scans were 4 s and 1024, respectively. Two-pulse phase-modulated decoupling was applied during evolution and both detection periods. The phase modulation angle was 15°, and the flip-pulse length was 4.8–4.9 μs. The applied notation frequency of the B 1 (1H) field was ω 1/2π = 89.3 kHz. The 13C scale was calibrated using glycine as external standard (176.03 ppm; low-field carbonyl signal).
The 13C CP/MAS NMR spectra were quantified according to Wilson (1987) based on the area of the appropriate peak relative to the total area. Additionally, the alkyl-C/O/N-alkyl-C ratio (Baldock et al. 1997) was calculated.
Statistical analyses
One-way ANOVA (analysis of variance), followed by LSD post hoc tests, was used to compare individual characteristics (C and N content, C:N ratio) of individual treatments. One-way ANOVA, followed by Tukey post hoc tests, was used to compare decomposition of individual species over time from initial litter to decomposed litter in the soil OL. Computations were made using the statistical program Statistica 13. Data from TMAH-Py-GC-MS were subjected to principal component analysis (PCA). Computations were done using the statistical program Canoco 4.5 (Šmilauer and Lepš 2014).
Results
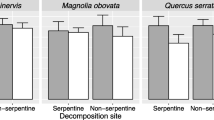
A significant decrease of litter mass during decomposition for both previously exposed and unexposed litter was observed for the investigated tree species (Q. robur and A. glutinosa; Fig. 1b, c). Only C. epigeios did not show a significant difference in litter mass at the end of the experiment as compared to initial litter (Fig. 1a). After one year of litter decomposition in the soil OL, the treatments that were previously exposed to ambient conditions and the treatments without prior exposure did not significantly differ in litter mass loss, except for Q. robur, where the previously exposed litter decomposed faster (Fig. 1b). However, the other two plants exhibited remarkable trends: while the mass of previously exposed litter of C. epigeios showed a clear decreasing trend after one year in the soil OL, litterbags with unexposed litter even showed a trend of a slight increase in mass after one year in the soil OL (Fig. 1a) most probably because of ingrowth of fungal hyphae (own observation). Opposite to that, exposed litter of A. glutinosa showed a decelerated decomposition in the soil OL in comparison to previously unexposed litter (Fig. 1c).
Total litter ash-free dry remaining mass of C. epigeios (a), Q. robur (b) and A. glutinosa (c). Columns represent means and bars represent SEM. Means marked by the same letter are not statistically different (one-way ANOVA, Tukey post hoc test P < 0.05). White columns represent ash-free remaining mass of litter that was exposed for one year to ambient conditions (1Y exp.) before it was placed for one year into the soil organic layer (1Y in OL). Grey columns represent ash-free remaining mass of litter directly placed into the soil organic layer for one year (1Y in OL)
The C and N contents and the C:N ratio of initial litter did not change after one year of exposure except for a decrease in the C:N ratio of C. epigeios (Table 1). The C content and the C:N ratio decreased after one year of decomposition in the soil OL for C. epigeios and Q. robur, but these characteristics did not differ between litter that was previously exposed to ambient conditions and that without previous exposure (Table 1). The N content decreased only for A. glutinosa litter after one year of decomposition in the soil OL for both treatments (exposed and unexposed). This was also reflected in the C:N ratio of decomposed A. glutinosa litter. Whilst the C content decreased after one year of decomposition in the soil OL, the C:N ratio did not change for that period of time (Table 1). This observation was valid for both examined treatments.
Changes in chemical composition of litter varied between the tree species. Q. robur showed a relative increase in aliphatic components but a relative decrease in aromatic components after exposure to ambient conditions (Table 2). In case of A. glutinosa, aliphatic and aromatic components relatively increased and carbohydrates decreased after one year of exposure to ambient conditions. However, the differences in composition of previously exposed and unexposed litter were negligible for both species after decomposition in the soil OL (Table 2).
