Abstract
Aims
Struvite (MgNH4PO4.6H2O) recovered from wastewater can be used as fertilizer. The agronomic effectiveness of struvite has mostly been evaluated using ground fertilizer mixed through soil. However, fertilizers are most commonly applied in granular form in the field. In this study, we assessed the dissolution and effectiveness of different struvites when applied in granular or powdered form.
Methods
Phosphorus (P) diffusion in soil, determined using a visualization technique and chemical analyses, and P uptake by 6-week old wheat was compared for soluble fertilizer (monoammonium phosphate, MAP), a commercial struvite and three synthesized struvites with different excess MgO, in both granular and ground form.
Results
Ground struvite mixed through soil quickly dissolved and its agronomic effectiveness was similar to that of MAP. For pure granular struvite, the granule dissolution rate ranged from circa 0.03 mg d−1 in alkaline soil to 0.43 mg d−1 in acidic soil. Excess base in the struvite fertilizer reduced its dissolution rate. The P uptake by wheat followed the order MAP > > struvite ≥ control (no P), with no significant difference between the control and the struvite treatment in alkaline soil.
Conclusions
Both fertilizer characteristics (particle size, excess base) and soil pH strongly affect the dissolution rate of struvite and hence its agronomic effectiveness.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wastewater streams contain large amounts of phosphorus (P), and there is a double incentive to remove P from waste streams. Firstly, removal of P before the water is returned to the environment is essential to reduce and prevent eutrophication. Secondly, phosphate rock, the primary raw material for P fertilizer production, is a limited resource, and recycling P is therefore vital for the sustainable production of food in the future (Cordell et al. 2011). Struvite (MgNH4PO4.6H2O) is a phosphate mineral that often spontaneously precipitates in wastewater treatment plants and may cause process and maintenance issues. However, in the past decade, struvite precipitation has gained interest as a method for P recovery from wastewater, as it has potential for use as P fertilizer (Le Corre et al. 2009).
There are different processes used, or being developed, to recover P from wastewater through struvite precipitation. In terms of reagents used, two groups can be distinguished. In the first group, a magnesium (Mg) salt, most commonly MgCl2, is used as the Mg source and the pH is adjusted with NaOH. These Mg salts are water soluble and react quickly with the P in the wastewater during the crystallization process, and allow the formation of a product with high purity (nearly pure struvite) (e.g. Britton et al. 2005). Alternatively, MgO or Mg(OH)2 is used as the Mg source, which is cheaper than Mg salts and also removes or reduces the need for NaOH to raise solution pH. However, it reacts slower than Mg salts as it has to dissolve first and is usually added in excess to promote the struvite precipitation resulting in a product containing excess MgO or Mg(OH)2 (Chimenos et al. 2003; Capdevielle et al. 2013).
The solubility of struvite in water has been well characterized and decreases with increasing pH below pH 9 (Bhuiyan et al. 2007; Booker et al. 1999). However, less is known about the solubility and release of P from struvite when applied to soil. Because of its low solubility compared to commercial acidulated P fertilizers (such as ammonium phosphates and superphosphates), it is typically considered a slow release fertilizer. However, it has been argued by several authors that plant availability of struvite P is similar to that of fully soluble fertilizers even in the short term. Cabeza et al. (2011) compared P uptake by maize for different P sources and found that struvite was as effective as triple superphosphate (TSP) in two soils with acidic or near-neutral pH. Johnston and Richards (2003) evaluated P availability to ryegrass for 11 different precipitated phosphates in two soils with near-neutral pH and found no difference between struvite and monocalcium phosphate sources. In a study with six urine-derived struvites (Antonini et al. 2012), P uptake by ryegrass was similar or even higher in struvite-treated soil than in soil treated with a commercial mineral fertilizer (chemical composition not specified). Bonvin et al. (2015) assessed P uptake by ryegrass from urine-derived struvite using 33P labelled fertilizer in an acidic soil (pH in water 5.4) and found that similar amounts of P in the plant were derived from the struvite fertilizer as from a water-soluble reference fertilizer (KH2PO4).
