Abstract
Background and aims
Tetrastigma hemsleyanum is a rare medicinal plant in China. Because it requires very strict environmental conditions to grow, it usually takes 3–5 years to form calabash-shaped roots and is difficult to meet the demand of Chinese medical market. Although it is known that endophytes can enhance the growth of the host plants, the molecular mechanism of the interplay between endophytes and their host plants growth is not completely understood yet. The aim of this study was to determine if endophytes can reduce the growth cycle and promote the medicinal ingredients in Tetrastigma hemsleyanum, and elucidate its underlying molecular mechanism.
Methods
Endophytic fungi were isolated and identified from the calabash-shaped root of Tetrastigma hemsleyanum. The fermentation broth of 6 endophyte strains (named as TH09, TH12, TH14, TH15, TH17 and TH26) were added to the MS medium to culture axenic plantlets. After 30 days of culture, the net growth, expansin gene expression and the flavonoid content were measured.
Results
From the calabash-shaped roots, we isolated 31 endophytic fungi belonging to 10 genera: Fusarium, Rhizopycnis, Colletotrichum, Cylindrocarpon, Emericellopsi, Penicillium, Plectosphaerella, Aspergillus tubingensis, Alternaria and Sarocladium, of which Fusarium is the dominant genus. Addition of the fermentation broth from strains TH12, TH15 and TH26 significantly increased the plant growth. Strains TH15 and TH26’s fermentation broth increased the expression of Th-exp (an expansin gene), while addition of the fermentation broth from TH14 increased Th-exp expression in leaves and stems, but decreased Th-exp expression in the root. Flavonoid content was increased in the presence of fermentation broth from strains TH09, TH14, TH17 and TH26. Particularly, the fermentation broth from TH26 can promote plant growth, enhance Th-exp expression and increase the flavonoid content in Tetrastigma hemsleyanum.
Conclusions
The results demonstrated that endophytic fungi from the calabash-shaped root of Tetrastigma hemsleyanum can regulate plant growth, expression of expansin gene and flavonoid content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tetrastigma hemsleyanum Diels & Gilg ex Diels (Tetrastigma hemsleyanum) is a rare medicinal plant in China. The whole plant can be used as Chinese herbal medicine with the calabash-shaped root having the highest medicinal value. Its calabash-shaped root contains a variety of medicinal ingredients including flavonoids, flaconoids, vitamins and amino acids (Sun et al. 2013; Fan et al. 2014; Lin et al. 2016), and has detoxification, anti-inflammatory, pain relieving and anti-tumor effect (Xu et al. 2008; Peng et al. 2015; Xiong et al. 2015; Peng et al. 2016). Tetrastigma hemsleyanum is mainly distributed in the south of the Yangtze River including Zhejiang, Jiangxi, Fujian, Yunnan and Guangxi provinces in China (Qian et al. 2015). It requires very strict environmental conditions, i.e. it needs to grow in the mountainous forests in the shaded side at about 700 m above the sea level. The average annual atmosphere temperature that is favorable for its growth is approximately 18 °C. It usually takes 3–5 years to form calabash-shaped roots under natural conditions. Due to its strict growth conditions and overcollection, Tetrastigma hemsleyanum has become an endangered species in China and is difficult to meet the demand of Chinese medical market (Zhu and Yan 2014; Du et al. 2015).
Endophytic fungi or endophytes exist widely inside the healthy tissues of living plants, and are important components of plant micro-ecosystems. In the long process of evolution, some endophytes and the host plants are mutually beneficial. Many studies indicate that there are three types of beneficial interactions between endophytes and their host plants: (1) endophytes absorb nutrients from the host plants for their own growth, and at the same time produce metabolites to promote the growth of the host plants. (2) endophytes enhance resistance of the host plants to pests, drought, high temperature and other biotic and abiotic stresses, (3) endophytes accumulate secondary metabolites, including bioactive compounds (Hardoim et al. 2015; Saikkonen et al. 2016; Jia et al. 2016; Compant et al. 2016). The potential of some endophytes can be served as fertilizers to promote plant growth, including improving roots development (Hardoim et al. 2015; Compant et al. 2016; Khan et al. 2016). Although endophytes have been previously extensively studied, there have been no prior reports on endophytes from Tetrastigma hemsleyanum. Thus, it is important to identify endophytes in Tetrastigma hemsleyanum and explore the possibility which endophytes can be used in the artificial cultivation to reduce the growth cycle of Tetrastigma hemsleyanum and increase its Chinese herbal medicine resources.