After one year of exposure of C. epigeios litter to ambient conditions, aliphatic components relatively increased and aromatic components decreased (Table 2). C. epigeios showed differences in chemical composition of previously exposed and unexposed litter after a year of decomposition in the soil OL. For both treatments, aliphatic and aromatic components relatively increased and carbohydrates decreased during decomposition. In exposed litter, however, the aliphatic components showed relatively higher abundances and carbohydrates dropped more rapidly than in unexposed litter (Table 2). This result was also projected in the alkyl-C/O/N-alkyl-C ratio of C. epigeios, where the previously exposed litter showed values that were almost twice as high (Table 2).
The PCA ordination diagram, based on the composition of major chemical groups of aromatic compounds in litter, revealed that differences in litter decomposition of previously exposed and unexposed litter occurred only in case of C. epigeios (Fig. 2).
Principal component analysis (PCA) ordination diagram based on composition of major chemical groups of aromatic compounds in litter determined by TMAH-Py-GC-MS. Treatments are marked with circles. Initial litter: C = C. epigeios; Q = Q. robur; A = A. glutinosa. 1 year of decomposition in the soil organic layer: CD = C. epigeios; QD = Q. robur; AD = A. glutinosa. 1 year of ambient exposure +1 year of decomposition in the soil organic layer: CFD = C. epigeios; QFD = Q. robur; AFD = A. glutinosa
Discussion
The litter decomposition of all three observed species in the soil OL was influenced by prior exposure to ambient conditions. While the litter of Q. robur decomposed faster and the litter of C. epigeios tended to decompose faster when it was previously exposed to ambient conditions (Fig. 1a, b), the decomposition of exposed litter of A. glutinosa tended to be rather hindered in comparison to non-exposed litter (Fig. 1c). We assume that the initial litter quality may be the key for unravelling these different responses to ambient exposure: After one year of exposure to ambient conditions, C. epigeios litter had a significantly lower C:N ratio than initial litter (Table 1) and the relative contribution of aliphatic components increased, while the contribution of aromatic components decreased (Table 2). A relative decrease in aromatic components and an increase in aliphatic components were also observed in exposed litter of Q. robur. As was postulated in a previous study (Frouz et al. 2011), the photodegradation of photochemical reactive phenolic compounds, including lignin (e.g. Moorhead and Callaghan 1994; Rozema et al. 1997), may have a decisive impact on the observed chemical changes in exposed litter that enhanced the later decomposition in soil. Although photodegradation was likely responsible for the relative decrease in aromatic compounds in exposed Q. robur litter as compared to the unexposed litter after the first year and the mass loss of exposed litter was significantly higher than that of the unexposed litter after the subsequent year in the soil OL, differences in the chemical composition as revealed by NMR spectroscopy and Py-GC-MS of both treatments were very small. An explanation for this observed pattern may be provided by the chemical changes of C. epigeios during decomposition (Table 2).
From all species, the litter of C. epigeios decomposed most slowly in the soil OL as no significant decrease in mass loss was observed in both exposed and unexposed litter (Fig. 1a). However, different trends in decomposition of exposed and unexposed litter were observed and exposure to ambient conditions resulted in substantial changes in its chemical composition. Ultimately, ambient conditions affected subsequent litter decomposition to such an extent that the alkyl-C/O/N-alkyl-C ratio (Table 2) was almost twice as high as compared to the unexposed litter and only C. epigeios showed significant differences in aromatic compounds at the end of the experiments (Fig. 2). The initial litter of C. epigeios did not have an exceptionally high content of aromatic components in comparison to the other investigated species, but a relatively high content of carbohydrates. Therefore, we assume that the differences in decomposition of exposed and unexposed litter in the soil OL may be based on photodegradation of lignin that disintegrated lignocellulose complexes. Because of a relatively high contribution of carbohydrates in C. epigeios litter, this process can be crucial for a faster microbial uptake of carbohydrates later in the soil OL. In this respect, rather the amount of carbohydrates associated with lignin might be crucial for decomposition than the sole quantity of lignin (Angst et al. 2016). If most of the lignin present in the litter of C. epigeios was associated with carbohydrates, photodegradation should have highly affected the subsequent litter decomposition in the soil OL through the previous release of carbohydrates. In Q. robur, however, photodegradation of lignin in the first year probably did not substantially affect the accessibility of carbohydrates. This inference is supported by a substantially higher content of aromatic compounds and a lower amount of carbohydrates in initial litter of Q. robur as compared to the grass species. It follows that decomposition in the soil OL after exposure to ambient conditions was not necessarily faster than that of the unexposed litter because less lignocellulose complexes were disintegrated by photodegradation. This suggests that the impact of photodegradation on subsequent litter decomposition in the soil OL is to some extent dependent on the chemical properties of the respective litter material.