Almost all studies that compared the effectiveness of struvite with that of soluble fertilizer, including the ones cited above, used ground fertilizers mixed through soil. However, commercial struvite-based fertilizers are usually in granular form. For instance, the struvite fertilizer Crystal Green® (Ostara) is available as a granular fertilizer with size guide numbers (SGN) from 100 to 350, with SGN 240 (i.e. mean particle diameter of 2.4 mm) recommended for agricultural use. In granular form, the dissolution of struvite would likely be much slower than for a powder mixed through soil, and the availability to plants would therefore likely be lower than for soluble fertilizers, at least in the short term. For instance, using 4D X-ray tomography, it was shown that only about 30 % of a struvite granule (with diameter around 3.6 mm) had dissolved in a slightly acidic soil after 14 weeks of incubation in the presence of plant roots (Ahmed et al. 2015). To our knowledge, only one study so far assessed the effectiveness of granular struvite for plant uptake. Talboys et al. (2016) carried out a pot trial with spring wheat and buckwheat to compare P availability of granular struvite (with a diameter of 2.4 mm) and diammonium phosphate (DAP) in a soil with pH 6.0. They found that after 30 d, there was little difference in uptake from struvite and DAP for buckwheat, but for spring wheat, the P uptake for the struvite treatment was only 30 % of that of the DAP treatment. In an experiment of longer duration (90 d), they found similar yield and P uptake by spring wheat for struvite and TSP, even though only 26 % of the struvite had dissolved, as determined by recovery of the residual struvite granules at the end of the experiment. This can likely be explained by the response curve reaching a plateau, in which case differences in P supply by treatments cannot be well distinguished (Barrow 1985).
Overall, there is very little information on the P release and availability to plants when struvite is added to the soil in granular form. The aim of the study was to assess how granulation and base excess in the fertilizer affects the dissolution rate and availability in various soils. We hypothesized that the dissolution and hence plant availability of P would be much slower for granular than for ground struvite, especially if the struvite contains excess base or when used in high pH soils.
Materials and methods
Soils
Selected physical and chemical properties of the soils used are given in Table 1. The soils were collected from the top layer (0–10 cm), air dried and sieved to <2 mm before use. Soil pH was determined in 0.01 M CaCl2 (L:S 5 L kg−1). Total C was measured with a dry combustion method (Matejovic 1997). The CaCO3 content was determined with a pressure calcimeter method (Martin and Reeve 1955). Particle size analysis was determined using the pipette method after oxidation of organic matter by hydrogen peroxide and destruction of carbonate by acetic acid (McKenzie et al. 2002). The cation exchange capacity (CEC) at pH 7.0 (ammonium acetate) and oxalate-extractable Al and Fe concentrations were determined according to Rayment and Higginson (1992). Isotopically exchangeable P (E value) was determined in a 0.5 mM CaCl2 extract at L:S 10 L kg−1 with 3 days of equilibration (Degryse and McLaughlin 2014).
Fertilizers
The fertilizers used in the experiments (Table 2) were commercial monoammonium phosphate (MAP) fertilizer (Incitec Pivot), a commercial struvite fertilizer (Crystal Green™, SGN 240) and a range of synthesized struvites. A pure struvite sample was synthesized according to a method modified from Kontrec et al. (2005). Solutions of 0.04 M NH4H2PO4 and 0.04 M MgCl2 were brought to pH 9.04 with 1 M NaOH, mixed in equal volumes and stirred for one hour. The precipitate was filtered and rinsed with deionized water. Two other struvites with excess MgO were made by adding MgO in increasing amounts to a NH4H2PO4 solution: 1.5 or 3.0 g MgO was added to 250 mL of a 0.1 M NH4H2PO4 solution. The suspension was stirred for one hour and the precipitate was filtered and rinsed with deionized water. The struvites were pelletized by extrusion through a 3-mm diameter cylindrical die to produce granules with a weight around 50 mg.
The pH and solubility of the products was measured in water at a liquid:solid ratio of 200 L kg−1 after 2 h of equilibration. The total composition was determined by acid dissolution in 3.2 M HNO3. The solutions were analysed for Mg and P using inductively coupled plasma – optical emission spectrometry (ICP-OES, Perkin Elmer Optima 7300 DV).
Petri dish experiments
Three Petri dish experiments were carried out in which the solubilisation of struvite in soil was assessed by comparing the P diffusion to that of P added as soluble fertilizer (MAP).
Effect of excess base (Exp 1)
The diffusion of P from the pure struvite and the two struvites with excess base was determined in the Monarto (M) soil (Table 1). The soil was wetted to field capacity and Petri dishes (diameter of 5.5 cm) were filled with the moist soil. A MAP granule or struvite pellet containing 8 mg P was added in the centre of a soil-filled Petri dish, in a 5-mm deep hole that was carefully closed after the fertilizer application. Each treatment was replicated two times. The Petri dishes were incubated at 25 °C, and P diffusion was visualized at 1, 3, 7 and 28 days after fertilizer application according to the method of Degryse and McLaughlin (2014). Briefly, an Fe-oxide impregnated paper was exposed to the soil surface for 5 to 30 min. The P captured on the paper was coloured using a modified malachite-green method, and the dried papers were scanned and analysed with image processing software to quantify the size of the P diffusion zone. At 28 days, the soils were sampled in concentric circles around the granule (0–7.5, 7.5–13.5, >13.5 mm from the fertilizer application site). The samples were analysed for pH, solution concentration in a 0.5 mM CaCl2 extract, E values (isotopically exchangeable P), and total P as described by Degryse and McLaughlin (2014).