On the other hand, expansins were discovered as cell wall proteins that mediate plant growth by loosening the cell walls without lysis of wall polymers. These proteins consist of four sub families: α-expansin, β-expansin, expansin-like A and expansin-like B. It is known that expansins are required in almost all plant physiological development aspects from germination to fruiting (Cosgrove et al. 2002; Cosgrove 2015; Marowa et al. 2016).
Although it is well-known that endophyte can enhance the host plant growth, the molecular mechanism of the interplay between endophyte and their host plant growth is not completely understood yet. It is unknown if the host gowth-promotion activated by endophyte is correlated with expansin gene expression. Besides, during the long process of co-evolution, endophyte can be directly or indirectly involved in the synthesis process of the host plant secondary metabolites, and even can produce metabolites that are the same or similar to those produced by the host (Hardoim et al. 2015; Jia et al. 2016). Thus, it is possible that endophyte can affect the contents of secondary metabolites (e.g. flavonoids) in Tetrastigma hemsleyanum.
To test these hypotheses, we isolated and characterized endophytes from the calabash-shaped root of Tetrastigma hemsleyanum. The effects of some endophytes on the plant growth, expansin gene expression and the content of flavonoids were investigated.
Materials and methods
Materials
Tetrastigma hemsleyanum was collected from the mountainous area in Jingnin County, Zhejiang Province in China and provided by Lishui Academy of Agricultural Sciences (Zhejiang Province, China), and planted in a glass conservatory in Hangzhou Normal University. The plantlet via tissue culture was derived from the previous study (Du et al. 2015).
Isolation and purification of endophytic fungi from the calabash-shaped root
The calabash-shaped roots (with diameter of 1.5 cm or so) derived from the plants that have been grown under natural conditions for more than three years were flushed with the tap water to remove the surface sediment, and soaked in detergent for 10 min. After rinse, the calabash-shaped root was soaked in sodium hypochlorite (1:2 dilution of the saturated solution) for 30 min, and finally the calabash-shaped root was rinsed 3 times with sterile water. The calabash-shaped root was cut into small pieces of 3–5 mm of length and width, and placed in PDA medium (containing 50 mg/L streptomycin, 200 mg/L peeled, diced, and filtered slag of potato, 20 g/L dextrose and 14 g/L agar) with the cutting surface toward the medium. Each petri dish of medium had 4–6 root segments of calabash-shaped root. The root segments were cultured at 28 °C and dark condition for 5 days. The mycelium grown from the cutting surface was picked and transferred to PDA medium containing 50 mg/L streptomycin. This process was repeated for 3–5 times to obtain purified colonies. As a control, the sterile water from last rinse was plated on the medium. Besides, the sterile-treated uncut calabash-shaped root was first spread on the medium and then removed from the medium. Last the petri dishes with medium were also placed at 28 °C to ensure that surfaces of roots were sterile.
Morphological and molecular identification of endophytic fungi
Morphological identification of endophytic fungi was performed based on colony’s surface morphology, edge shape, color and growth rate (Dai 1987). Fungal genomic DNA was extracted using fungal genomic DNA extraction kit (Sangon Biotechnology Co., Ltd., Shanghai, China). Based on the rDNA internal transcribed spacer region (Valente et al. 1996), primers of ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) were designed and synthesized by Sangon Biotechnology. The PCR was performed in a 50 μL reaction system containing 2 μL of DNA template (about 100 ng/μL), 2 μL of each primers (10 μmol/L), 1 μL of dNTP mix (10 mmol/L, Promega, Madison, WI, USA), 0.2 μL High fidelity Platinum® Taq DNA polymerase (5 U/μL, Invitrogen, Carlsbad, CA, USA), 5 μL 10 × High Fidelity PCR buffer, 2 μL 50 mmol/L MgSO4, 35.8 μL nuclease-free distilled water. Amplification was conducted by one cycle of 95 °C for 2 min, followed by 30 cycles of 95 °C for 45 s, 55 °C for 45 s and 72 °C for 45 s, and a final extension of 72 °C for 10 min. PCR product was purified and ligated to the vector pMD19-T (TaKaRa Biotechnology Co., Ltd., Shiga, Japan). The ligation products were transformed into competent E. coli DH5α cells. Positive clones were selected and insertions in plasmid were sequenced. The sequences were analyzed by NCBI BLAST online software (http://www.ncbi.nlm.nih.gov/blast) to determine the species of isolated endophytic fungi. MEGA6 software (http://www.megasoftware.net) was used to generate phylogenetic tree.