A further factor that supports the abovementioned indications and might explain the observed variation in the impact of photodegradation on litter decomposition is the C:N ratio of the litter, potentially also explaining why some plant species hold marcescent litter. The species examined in the current study varied in the amount of marcescent litter and also in its quality. Whilst A. glutinosa produces litter with a low C:N ratio that completely falls in autumn, Q. robur with a substantially higher C:N ratio holds part of its litter marcescent. The grass C. epigeios holds most of its dead biomass marcescent for a substantial amount of time. Its litter had by far the highest C:N ratio and, as described above, the difference in chemical composition of exposed and unexposed litter was highest from all of the studied species (Fig. 1). Thus, abiotic factors (i.e. solar radiation) may play a more important role for subsequent decomposition in litter with high C:N ratios, which is generally more resistant to microbial decay. As photodegradation may lead to the breakdown of phenolic macromolecules and to the disintegration of lignocellulose complexes, litter biomass becomes more susceptible and available to microbial utilization. Such a process has previously been described as photo-facilitation (Frouz et al. 2011; Yanni et al. 2015; Austin et al. 2016). These findings imply, that marcescence can be a way of how species with poorly decomposable leaves indirectly decrease the C:N ratio and thus stoichiometric differences to the soil microbial biomass, making the plant material better decomposable.
Contrary to C. epigeios and Q. robur, the litter of A. glutinosa has sufficient amounts of N (i.e. low C:N ratio) and is thus well utilizable by microbes. This may also explain the non-existence of naturally marcescent leaves of this species. From the 13C NMR spectra (Table 2), we assume that litter of A. glutinosa was not substantially influenced by photodegradation because the relative amount of aromatic components did not decrease. Only carbohydrates decreased after exposure to ambient conditions. Because carbohydrates have not been recognized as photochemically reactive (Austin and Ballaré 2010), their decrease resulted most probably from leaching of soluble compounds (e.g., carbohydrates and nutrients), which represents an important process during litter decomposition (Kaiser et al. 2002; Cepáková et al. 2016). Thus, leaching of soluble OM was probably responsible for the observed decrease in mass loss of A. glutinosa litter after one year of exposure to ambient conditions (Fig. 1c). Interestingly, this observed decrease was not manifested in a greater mass loss at the end of the experiment, where previously unexposed litter tended to decomposed faster (Fig. 1c). Ibrahima et al. (1995) proposed that leaching concentrates cellulose and lignin in litter and depletes sugars. A loss of water-soluble OM may thus result in less degradable residual OM that consequently decomposes slower than intact material (Parsons et al. 1990). Further, UV radiation is known to negatively affect activity and abundance of the microbial community that is associated with exposed litter (Brandt et al. 2007). This may also be a reason why exposed A. glutinosa litter tended to decompose slower in comparison to unexposed litter.
Conclusion
The response of litter to exposure to ambient conditions differed between the investigated species. Ambient conditions facilitated subsequent litter decomposition in the soil OL for the tree Q. robur and for the grass C. epigeios, whereas the decomposition of litter from the tree A. glutinosa showed a rather negative response to previous ambient exposure. These results, combined with our chemical analyses, indicate that the exposure of litter to ambient conditions seems to be most relevant for species with litter of a comparably low quality, such as Q. robur and C. epigeios. Keeping litter standing may provide more favourable conditions for insolation that may lead to photodegradation of recalcitrant structures and subsequent faster decomposition in the soil OL. Although our inferences hint at a possible explanation for the existence of marcescent litter, it still remains uncertain whether our data exhaustively explain its ecological relevance as other factors may also play a substantial role (e.g. soil biota abundance and activity, thickness and leaf area of decomposed litter). We encourage future experiments to employ similar methods as used in the present study on larger sets of representative species to which marcescence is characteristic.