Granular versus ground struvite in different soils (Exp 2)
The effect of physical form (granular or ground) and soil properties on struvite solubilisation was assessed by comparing P solubility of struvite (Crystal Green) and MAP in three soils (Monarto, Bordertown (B) and Dooen (D); Table 1). The soils were wetted to field capacity and Petri dishes (diameter of 5.5 cm) were filled with the moist soil. A fertilizer granule containing 2.7 mg P was added in the centre of a soil-filled Petri dish, in a 5-mm deep hole. For the mixed treatments, ground fertilizer (<0.15 mm) providing 5.4 mg P was mixed through the soil. Each treatment was replicated three times. The Petri dishes were incubated at 25 °C, and P diffusion was visualized at 1, 2, 5, 14 and 28 days after fertilizer application. At 28 days, the soils with granular fertilizer treatments were concentrically sampled (0–7.5, 7.5–13.5, >13.5 mm from the fertilizer application site), while a single sample of each replicate was used for the homogeneous treatments. The soil samples were analysed as described above.
Simulation of pot trial treatments (Exp 3)
In this experiment, the same soils were used as in the pot trial (see below), i.e. Mt. Compass (MC) and Black Point (BP), and the fertilizer treatments simulated those of the pot trial: MAP fertilizer in granular or ground form, struvite (Crystal Green) in granular or ground form, and struvite with excess base (Str2 in Table 2) in pelletized form. The soils were wetted to field capacity and Petri dishes (diameter of 5.5 cm) were filled with the moist soil. For the treatments with granules/pellets, a granule/pellet containing 3.5 mg P was added in the centre of a soil-filled Petri dish, in a 5-mm deep hole. For the mixed treatments, ground fertilizer was mixed through the soil at the same rate. Each treatment was replicated three times. The Petri dishes were incubated at 25 °C, and P diffusion was visualized at 1, 3, 7, 14, 28 and 60 days after fertilizer application. At 60 days, the soils with granular fertilizer treatments were concentrically sampled in two sections (<7.5 and >7.5 mm from the fertilizer application site), while a single sample of each replicate was used for the homogeneous treatments. The soil samples were analysed as described above.
Pot experiment
A pot trial was carried out in which wheat (Triticum aestivum L. cv. Frame) was grown for six weeks on the alkaline BP soil or acidic MC soil. Phosphorus was added at a rate of 15 mg P kg−1, either as MAP (SGN 290), struvite (Crystal Green, SGN 240) or struvite made with excess MgO (Str2) in granular form, or as MAP or struvite ground and mixed through soil. Also a control treatment with no added P was included. Each treatment was replicated three times.
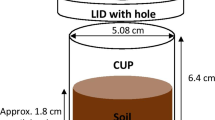
Air dried, sieved soils were moistened with basal fertilizer solution to field capacity. The basal fertilizer solutions were made up using urea, K2SO4, MgSO4, MnCl2.4H2O, CuSO4.5H2O, ZnCl2, (NH4)6Mo7O24, and (Mt Compass only) Ca(NO3)2 and supplied (in mg kg−1): 50 N, 50 K, 33 S, 10 Mg, 0.05 Mo and (for Black Point) 2 Mn, 2 Cu, 2 Zn or (for Mt. Compass): 20 Ca, 0.5 Mn, 0.5 Cu, 0.5 Zn. For the treatments with ground fertilizer, the ground MAP or struvite fertilizer was mixed through the soil, before pots (12 cm diameter) were filled with 1 kg of the soil. For the treatments with granular fertilizer, the granules (three per pot for the MAP treatment and seven per pot for the struvites) were applied at equidistant points 3 cm below the soil surface, 2 cm from the border of the pot. After two days, four pre-germinated wheat seedlings were planted at 1 cm depth, and thinned to two after 10 days. Plants were grown in a temperature-controlled growth room (12/12 h, 20/15 °C day/night cycle) and watered daily to field capacity.
After six weeks of growth, shoots were harvested, washed and oven dried at 65 °C. Dry shoots were weighed, ground and a sub-sample digested in aqua regia. The digests were analysed for concentrations of P and other plant nutrients (B, Ca, Cu, Fe, Mg, Mn, S and Zn) by ICP-OES.
Statistical analysis
Statistical differences were determined by analysis of variance (ANOVA) followed by post-hoc Duncan’s test at α = 0.05 using SPSS software version 21 (SPSS Inc., Chicago, US).