Preparation of endophyte’s fermentation broth
Six strains (named as TH09, TH12, TH14, TH15, TH17 and TH26) that were isolated in more than 3 experiments in plant replicates were cultured on solid PDA medium for 5 days. A hole punch was used to obtain three pieces of fungal plug with mycelium (with a diameter of 0.5 cm) from the edge of the colony. Subsequently, the fungal plug with mycelium was inoculated into 100 mL PDA liquid medium and grown at 28 °C with shaking (100 r/min) for 5 days. The culture broth was filtered through a Whatman filter paper with 15 cm diameter (Hangzhou Xinhua Paper Industry Co., Ltd., China). The filtrate (i.e. fermentation broth) was mixed with MS liquid medium (Murashige and Skoog 1962) at the ratio of 1:9 and sterilized after agar was added. This solid medium was used for the cultivation of Tetrastigma hemsleyanum plants. As a control, an equal volume of sterile water (instead of the fermentation broth) was mixed with MS medium.
Analysis of the plant growth
Axenic branches with stems and leaves (about 0.3 g) were cut from the plantlets with consistent morphology and growth. Then the branches were inserted into the medium with or without endophyte’s fermentation broth. After 30 days of cultivation (25 °C at day, 18 °C at night, 2000 lx, 12 h/d), the plantlets were washed agar from the root and fresh weight was measured.
Analysis of expansin gene expression
RNA was extracted from leaves, stems and roots of the plants cultured for 30 days in the presence or absence of fungal fermentation broth. First strand cDNA was synthesized using a SuperMix kit (TransGen Biotech, Beijing, China). Actin was amplified as an internal control using the primers actin-F (5′-GCCCTTGACTATGAGCAGGA-3′) and actin-R (5′-GAAAAGGACTTCAGGGCAGC-3′) (Huang et al. 2015). Based on our previous study, the expansin gene Th-exp. (GenBank accession number KP693606) was amplified by the primers Thexp-F (5′-CCCTGGTAGATGGCGAACTT-3′) and Thexp-R (5′-AACCGCCACTAATTTCTGCC-3′) (Song et al. 2016). Bestar SybrGreen qPCR Mastermix Kit (DBI Bioscience Company, Shanghai, China) was used for analysis of gene expression. Step One Plus™ Real-Time PCR System (Applied Biosystems, Waltham, MA, USA) was used for PCR with the following parameter: one cycle of 95 °C for 2 min followed by 40 cycles of 95 °C for 10 s, 55 °C for 30 s and 72 °C for 30 s, and a final extension of 72 °C for 10 min. Relative expression of Th-exp. was calculated as reported by Schmittgen and Livak (2008).
Measurement of flavonoid content
Total content of flavonoid was measured as described previously (Du et al. 2015). Briefly, the leaves, stems and roots were dried and homogenized into powder by a homogenizer. The powder (0.1 g) was placed into a 5 mL-centrifuge tube containing 1.5 mL 50% ethanol. After extraction using ultrasonic extraction instrument for 0.5 h and centrifugation at 11000 r/min, 50% ethanol was added to the volume of 2.5 mL. Flavonoid solution (1.0 mL) was absorbed and transferred into a 25 mL-volumetric flask, followed by addition of 50% ethanol to 9 mL and added 1 mL of 5% NaNO2 solution. Following 6 min of standing, 10% of Al(NO3)3 solution (1 mL) was added and mixed. After another 6 min of standing a 10% NaOH solution (10 mL) was added, 50% ethanol was used to reach the final volume of 25 mL. Following mixing and standing for 15 min, the samples’ absorbance was measured at 500 nm using a UV spectrophotometer and the total flavonoid content in each sample was calculated based on the standard curve. Rutin (Sangon Biotechnology) was used as the standard for total flavonoid.
Statistical analysis
The experiments for the determination of fresh weight, Th-exp. expression and flavonoid content were carried out in triplicate. The results were expressed as means ± standard deviation. Statistical analysis of data was performed by SPSS 19.0 using t-test to compare differences between the mean values of control and treatment.
Results
Isolation of endophytic fungi from the calabash-shaped root
After 3 days of growth on PDA medium, mycelium started to grow from the cutting site of the calabash-shaped root (Suppl. Fig. 1a). In contrast, there was not mycelium growth in the control petri dishes, suggesting that the calabash-shaped root surface was thoroughly sterilized and growing mycelia were endophytes. After subculturing the mycelium on PDA plates 3–5 times, single pure colony can be obtained (Suppl. Fig. 1b). Based on the morphology, color and growth characteristics of hyphaes, colonies and spores, a total of 31 strains were isolated from 8 separate experiments. Six strains (named as TH09, TH12, TH14, TH15, TH17 and TH26) were isolated from more than 3 independent experiments in plant replicates, and thus could represent the dominant endophytes.