Abbreviations
- ANOVA:
-
Analysis of variance
- C:
-
Carbon
- CP/MAS:
-
Cross polarization magic angle spinning
- GC-MS:
-
Gas chromatography-mass spectrometry
- N:
-
Nitrogen
- NMR:
-
Nuclear magnetic resonance
- OL:
-
Organic layer
- OM:
-
Organic matter
- PCA:
-
Principal component analysis
- Py:
-
Pyrolysis
- TMAH:
-
Tetramethylammonium hydroxide
- UV:
-
Ultraviolet
References
Abadía A, Gil E, Morales F, Montanés L, Montserrat G, Abadía J (1996) Marcescence and senescence in a submediterranean oak (Quercus subpyrenaica E.H. Del Villar): photosynthetic characteristics and nutrient composition. Plant Cell Environ 19:685–694. doi:10.1111/j.1365-3040.1996.tb00403.x
Angst G, Heinrich L, Kögel-Knabner I, Mueller CW (2016) The fate of cutin and suberin of decaying leaves, needles and roots - inferences from the initial decomposition of bound fatty acids. Org Geochem 95:81–92. doi:10.1016/j.orggeochem.2016.02.006
Austin AT, Ballaré CL (2010) Dual role of lignin in plant litter decomposition in terrestrial ecosystems. Proc Natl Acad Sci U S A 107:4618–4622. doi:10.1073/pnas.0909396107
Austin AT, Vivanco L (2006) Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 442:555–558. doi:10.1038/nature05038
Austin AT, Araujo PI, Leva PE (2009) Interaction of position, litter type, and water pulses on decomposition of grasses from the semiarid Patagonian steppe. Ecology 90:2642–2647. doi:10.1890/08-1804.1
Austin AT, Méndez MS, Ballaré CL (2016) Photodegradation alleviates the lignin bottleneck for carbon turnover in terrestrial ecosystems. Proc Natl Acad Sci 201516157. doi:10.1073/pnas.1516157113
Baldock JA, Oades JM, Nelson PN, Skene TM, Golchin A, Clarke P (1997) Assessing the extent of decomposition of natural organic materials using solid-state 13C NMR spectroscopy. Aust J Soil Res 35:1061–1083. doi:10.1071/s97004
Brandt LA, King JY, Milchunas DG (2007) Effects of ultraviolet radiation on litter decomposition depend on precipitation and litter chemistry in a shortgrass steppe ecosystem. Glob Chang Biol 13:2193–2205. doi:10.1111/j.1365-2486.2007.01428.x
Cepáková Š, Tošner Z, Frouz J (2016) The effect of tree species on seasonal fluctuations in water-soluble and hot water-extractable organic matter at post-mining sites. Geoderma 275:19–27. doi:10.1016/j.geoderma.2016.04.006
Dunberg A (1982) Why beech and oak trees retain leaves until spring: a comment on the contribution by Otto and Nilsson. Oikos 39:275–277. doi:10.2307/3544497
Frouz J, Cajthaml T, Mudrák O (2011) The effect of lignin photodegradation on decomposability of Calamagrostis epigeios grass litter. Biodegradation 22:1247–1254. doi:10.1007/s10532-011-9479-8
Frouz J, Livečková M, Albrechtová J, Chroňáková A, Cajthaml T, Pižl V, Háněl L, Starý J, Baldrian P, Lhotáková Z, Šimáčková H, Cepáková Š (2013) Is the effect of trees on soil properties mediated by soil fauna? A case study from post-mining sites. For Ecol Manag 309:87–95. doi:10.1016/j.foreco.2013.02.013
Gehrke C, Johanson U, Callaghan TV, Chadwick D, Robinson CH (1995) The impact of enhanced ultraviolet-B radiation on litter quality and decomposition processes in Vaccinium leaves from the subarctic. Oikos 72:213–222. doi:10.2307/3546223
Helingerová M, Frouz J, Šantrůčková H (2010) Microbial activity in reclaimed and unreclaimed post-mining sites near Sokolov (Czech Republic). Ecol Eng 36:768–776. doi:10.1016/j.ecoleng.2010.01.007