Results
Fertilizer composition and solubility in water
Phosphorus in the MAP fertilizer was nearly fully soluble in water (Table 2). For the commercial struvite (Str), only 3 % of the total P was soluble in water at a liquid:solid ratio of 200 L kg−1. The synthesized pure struvite (Str0) had a slightly lower pH and slightly higher P solubility than the commercial struvite. The composition of both these struvites (Str and Str0) was close to the theoretical composition of pure struvite (12.6 % P and 9.9 % Mg). The observed solubility of the struvites was in good agreement with the expected solubility given a pK sp of 13.2 (23 mg P L−1 at pH 9.3, corresponding to 0.4 % P on fertilizer basis at a liquid:solid ratio of 200 L kg−1), which is within the range of published solubility products of struvite (Le Corre et al. 2009). Excess base in the struvite decreased the total P concentration and resulted in higher pH (around 10) and slightly lower P solubility than for the pure struvite (Table 2). XRD analysis showed that Str2 was a mixture of struvite and brucite (Mg(OH)2) (details not shown), indicating that hydration of MgO occurred during the struvite precipitation process.
Effect of excess base on struvite dissolution in soil
Even though the excess base only had a small effect on the P solubility in water (Table 2), a very clear effect was observed on the struvite dissolution in soil. At any given time, the P diffusion zone visualized around the granule was larger for the struvite without excess base (Str0) than for the struvites with excess base (Str1 and Str2; Fig. 1).
This effect of excess base on struvite solubilisation was also very clear from the chemical measurements carried out on soil concentrically sampled around the application site at the end of the 4-week incubation (Fig. 2, Table 3). The solution concentrations at >7.5 mm from the application site decreased and the fraction of P recovered at <7.5 mm from the application site increased with increasing excess base in the struvite, which was likely related to the higher pH maintained near the application site, resulting in lower solubility of struvite. All three struvites showed much less P diffusion than the treatment with soluble fertilizer (MAP), which can be attributed to incomplete dissolution of struvite. To obtain an estimate of the extent of struvite dissolution, the amount of “fertilized” soil in the MAP and struvite treatment was compared. Given the radial diffusion geometry in the Petri dishes, the amount of soil in the high P zone is proportional to the area of this zone, and hence the percentage of dissolved struvite (Dissol) was estimated as follows:
Percentage of added P recovered at distance of less or more than 7 mm from the fertilizer application site at 28 days after fertilizer application in the Monarto soil. Phosphorus was applied at a rate of 8 mg P, as monoammonium phosphate (MAP) or as struvite without (Str0) or with low (Str1) or high excess base (Str2). Error bars are standard deviations. The filled part of the bar represents the non-exchangeable P (Exp 1)
where A struvite and A MAP is the area of the high P zone (exceeding the visualization threshold which roughly corresponds to a solution concentration around 0.3 mg P L−1; Degryse and McLaughlin 2014) for the struvite and MAP treatments, respectively. Using this approach, it was estimated that 26 % of the struvite without excess Mg was dissolved after 28 d, compared to 15 % for the struvite with low excess and 8 % for the struvite with high excess base.
The effect of excess base on struvite solubilisation in soil was confirmed in the third Petri dish experiment (Table 4) which simulated the treatments of the pot trial. In both soils, the P solution concentration at >7.5 mm was lower and the percentage of applied P remaining at <7.5 mm from the application site higher for the struvite with high excess base (Str2) than for the commercial, nearly pure struvite (Str). Based on the visualization results at 28 d, it was estimated that 44 % of the pure struvite but only 0.2 % of the struvite with excess base had dissolved at 28 d in the acidic MC soil. In the alkaline BP soil, the difference between struvites was smaller, with an estimated dissolution of 2.1 % for the pure struvite compared to 0.6 % for the struvite with excess base.
It is noteworthy that the percentage of P recovered in isotopically exchangeable form was often considerably higher than the percentage of struvite estimated to be dissolved at the end of the experiment. For instance, in the BP soil, it was estimated from the visualization results that <5 % of the struvite had dissolved by the end of the experiment for both struvites, but more than 30 % was isotopically exchangeable (Table 4). This discrepancy can be explained by the high L:S ratio used during isotopic exchange, which promotes dissolution of struvite, compared to the in situ conditions in the Petri dish.
Effect of physical form (powdered/granular) on struvite dissolution in soil
As discussed above, there were clear differences in P diffusion between struvite and MAP when the fertilizers were applied as granules/pellets. However, this was not the case when the fertilizers were ground and mixed through soil (Fig. 3). Both in the second (soils M, B, D) and third Petri dish experiment (soils MC and BP), there was little difference in P solubility based on the visualisation results (Fig. 3). Chemical measurements at the end of the incubation confirmed these findings. In the second experiment, the P solution concentration in the ground struvite treatment was not significantly different from that in the ground MAP treatment for the Bordertown soil (2.5 mg P L−1) and was even higher than in the MAP treatment for the two other soils (2.2 vs 1.4 mg L−1 in Dooen and 4.0 vs 2.2 mg L−1 in Monarto), which may have been related to the pH difference. In the third experiment, there was no significant difference in P solubility between the ground MAP and struvite treatments in either of the soils (Table 4).