Classification of the endophytic fungi
PCR amplification of specific sequences for identification of organisms has become common. The rDNA internal transcribed spacer (ITS) region is indispensable for species recognition in fungi (Schoch et al. 2012). PCR was performed using the genomic DNA of the 31 strains as template, and ITS1 and ITS4 as primers. PCR products with a size ranging from 500 to 750 bp were obtained from all the 31 strains (Suppl. Fig. 2). PCR products were cloned and sequenced, resulting in ITS rDNA signature sequences. The 31 ITS sequences have been deposited into GenBank database and their accession numbers are KY607733-KY607763, repectively. BLAST analysis showed that 31 strains were highly homologous to the known fungi in the GenBank and belonged to 10 genera (one strain is “uncultured fungus”): Fusarium (10 strains), Rhizopycnis (8 strains), Cylindrocarpon (3 strains), Colletotrichum (2 strains), Plectosphaerella (2 strains), Emericellopsis (1 strain), Penicillium (1 strain), Aspergillus (1 strain), Alternaria (1 strain) and Sarocladium (1 strain) (Suppl. Table 1). The most frequently isolated strains belonged to the genus Fusarium, followed by Rhizopycnis. Among the 8 strains in Fusarium, the sequencing lengths of ITS in strain TH17, TH22 and TH23 were 588 bp, and they shared high similarity (99% identities). All ITS sequencing lengths of strain TH15, TH21, TH26, TH27 and TH28 were 544 bp, and they had high ITS sequence similarity (99% identities). Among the 6 strains in Rhizopycnis, ITS sequences of TH14 were highly similar to TH16 and TH19 (99% identities), while those of TH24 were highly similar to TH06 and TH08 (99% identities). In addition, although TH15 and TH21’s ITS sequence (Fusarium, 544 bp), TH16 and TH19’s ITS sequence (Rhizopycnis, 540 bp), TH06 and TH08’s ITS sequence (Rhizopycnis, 541 bp), TH12 and TH13’s ITS sequences (Plectosphaerella, 558 bp) were identical, the mycelium and colony morphology were different. MEGA6 software (algorithms Neighbour-Joining) was used for phylogenetic analysis. Phylogenetic trees based on ITS rDNA sequences from 31 endophytic fungi formed distinct clades and showed their evolutionary relationships (Fig. 1).
Effect of endophytic fungi on plant growth
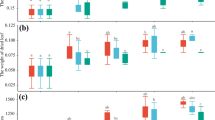
To test the effect of endophytes on Tetrastigma hemsleyanum growth, the fermentation broth of 6 strains were added to the MS medium to culture axenic plantlets. After 30 days of culture, the net growth was measured. The plantlets grown on the medium containing fermentation broth of TH12, TH15 and TH26 had slightly larger leaves, longer roots and the plant grew faster compared to the control (absence of fungi’ fermentation broth) (Fig. 2). The net fresh weight was significantly different after treatment with TH09, TH12, TH14, TH15 and TH26’s fermentation broth compared to the untreated control (p < 0.05). The net fresh weight increase of the plants cultured in the presence of strains TH12, TH15 and TH26’s fermentation broth was significantly higher than the control. However, the fermentation broth from TH17 did not affect the plant growth, while the fermentation broth from TH09 and TH14 inhibited plant growth (Table 1). Especially, addition of TH14’s fermentation broth even resulted in the yellow and wilt leaves.
Effect of endophytic fungi on the expansin gene expression
Quantitative RT-PCR was employed to evaluate the Th-exp. (an expansin gene) expression. Relative expression of Th-exp. normalized to actin was calculated as 2-△△CT (Schmittgen and Livak 2008). There were significant differences between the control group and all treatments except the leaves from the group treated with TH17’s fermentation broth (p < 0.05). Strains TH15 and TH26’s fermentation broth increased the expression of Th-exp., and expression of Th-exp. was down-regulated in the presence of fermentation broth from strains TH09 and TH12 in the leaves, stems and roots. Strain TH17’s fermentation broth reduced the expression of Th-exp. in stem and root, while did not significantly affect expression of Th-exp. in leaves. Addition of the fermentation broth from TH14 increased Th-exp. expression in leaves and stems, but decreased Th-exp. expression in the root (Fig. 3).