Ibrahima A, Joffre R, Gillon D (1995) Changes in litter during the initial leaching phase: an experiment on the leaf litter of Mediterranean species. Soil Biol Biochem 27:931–939. doi:10.1016/0038-0717(95)00006-Z
Kaiser K, Guggenberger G, Haumaier L, Zech W (2002) The composition of dissolved organic matter in forest soil solutions: changes induced by seasons and passage through the mineral soil. Org Geochem 33:307–318. doi:10.1016/S0146-6380(01)00162-0
Michalzik B, Kalbitz K, Park JH, Solinger S, Matzner E (2001) Fluxes and concentrations of dissolved organic carbon and nitrogen - a synthesis for temperate forests. Biogeochemistry 52:173–205. doi:10.1023/A:1006441620810
Mingo A, Oesterheld M (2009) Retention of dead leaves by grasses as a defense against herbivores. A test on the palatable grass Paspalum dilatatum Oikos 118:753–757. doi:10.1111/j.1600-0706.2008.17293.x
Moorhead DL, Callaghan T (1994) Effects of increasing ultraviolet B radiation on decomposition and soil organic matter dynamics: a synthesis and modelling study. Biol Fertil Soils 18:19–26. doi:10.1007/BF00336439
Otto C, Nilsson LM (1981) Why do beech and oak trees retain leaves until spring? Oikos 37:387–390. doi:10.2307/3544134
Parsons WFJ, Taylor BR, Parkinson D (1990) Decomposition of aspen (Populus tremuloides) leaf litter modified by leaching. Can J For Res 20:943–951. doi:10.1139/x90-127
Rozema J, Tosserams M, Nelissen HJM, Heerwaarden L, Broekman RA, Flierman N (1997) Stratospheric ozone reduction and ecosystem processes: enhanced UV-B radiation affects chemical quality and decomposition of leaves of the dune grassland species Calamagrostis epigeios. Plant Ecol 128:285–294. doi:10.1023/A:1009723210062
Sampedro I, Cajthaml T, Marinari S, Petruccioli M, Grego S, D’Annibale A (2009) Organic matter transformation and detoxification in dry olive mill residue by the saprophytic fungus Paecilomyces farinosus. Process Biochem 44:216–225. doi:10.1016/j.procbio.2008.10.016
Šmilauer P, Lepš P (2014) Multivariate analysis of ecological data using CANOCO 5. Cambridge University Press, Cambridge
Uselman SM, Snyder KA, Blank RR, Jones TJ (2011) UVB exposure does not accelerate rates of litter decomposition in a semi-arid riparian ecosystem. Soil Biol Biochem 43:1254–1265. doi:10.1016/j.soilbio.2011.02.016
Wilson MA (1987) NMR techniques and applications in geochemistry and soil chemistry. Pergamon Press, Oxford
Yanni SF, Suddick EC, Six J (2015) Photodegradation effects on CO2 emissions from litter and SOM and photo-facilitation of microbial decomposition in a California grassland. Soil Biol Biochem 91:40–49. doi:10.1016/j.soilbio.2015.08.021
Acknowledgements
This study was supported by the grant No. P504/12/1288 from the Czech Science Foundation and funds from the team project GAJU/04-158/2016/P at the University of South Bohemia. We also thank Jitka Hubačová for help with conducting the experiment, Carsten W. Mueller from the Technical University Munich, Chair of Soil Science for the possibility to write the manuscript at his department, Veronika Jílková for reading the manuscript, and two anonymous reviewers, whose comments helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ingrid Koegel-Knabner.
Rights and permissions
About this article
Cite this article
Angst, Š., Cajthaml, T., Angst, G. et al. Retention of dead standing plant biomass (marcescence) increases subsequent litter decomposition in the soil organic layer. Plant Soil 418, 571–579 (2017). https://doi.org/10.1007/s11104-017-3318-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3318-6