Visualized P diffusion zone at 28 days after fertilizer application in different soils (see Table 1) for struvite (Str) or monoammonium phosphate (MAP) added to the soil as a granule in the centre of the Petri dish or ground and mixed through the soil (Exp 2 and 3)
Effect of soil properties on struvite solubilisation
In all three experiments, there was a clear difference in P diffusion between the MAP and struvite treatment. In the MAP treatment, most movement occurs in the first couple of days which can be attributed to saturation of the adsorption sites, but after this initial phase, adsorption reactions limited further movement (Fig. 4). In contrast, the diffusion for the struvite treatments was slower and proceeded more gradually, which can be explained by ongoing dissolution of the struvite.
The diameter of the P diffusion zone as function of time for the MAP or struvite (Str: no excess base; Str2: with excess base) as function of time in five soils. In soils M, B and D, P was applied at a rate of 2.7 mg P, as monoammonium phosphate (MAP) or as struvite (Str) (Exp 2). In soils MC and BP, P was applied at a rate of 3.5 mg P as MAP or as struvite without (Str) or with excess base (Str2) (Exp 3). Error bars give standard deviations
Using Equation (1), the solubilisation of struvite as function of time was estimated (Fig. 5). A near-linear trend was observed and the dissolution rate was hence expressed by the slope of this linear relationship. The fastest dissolution rate was observed in the acidic soils (B and MC), in which around 50 % of the granular struvite was dissolved after 4 weeks, corresponding to a dissolution rate of ca 0.43 mg struvite d−1 for each granule. The high pH soils showed much slower dissolution, with only 3 % of the granular struvite dissolved after 4 weeks for the BP soil, corresponding to a granule dissolution rate of 0.03 mg struvite d−1.
a The fraction of struvite that is dissolved as a function of time, as estimated from the area of the P diffusion zone for struvite relative to monoammonium phosphate (Eq. 1) in soils M, B and D (Exp 2) and soils MC and BP (Exp 3). b The dissolution rate of struvite granules, as estimated from the slope of the linear regression lines (through zero) in Fig. 5a, as function of the soil pH
Pot trial
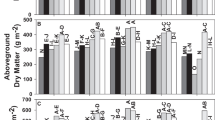
Adding MAP fertilizer significantly increased the yield, shoot P concentrations and P uptake in both soils (Fig. 6). In the MC soil, there was no significant difference between granular and ground MAP, while in the BP soil, the yield was higher for granular than for ground MAP. The Petri dish experiment indicated that there was no difference in P fixation between ground and granular treatments, since in both cases, about half of the P was still in labile (isotopically exchangeable) form after 60 d (Table 4). We hypothesize the higher availability for the granular treatment in the BP soil was due to a “banding effect”. With granular P, the fertilizer was positioned closer to the seed, and this would have resulted in the roots being exposed to higher P concentrations in the early growth stages than for the ground treatment in which the fertilizer was mixed throughout the soil and therefore more diluted. This banding effect would have mattered less in the MC soil because of the weaker P sorption and hence higher P mobility in this soil (cf. Fig. 3).
The dry matter yield (DMY), P concentration in the shoot and P uptake in soils MC and BP without added P (Ctr) or with P added at 15 mg P kg−1 as monoammonium phosphate (MAP), struvite without (Str) or with excess base (Str2) in granular form or as MAP or Str ground and mixed through soil. Different letters indicate significant differences (P ≤ 0.05)
For the granular treatments, the P availability was much lower for the struvite than for the MAP treatments. In the BP soil, the yield and P uptake was not significantly higher for the struvite treatments than for the control treatment. In the MC soil, the yield and P uptake in the struvite treatments was higher than in the control treatment, but much lower than in the MAP treatment, and was higher for the struvite without than with excess base. In contrast, for the treatments in which the fertilizer was ground and mixed through soil, there was no significant difference between the MAP and struvite treatments.
Discussion
Struvite dissolution in soil depends on the particle size
The dissolution of struvite was much slower for struvite added to the soil in granular form than for ground (<0.15 mm) struvite mixed through soil. For granular struvite without excess base, the estimated dissolution after 4 weeks incubation in soil ranged from 2.7 to 48 % in five soils with a wide range of pH values (Fig. 5). When the struvite was ground and mixed through soil, the chemical results indicated that P solubility was similar in both treatments at the end of the incubation, and the P diffusion visualizations indicated that this was already the case after 1 day of incubation (similar colour value for both treatments).
This difference between granular or ground struvite can be explained by the increasing soil:fertilizer contact with decreasing particle size. When the struvite is ground and mixed, the adsorption of P on the solid phase and the pH buffering of the soil allow for quick dissolution of the struvite. However, when struvite is in granular form, diffusion of dissolved P from the particle surface into the soil becomes the rate-limiting process. This is similar to the dissolution of phosphate rock in soil (Kirk and Nye 1986). Several studies have shown that reducing the particle size of rock phosphate increases its dissolution and improves its agronomic effectiveness (e.g. Alston and Chin 1974).