Effect of endophytic fungi on flavonoid content
Flavonoid is the secondary metabolites that are widespread in many medicinal plants, and includes different components (e.g., quercetin, naringenin and kaempferol). It has been reported that flavonoid has medicinal value in Tetrastigma hemsleyanum (Sun et al. 2013). The determination of total flavonoid contents in plant was conducted by spectrophotometric method. There were significant differences between the control group and the groups treated with fermentation broth from TH09, TH14, TH17 and TH26 (p < 0.05). Flavonoid contents in leaves, stems and roots were increased in the presence of fermentation broth from TH09, TH14, TH17 and TH26. In contrast, fermentation broth from TH12 and TH15 did not have significant effect on the flavonoid contents (Fig. 4).
In summary, the effect of fermentation broth from 6 strains on plant growth, Th-exp. gene expression and flavonoid content were summarized in Table 2. Treatments with the fermentation broth from TH15 and TH26 lead to the up-regulation of Th-exp. expression in leaves, stems and roots, while treatment with TH09 decreased Th-exp. expression in leaves, stems and roots. The effects of these three strains on plant growth coincide with the effects on Th-exp. expression. Besides, the effect of TH26 on flavonoid coincides with its effect on plant growth, while the effects of other five strains on flavonoid are not consistent with plant growth. Remarkably, strain TH26 has promoting activity on plant growth, Th-exp. expression and flavonoid synthesis.
Discussion
Firstly, endophyte is a huge microbial system widely present in different tissues of plants. Frohlich and Hyde (1999) reported that a total of 189 species of fungi were isolated from the six palms. Their results indicate that 33:1 would be a more accurate estimate of the ratio of host specific fungal to palm species in the tropics. Chen et al. (2013) confirmed that endophytic xylariaceous fungi are the dominant fungi in several Dendrobium species of tropical regions in China. The diversity, taxonomy and distribution of culturable endophytic xylariaceous fungi associated with seven medicinal species of Dendrobium (Orchidaceae) were investigated. Among the 961 endophytes isolated, 217 xylariaceous fungi (morphotaxa) were identified using morphological and molecular methods. Mousa et al. (2015) reported that 215 bacterial endophytes were isolated from diverse maize genotypes. To evaluate the diversity of endophytic fungi from the leaves of the common bean, twenty-seven different taxa were identified and the results showed that Colletotrichum was the most commonly isolated genera from the common bean (Gonzaga et al. 2015). Eighty nine endophytic nitrogen fixing bacteria were isolated from the leaves, stems and roots of industrial variety, wild and chewing sugarcane plants grown for 6 weeks in nitrogen (N)-free sand (Muangthong et al. 2015). Characterization and evaluation of fungal community changes showed that the sugarcane fungal community is composed of at least 35 different genera, mostly in the phylum Ascomycota (Romão-Dumaresq et al. 2016). In this study, we isolated 31 strains of endophytes from the calabash-shaped root of Tetrastigma hemsleyanum. Morphological and ITS rDNA sequence analysis showed that these 31 strains belonged to 10 genera, suggesting a great diversity of endophytes in calabash-shaped root of Tetrastigma hemsleyanum.
Secondly, it is clear that endophyte can affect the host plant seed germination, seedling survival, growth and tillering, inflorescence development, and biomass (Guo and Wang 2001; Hardoim et al. 2015). The seed biology and seedling growth of endophyte-infected and uninfected perennial ryegrass (Lolium perenne) and tall fescue (Festuca arundinacea) were evaluated. Seeds from infected plants of both species exhibited a higher rate of germination than seeds from uninfected plants. Infected perennial ryegrass and tall fescue plants produced significantly more biomass and tillers than uninfected plants (Clay 1987; Clay and Holah 1999). Malinowaki and Beleaky (2000) showed that cool-season grasses infected with endophytic fungi had longer leaf growing season, more developed root system, more ideal plant shape and stronger ability to survive. The endophytes associated with Orchidacea can promote the growth of the host plants, the germination of seeds and the development of the young heterotrophic plantlets (Guo and Wang 2001). A dark-septate endophytic fungus EF-37 isolated from the roots of Saussurea involucrate showed a positive effect on plant root development and plant growth (Wu et al. 2010). On the other hand, expansins have versatile developmental roles including the control of organ size, morphology and abscission (Cosgrove et al. 2002; Marowa et al. 2016). In Arabidopsis thaliana, expansin can modulate leaf growth and pedicel abscission (Cho and Cosgrove 2000). Further, inducible repression of multiple expansin genes leads to growth suppression during leaf development (Goh et al. 2012). We showed that endophytes TH15 and TH26 can promote the plant growth, TH17 had no effect on the plant growth, while TH09 can inhibit the growth of Tetrastigma hemsleyanum. Meanwhile, treatment with the fermentation broth from TH15 and TH26 led to the up-regulation of Th-exp. expression in leaves, stems and roots. In contrast, treatment with TH09 decreased Th-exp. expression (Table 2). The effects of these three strains on plant growth coincide with the effects on Th-exp. expression, suggesting that promotion or inhibition of the plant growth by these endophytes may be due to the promotion or inhibition of Th-exp. expression. However, TH12 promotes plant growth although Th-exp. expression is inhibited. Expansin is encoded by a gene family (Sampedro and Cosgrove 2005). In Tetrastigma hemsleyanum, there could be other expansin genes in addition to Th-exp. We speculate that TH12 inhibited the expression of Th-exp. gene, but maybe not affect or even increased the expression of other expansin genes, thereby promoting the plant growth. In addition, TH14 enhanced Th-exp. expression in leaves and stems. However, TH14 reduced Th-exp. expression in the roots, which could affect the root absorption function and be one of the reasons why TH14 inhibits the plant growth. In particularly, there were reports that endophytes can have a positive, negative or neutral effect on the plant (Hardoim et al. 2015). Different strains of fungi exhibited different effects (promoting or inhibiting) on the growth of Tetrastigma hemsleyanum. The underlying mechanism remains to be further explored.
Some results indicated that endophytes could increase growth of host plants by secreting or increasing hormones (Guo and Wang 2001) or by obtaining nutritional elements such as nitrogen and phosphorus that are useful for plants (Zhang et al. 2006; Hartley and Gange 2009). A few other studies also reported that some endophytes could promote the growth and fitness of the host plants by activating the expression of a certain enzymes and genes. For example, Piriformospora indica increased the growth of tobacco roots by stimulating the expression of nitrate reductase and the starch-degrading enzyme (Sherameti et al. 2005). Our results indicated that endophytes could also promote the host plant growth by up-regulating the expression of expansin genes.
Thirdly, some reports show that endophyte isolated from Taxus can produce Taxol under in vitro axenic culture conditions (Stierle et al. 1993; Gangadevi and Muthumary 2009; Garyali et al. 2013). In addition, it has been reported that production of antioxidant compounds (e.g., flavonoids and other phenolic antioxidants) were increased in endophyte-infected plants (Stierle AA and Stierle DB 2015; Negreiros de Carvalho et al. 2016). Analysis of the chemical composition of Saussurea involucrata seedlings showed that the level of rutin was higher in plants cultivated with endophyte compared to the controls (Wu et al. 2010). Our results showed that the total flavonoid content in Tetrastigma hemsleyanum were higher (the highest reached about two folds) when the plant was cultured in the presence of fermentation broth from fungi TH09, TH14, TH17 and TH26, which is consistent with previous reports that endophyte can increase the synthesis of secondary metabolites in host plants. Although fungi TH12 and TH15 have no effect on the flavonoid content, these two strains can promote the host plant growth. It is worth noting that strain TH26 has promoting activity on plant growth, Th-exp. expression and flavonoid synthesis. Thus, this strain has supernal application value in the regulation of plant growth and flavonoid content.
In summary, to the best of our knowledge, this is the first report showing that endophytic fungi can promote host plant growth, up-regulate expansin gene expression and increase the synthesis of medicinal ingredient flavonoid in Tetrastigma hemsleyanum.