Struvite dissolution in soil depends on soil characteristics
The rate at which struvite dissolved was highly dependent on soil pH. In the most acidic soils, the dissolution rate of struvite granules (SGN 240) was estimated to be around 0.43 mg d−1. In the high-pH soils, the granule dissolution rate was <0.05 mg d−1 (Fig. 5). This can be explained by the pH dependent solubility of struvite. The minimum solubility of struvite is around pH 9–11. Below pH 8, there is a sharp increase in solubility with decreasing pH (e.g. Nelson et al. 2003). Hence, the lower soil pH results in higher solution concentrations of P at the soil:struvite interface and hence faster dissolution. Similar findings have been reported for the dissolution of rock phosphate in soil (Bolan and Hedley 1990; Kanabo and Gilkes 1987).
Few other studies have assessed the effect of soil pH on struvite solubilisation. Achat et al. (2014) evaluated effectiveness of recycled P products, including struvite, in different soils. They found that isotopically exchangeable P was similar for struvite as for soluble fertilizer (TSP), irrespective of pH. Finely ground fertilizer (<100 μm) mixed through soil was used in this study, which explains the full solubilisation of struvite and the lack of a pH effect, since under these conditions solubilisation of struvite proceeds quickly even in high pH soils.
While soil pH appears to be the main determinant, it is likely that other soil properties may also affect the dissolution rate of granular struvite. For instance, at the same pH, dissolution of struvite would likely be promoted by higher P sorption capacity, as has been found to be the case for rock phosphate. Smyth and Sanchez (1982) studied rock phosphate dissolution in seven acid soils from the Cerrado of Brazil and showed that phosphate rock was most reactive in soils with high P sorption capacity. Similarly, Babare et al. (1997) found that the extent of rock phosphate dissolution in 28 acidic pasture soils was positively correlated with the P buffering capacity of the soil. The higher dissolution rate for soils with strong P sorption can be attributed to the sorption sites acting as a sink for dissolved P and hence promoting dissolution. Furthermore, a high CEC may also promote dissolution as it would provide a sink for Mg, similarly to the effect of Ca-sink size on the dissolution of rock phosphate (Robinson et al. 1992).
Excess base in the struvite reduces its dissolution rate in soil
Excess base (Mg(OH)2, brucite) in the struvite reduced its dissolution rate in soil (Figs. 1 and 4). This can be explained by the higher pH and the higher Mg concentrations at the granule:soil interface, resulting in lower solubility of the struvite. It is likely that the effect of excess base on the struvite dissolution rate would also depend on the soil properties, e.g. on the pH buffering capacity. For instance, the difference in dissolution between Str (nearly pure struvite) and Str2 (with excess base) was much larger in the very sandy MC soil than in the BP soil, which was likely related to the low pH buffering capacity of the MC soil. The pH measured on the soil near the granule application site was much higher for Str2 than for Str in the MC soil, explaining the much slower dissolution of Str2, while the difference was small in the BP soil (Table 4).
Most studies have assessed P availability of struvite in soils using ground struvite mixed through soil, and have reached the conclusion that there is little difference between struvites of different origin and different degree of purity (Cabeza et al. 2011; Johnston and Richards 2003). However, this conclusion likely does not apply to commercial granular struvites, since the struvite dissolution rate depends on excess base in the case of granular fertilizers.
Agronomic effectiveness of struvite depends on its dissolution rate
We found that struvite and soluble fertilizer (MAP) were equally effective in supplying P to wheat when the fertilizers were ground and mixed through soil. However, when the fertilizers were used in granular form, as is usually the case in agricultural applications, the soluble fertilizer was much more effective. In the BP soil, there was no significant difference in P availability between the struvite treatments and the control treatment without added P. This can be explained by the very slow dissolution of struvite in this soil. From the dissolution rates derived from the Petri dish experiments, it is estimated that only 5 % (Str) or 1 % (Str2) of the struvite would have dissolved over the duration of the pot experiment (6 weeks). In the MC soil, the P availability was higher for the pure struvite than for the struvite with excess base (Str2), but much lower than for MAP. The much lower P availability for Str2 than for MAP can be explained by its slow dissolution (estimated to be ~1 % over the duration of the pot trial based on the results of the Petri dish experiment; Table 4). For the pure struvite, the much lower P availability than for MAP may seem surprising given its relatively fast dissolution in this acid soil (estimated to be ~60 % over the duration of the 6-weeks pot trial, cf. Fig. 5). However, the P supply at an early stage is critical for optimum crop yield (Grant et al. 2001) and in the first couple of weeks, less than 20 % of the struvite would have been dissolved and the P availability would have been small compared to that of the soluble fertilizer.
Almost all studies that compared struvite with soluble P fertilizer sources came to the conclusion that struvite has equal agronomic effectiveness as soluble fertilizers (Antonini et al. 2012; Cabeza et al. 2011; Johnston and Richards 2003; Plaza et al. 2007). However, all of these studies used struvite in ground form mixed through soil. Our results demonstrate that when struvite is ground and mixed through soil, it dissolves quickly and the P supply is similar as that of soluble fertilizer applied at the same P rate. However, when struvite is applied in granular form, it dissolves much slower and its agronomic effectiveness initially is lower than that of soluble fertilizers. Ackerman et al. (2013) compared the agronomic effectiveness of struvite and MAP using fertilizer banded with the seed, and found much lower effectiveness for struvite than for MAP. They hypothesized that the difference with other literature reported studies, which all showed similar effectiveness for struvite and soluble fertilizers, was related to difference in soil properties or P rate used. However, given the findings of our study, it seems likely that this discrepancy was due to the method of fertilizer application (banded in Ackerman et al. (2013) versus mixed through soil in the other studies). As with granular fertilizers, the soil:fertilizer contact is reduced and the struvite dissolution would hence be slower when banding than when mixing ground fertilizer through soil. To our knowledge, the only other study that assessed plant availability of granular struvite is the recent study by Talboys et al. (2016). They also found that granular struvite is slow to dissolve and does not provide sufficient P for early crop growth.
It is to be expected that struvite would have similar agronomic effectiveness as soluble fertilizers once it is (nearly) fully dissolved. Based on our results, it is estimated that the time to reach near-complete dissolution may range from days to years depending on fertilizer, soil and plant properties. Granule size and excess base are likely the main struvite fertilizer characteristics affecting the dissolution rate, with slower dissolution for large granules or at high base excess. Soil pH appears to be the main soil property affecting the dissolution rate of struvite granules, but the capacity of the soil to buffer the pH and the products of dissolution (phosphate and Mg2+) would likely also play a role. Also, plant roots may affect the dissolution rate by acting as a sink and by modifying the chemical conditions of the rhizosphere. For instance, Talboys et al. (2016) found that buckwheat was better able to take up P from struvite than spring wheat, which was attributed to the higher exudation of organic acids by buckwheat, but might also be related to rhizosphere acidification (Zhu et al. 2002).
Despite the growing interest in struvite over the last decade and the introduction of commercial granular products, hardly any literature-reported studies have assessed the effectiveness of granular struvite fertilizers, and there is hence a scarcity of field-relevant data. Furthermore, while results for ground struvite have led to the conclusion that the purity of the struvite does not affect its agronomic effectiveness, it is unlikely that this conclusion holds for granular struvite, since excess base in the fertilizer strongly reduces its dissolution rate in soil. Additional field-scale studies need to assess the agronomic effectiveness and residual value of struvite fertilizers when applied in granular form.
References
Achat DL, Daumer ML, Sperandio M, Santellani AC, Morel C (2014) Solubility and mobility of phosphorus recycled from dairy effluents and pig manures in incubated soils with different characteristics. Nutr Cycl Agroecosyst 99:1–15
Ackerman JN, Zvomuya F, Cicek N, Flaten D (2013) Evaluation of manure-derived struvite as a phosphorus source for canola. Can J Plant Sci 93:419–424
Ahmed S, Klassen TN, Keyes S, Daly M, Jones DL, Mavrogordato M, Sinclair I, Roose T (2015) Imaging the interaction of roots and phosphate fertiliser granules using 4D X-ray tomography. Plant Soil:1–10
Alston A, Chin K (1974) Response of subterranean clover to rock phosphates as affected by particle size and depth of mixing in the soil. Anim Prod Sci 14:649–655
Antonini S, Arias MA, Eichert T, Clemens J (2012) Greenhouse evaluation and environmental impact assessment of different urine-derived struvite fertilizers as phosphorus sources for plants. Chemosphere 89:1202–1210
Babare A, Gilkes R, Sale P (1997) The effect of phosphate buffering capacity and other soil properties on North Carolina phosphate rock dissolution, availability of dissolved phosphorus and relative agronomic effectiveness. Anim Prod Sci 37:1037–1049
Barrow NJ (1985) Comparing the effectiveness of fertilizers. Fert Res 8:85–90