References
Chen J, Zhang LC, Xing YM, Wang YQ, Xing XK, Zhang DW, Liang HQ, Guo SX (2013) Diversity and taxonomy of endophytic xylariaceous fungi from medicinal plants of Dendrobium (Orchidaceae). PLoS One 8:e58268
Cho HT, Cosgrove DJ (2000) Altered expression of expansin modulates leaf growth and pedicel abscission in Arabidopsis thaliana. Proc Natl Acad Sci U S A 97:9783–9788
Clay K (1987) Effects of fungal endophytes on the seed and seedling biology of Lolium perenne and Festuca arundinacea. Oecologia 73:358–362
Clay K, Holah J (1999) Fungal endophyte symbiosis and plant diversity in successional fields. Science 285:1742–1745
Compant S, Saikkonen K, Mitter B, Campisano A, Mercado-Blanco J (2016) Editorial special issue: soil, plants and endophytes. Plant Soil 405:1–11
Cosgrove DJ (2015) Plant expansins: diversity and interactions with plant cell walls. Curr Opin Plant Biol 25:162–172
Cosgrove DJ, Li LC, Cho HT, Hoffmann-Benning S, Moore RC, Blecker D (2002) The growing world of expansins. Plant Cell Physiol 43:1436–1444
Dai FL (1987) Fungal morphology and classification. Beijing: Science Press (in Chinese)
Du S, Xiang T, Song Y, Huang L, Sun Y, Han Y (2015) Transgenic hairy roots of Tetrastigma hemsleyanum: induction, propagation, genetic characteristics and medicinal components. Plant Cell Tissue Organ Cult 122:373–382
Fan SM, Huang ZH, Lin J, Huang XC, Xu YF, Xu W (2014) Separation and quantitative determination of four flavonoids in Tetrastigma hemsleyanum. Zhong Yao Cai 37:2226–2230 (in Chinese)
Frohlich J, Hyde KD (1999) Biodiversity of palm fungi in the tropics: are global fungal diversity estimates realistic? Biodivers Conserv 8:997–1004
Gangadevi V, Muthumary J (2009) A novel endophytic Taxol-producing fungus Chaetomella raphigera isolated from a medicinal plant, Terminalia arjuna. Appl Biochem Biotechnol 158:675–684
Garyali S, Kumar A, Reddy MS (2013) Taxol production by an endophytic fungus, Fusarium redolens, isolated from Himalayan yew. J Microbiol Biotechnol 23:1372–1380
Goh HH, Sloan J, Dorca-Fornell C, Fleming A (2012) Inducible repression of multiple expansin genes leads to growth suppression during leaf development. Plant Physiol 159:1759–1770
Gonzaga LL, Costa LE, Santos TT, Araújo EF, Queiroz MV (2015) Endophytic fungi from the genus Colletotrichum are abundant in the Phaseolus vulgaris and have high genetic diversity. J Appl Microbiol 118:485–496
Guo SX, Wang QY (2001) Character and action of good strain on stimulating seed germination of Gastrodia elata. Mycosystema 3:408–412
Hardoim PR, van Overbeek LS, Berg G, Pirttilä AM, Compant S, Campisano A, Döring M, Sessitsch A (2015) The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol Mol Biol Rev 79:293–320
Hartley SE, Gange AC (2009) Impacts of plant symbiotic fungion insect herbivores: mutualism in a multitrophic context. Annu Rev Entomol 54:323–342
Huang L, Song Y, Xiang T, Sun Y, Wu P, Wang B (2015) Cloning and analysis of two actin gene fragments from Tetrastigma hemsleyanum Diels et Gilg. J Hangzhou Normal University (Natural Science edition) 14:51–55 (in Chinese)
Jia M, Chen L, Xin HL, Zheng CJ, Rahman K, Han T, Qin LP (2016) A friendly relationship between endophytic fungi and medicinal plants: a systematic review. Front Microbiol 7:906
Khan A, Hossain MT, Park HC, Yun D, Shim SH, Chung YR (2016) Development of root system architecture of Arabidopsis thaliana in response to colonization by Martelella endophytica YC6887 depends on auxin signaling. Plant Soil 405:81–96
Lin Z, Chen L, Qiu Q, Guo S (2016) Isolation and identification of antiproliferative compounds from the roots of Tetrastigma hemsleyanum against MDA-MB-435S cell lines. Pak J Pharm Sci 29:1171–1175
Malinowaki DP, Beleaky DP (2000) Adaptations of endophyte-infected cool-season grass to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Sci 40:923–940
Marowa P, Ding A, Kong Y (2016) Expansins: roles in plant growth and potential applications in crop improvement. Plant Cell Rep 35:949–965
Mousa WK, Shearer CR, Limay-Rios V, Zhou T, Raizada MN (2015) Bacterial endophytes from wild maize suppress Fusarium graminearum in modern maize and inhibit mycotoxin accumulation. Front Plant Sci 6:805
Muangthong A, Youpensuk S, Rerkasem B (2015) Isolation and characterisation of endophytic nitrogen fixing bacteria in sugarcane. Trop Life Sci Res 26:41–51
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue culture. Physiol Plant 18:100–127