Bhuiyan M, Mavinic D, Beckie R (2007) A solubility and thermodynamic study of struvite. Environ Technol 28:1015–1026
Bolan N, Hedley M (1990) Dissolution of phosphate rocks in soils. 2. Effect of pH on the dissolution and plant availability of phosphate rock in soil with pH dependent charge. Fert Res 24:125–134
Bonvin C, Etter B, Udert KM, Frossard E, Nanzer S, Tamburini F, Oberson A (2015) Plant uptake of phosphorus and nitrogen recycled from synthetic source-separated urine. Ambio 44:217–227
Booker N, Priestley A, Fraser I (1999) Struvite formation in wastewater treatment plants: opportunities for nutrient recovery. Environ Technol 20:777–782
Britton A, Koch FA, Mavinic DS, Adnan A, Oldham WK, Udala B (2005) Pilot-scale struvite recovery from anaerobic digester supernatant at an enhanced biological phosphorus removal wastewater treatment plant. J Environ Eng Sci 4:265–277
Cabeza R, Steingrobe B, Römer W, Claassen N (2011) Effectiveness of recycled P products as P fertilizers, as evaluated in pot experiments. Nutr Cycl Agroecosyst 91:173–184
Capdevielle A, Sýkorová E, Biscans B, Béline F, Daumer M-L (2013) Optimization of struvite precipitation in synthetic biologically treated swine wastewater—Determination of the optimal process parameters. J Hazard Mater 244:357–369
Chimenos J, Fernandez A, Villalba G, Segarra M, Urruticoechea A, Artaza B, Espiell F (2003) Removal of ammonium and phosphates from wastewater resulting from the process of cochineal extraction using MgO-containing by-product. Water Res 37:1601–1607
Cordell D, Rosemarin A, Schröder J, Smit A (2011) Towards global phosphorus security: A systems framework for phosphorus recovery and reuse options. Chemosphere 84:747–758
Degryse F, McLaughlin MJ (2014) Phosphorus diffusion from fertilizer: visualization, chemical measurements, and modeling. Soil Sci Soc Am J 78:832–842
Grant C, Flaten D, Tomasiewicz D, Sheppard S (2001) The importance of early season phosphorus nutrition. Can J Plant Sci 81:211–224
Johnston A, Richards I (2003) Effectiveness of different precipitated phosphates as phosphorus sources for plants. Soil Use Manag 19:45–49
Kanabo I, Gilkes R (1987) The role of soil pH in the dissolution of phosphate rock fertilizers. Fert Res 12:165–173.
Kirk G, Nye P (1986) A simple model for predicting the rates of dissolution of sparingly soluble calcium phosphates in soil. I The basic model J Soil Sci 37:529–540
Kontrec J, Babić-Ivančićand V, Brečević L (2005) Formation and morphology of struvite and newberyite in aqueous solutions at 25 and 37 °C. Coll Anthropol 29:289–294
Le Corre KS, Valsami-Jones E, Hobbs P, Parsons SA (2009) Phosphorus recovery from wastewater by struvite crystallization: A review. Crit Rev Environ Sci Technol 39:433–477
Martin AE, Reeve R (1955) A rapid manometeic method for determining soil carbonate. Soil Sci 79:187–198
Matejovic I (1997) Determination of carbon and nitrogen in samples of various soils by the dry combustion. Commun Soil Sci Plant Anal 28:1499–1511
McKenzie NJ, Coughlan KJ, Cresswell HP (2002) Soil physical measurements and interpretation for land evaluation. CSIRO Publishing, Collingwood.
Nelson NO, Mikkelsen RL, Hesterberg DL (2003) Struvite precipitation in anaerobic swine lagoon liquid: effect of pH and Mg: P ratio and determination of rate constant. Bioresour Technol 89:229–236
Plaza C, Sanz R, Clemente C, Fernández JM, González R, Polo A, Colmenarejo MF (2007) Greenhouse evaluation of struvite and sludges from municipal wastewater treatment works as phosphorus sources for plants. J Agric Food Chem 55:8206–8212
Rayment GE, Higginson FR (1992) Australian laboratory handbook of soil and water chemical methods. Inkata Press, Melbourne
Robinson JS, Syers JK, Bolan NS (1992) Importance of proton supply and calcium-sink size in the dissolution of phosphate rock materials of different reactivity in soil. J Soil Sci 43:447–459
Smyth T, Sanchez P (1982) Phosphate rock dissolution and availability in Cerrado soils as affected by phosphorus sorption capacity. Soil Sci Soc Am J 46:339–345
Talboys PJ, Heppell J, Roose T, Healey J R, Jones DL, Withers PJ (2016). Struvite: A slow-release fertiliser for sustainable phosphorus management? Plant Soil 401:109–123.
Zhu YG, He YQ, Smith SE, Smith FA (2002) Buckwheat (Fagopyrum esculentum Moench) has high capacity to take up phosphorus (P) from a calcium (Ca)-bound source. Plant Soil 239:1–8
Acknowledgments
This work was supported by The Mosaic Company. We also thank Ashleigh Broadbent, Bogumila Tomczak, and Colin Rivers for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: John Hammond.
Rights and permissions
About this article
Cite this article
Degryse, F., Baird, R., da Silva, R.C. et al. Dissolution rate and agronomic effectiveness of struvite fertilizers – effect of soil pH, granulation and base excess. Plant Soil 410, 139–152 (2017). https://doi.org/10.1007/s11104-016-2990-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2990-2