Negreiros de Carvalho PL, Silva Ede O, Chagas-Paula DA, Hortolan Luiz JH, Ikegaki M (2016) Importance and implications of the production of phenolic secondary metabolites by endophytic fungi: a mini-review. Mini Rev Med Chem 16:259–271
Peng X, Zhuang DD, Guo QS (2015) Induction of S phase arrest and apoptosis by ethyl acetate extract from Tetrastigma hemsleyanum in human hepatoma HepG2 cells. Tumour Biol 36:2541–2550
Peng X, Zhang YY, Wang J, Ji Q (2016) Ethylacetate extract from Tetrastigma hemsleyanum induces apoptosis via the mitochondrial caspase-dependent intrinsic pathway in HepG2 cells. Tumour Biol 37:865–876
Qian L, Dai D, Jiang H, Lin W (2015) Research progresses of the endangered medicinal plant Tetrastigma hemsleyanum Diels et Gilg. Acta Agriculturae Zhejiangensis 27:1301–1308 (in Chinese)
Romão-Dumaresq AS, Dourado MN, Fávaro LC, Mendes R, Ferreira A, Araújo WL (2016) Diversity of cultivated fungi associated with conventional and transgenic sugarcane and the interaction between endophytic Trichoderma Virens and the host plant. PLoS One 11:e0158974
Saikkonen K, Young CA, Helander M, Schardl CL (2016) Endophytic Epichloë species and their grass hosts: from evolution to applications. Plant Mol Biol 90:665–675
Sampedro J, Cosgrove DJ (2005) The expansin superfamily. Genome Biol 6:242
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W, Fungal Barcoding Consortium (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc Natl Acad Sci U S A 109:6241–6246
Sherameti I, Shahollari B, Venus Y, Altschmied L, Varma A, Oelmüller R (2005) The endophytic fungus Piriformospora indica stimulates the expression of nitrate reductase and the starch-degrading enzyme glucan-water dikinase in tobacco and Arabidopsis roots through a homeodomain transcription factor that binds to a conserved motif in their promoters. J Biol Chem 280:26241–262417
Song Y, Xiang T, Wu P, Wang B, Li J, Zhang T (2016) Molecular cloning, bioinformatics characterization and gene expression analysis of the full-length expansin gene cDNA from Tetrastigma hemsleyanum. Chin Tradit Herb Drugs 47:810–815 (in Chinese)
Stierle AA, Stierle DB (2015) Bioactive secondary metabolites produced by the fungal endophytes of conifers. Nat Prod Commun 10:1671–1682
Stierle A, Strobel G, Stierle D (1993) Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 260:214–216
Sun Y, Li H, Hu J, Li J, Fan YW, Liu XR, Deng ZY (2013) Qualitative and quantitative analysis of phenolics in Tetrastigma hemsleyanum and their antioxidant and antiproliferative activities. J Agric Food Chem 61:10507–10515
Valente P, Gouveia FC, de Lemos GA, Pimentel D, van Elsas JD, Mendonça-Hagler LC, Hagler AN (1996) PCR amplification of the rDNA internal transcribed spacer region for differentiation of Saccharomyces cultures. FEMS Microbiol Lett 137:253–256
Wu LQ, Lv YL, Meng ZX, Chen J, Guo SX (2010) The promoting role of an isolate of dark-septate fungus on its host plant Saussurea involucrata Kar. Et Kir. Mycorrhiza 20:127–135
Xiong Y, Wu X, Rao L (2015) Tetrastigma hemsleyanum (Sanyeqing) root calabash-shaped root extracts induces apoptosis in human cervical carcinoma HeLa cells. J Ethnopharmacol 65:46–53
Xu CJ, Ding GQ, Fu JY, Meng J, Zhang RH, Lou XM (2008) Immunoregulatory effects of ethyl-acetate fraction of extracts from Tetrastigma hemsleyanum Diels et. Gilg on immune functions of ICR mice. Biomed Environ Sci 21:325–331
Zhang HW, Song YC, Tan RX (2006) Biology and chemistry of endophytes. Nat Prod Rep 23:753–771
Zhu MX, Yan LH (2014) Resource investigation on rare and endangered she medicine Tetrastigma hemsleyanum in Zhejiang Province. Zhong Yao Cai 37:766–770 (in Chinese)
Acknowledgments
The work was supported by grants from the National Key Research and Development Project of China (Grant No. 2016YFC0503100), the Project of Science and Technology of Zhejiang Province for Commissioner (T Xiang), the Hangzhou Science and Technology Development Plan (Grant No. 20140432B05) and the Projects of Zhejiang Provincial Key Laboratory for Genetic Improvement and Quality Control of Medicinal Plants (Grant No. 201302).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Katharina Pawlowski.
Electronic supplementary material
ESM 1
(DOCX 2107 kb)
Rights and permissions
About this article
Cite this article
Song, Y., Wu, P., Li, Y. et al. Effect of endophytic fungi on the host plant growth, expression of expansin gene and flavonoid content in Tetrastigma hemsleyanum Diels & Gilg ex Diels. Plant Soil 417, 393–402 (2017). https://doi.org/10.1007/s11104-017-3266-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3266-1