Abstract
Aim
To characterize the response of sunflower to the low levels of Cd exposure encountered in agricultural soils.
Methods
Plants were exposed in hydroponics to low concentrations of Cd (2.5 nM or 20 nM) and sampled at four vegetative stages (6, 9, 14 and 19 expanded leaves). Plant growth, root Cd absorbing properties and Cd partitioning between organs were monitored along with Cd content in the xylem sap.
Results
Sunflower growth was not limited when exposed to 20 nM Cd. The amount of Cd taken up by the plant roots as well as the rate of Cd loading in xylem sap increased in direct proportion to the concentration of Cd2+ in the nutrient solution, suggesting that neither the root Cd absorbing capacities nor the root-to-shoot translocation of Cd were impacted by the level of Cd exposure. The partitioning of Cd between stem and leaves followed that of dry matter, regardless of the Cd treatment. The root-to-shoot partitioning of Cd at early growth stages differed from that prevailing later on.
Conclusions
In an agricultural context, the partitioning of Cd between sunflower organs does not appear to be affected by the level of Cd exposure during vegetative growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium is a non-essential element that is toxic for humans, plants and animals. Cadmium enters agricultural soils from atmospheric depositions and from agricultural inputs including phosphate fertilizers and sewage sludge. Because of the significant soil-to-plant transfer of Cd, around 90 % of Cd exposure in the non-smoking population is through food, mainly through the consumption of agricultural crops. Recent exposure estimates emphasized that the average weekly intake of Cd from food in Europe, China and the USA was close to the tolerable intake level of 2.5 μg kg−1 body weight recommended by the European Food Safety Authority (EFSA, 2009). As a consequence, plant-derived human intake of Cd should be reduced.
After oilseed rape, sunflower (Helianthus annuus L.) is the second most important oleaginous crop in France in terms of cultivated area (762 000 ha) and oil production (671 000 tons per year) (data 2011, http://www.cetiom.fr). Large amounts of oil cake (more than 700 000 tons per year) are made as by-products of seed crushing for animal feed. The problem of cadmium is of special concern in sunflower since this species accumulates more Cd than other grain crops (Grant et al. 2008; Simon 1998). Indeed, the concentration of Cd in sunflower oil cakes can reach levels close to the European regulatory limit for feed (i.e. 1 mg Cd kg−1, EC 2005/87) (Dauguet and Lacoste 2013). The cadmium accumulates in the animal’s liver and kidney, both of which are consumed by humans.
One way to decrease the plant-derived human exposure to Cd is to limit the transfer of the contaminant to food and feed crops, through dedicated agronomic actions. This requires a better understanding of the processes governing the availability of Cd in the soil as well as those controlling the allocation of Cd to edible plant organs. Processes governing the availability of Cd in the soil (e.g. sorption, complexation) have been extensively described (Christensen and Haung 1999; Helmke 1999). This resulted in practical recommendations to limit the concentration of Cd2+ at the root surface, including maintaining the soil pH above 7.0 by liming (Hough et al. 2003), and to the current development of a mechanistic model of Cd bioavailability (Custos et al. 2014; Lin et al. 2014). The processes that control the transfer of Cd from the soil solution to edible plant organs are also well documented. As listed in Clemens et al. (2013), uptake into the root cell symplast, sequestration in the root through vacuolar storage and xylem loading are key points controlling Cd accumulation in plant shoots. The molecular mechanisms behind these processes have been extensively investigated, especially in hyperaccumulators (Clemens et al. 2013). One major difficulty in formalizing this knowledge in a simulation model is linked to the dependence of the Cd allocation pathways on the level of Cd exposure.
Indeed, we know from experiments performed at the micromolar range that excess Cd in crops can disturb a series of physiological processes including respiration, photosynthesis and nitrogen assimilation, resulting in growth inhibition (di Toppi and Gabbrielli 1999). Plant response to Cd thus mainly consists in protecting the photosynthetic apparatus by limiting Cd uptake and sequestrating it in less bioactive compartments (Balestri et al. 2014). One important mechanism against Cd toxicity that plants have thus evolved is to limit the translocation of Cd to the shoots. As reviewed by Lux et al. (2011), this so-called sequestration of Cd in plant roots results from the restricted movement of Cd both in the root symplast, caused by the sequestration of Cd in vacuoles, and in the root apoplast, in relation with the development of apoplastic barriers. In shoots, the allocation of Cd to old leaves appears to be enhanced under Cd stress (De Maria et al. 2013) and preferential sequestration of excess Cd in stems has sometimes been proposed.
However, one can wonder whether these Cd-induced changes in the between-organ partitioning of Cd generally observed in the case of high Cd levels of exposure (μM range) also occur at levels encountered in agricultural soils, i.e. when the free ionic concentration of Cd is below 20 nM (Sauvé et al. 2000). The aim of the present work was to investigate if some processes involved in the plant response to Cd are detectable at low Cd exposure. A hydroponic culture was conducted to characterize the response of sunflower plants exposed to 2.5 and 20 nM Cd throughout the vegetative stage. Our objectives were to (i) check if plant growth and the root Cd absorbing properties changed between 2.5 and 20 nM Cd, (ii) compare the between-organ partitioning of Cd in the two levels of exposure, and (iii) examine whether the partitioning of Cd is linked with the Cd in the xylem sap.
Materials and methods
Design of the experiment
A sunflower cultivar that is widely cultivated in France (cv ES BIBA, Euralis) was continuously exposed in hydroponics to two concentrations of Cd and sampled at four vegetative stages, with five replicates per stage and per Cd treatment. Plant sampling was performed when plants had 6, 9, 14 and 19 expanded leaves (stages 1 to 4, equivalent to 16, 19, 32 and 36 on the BBCH scale), corresponding to an exposure to Cd of 14, 17, 21 and 27 days from transplanting (195, 244, 301 and 392 growing degree-days (GDD), with a base temperature of 6 °C), respectively. The two Cd concentrations (2.5 and 20 nM Cd2+) corresponded to the range of Cd2+ concentrations commonly found in the porewater of non-polluted agricultural soils (Sauvé et al. 2000).
Plant cultivation
Seeds were washed with H2O2 (6 % v/v), rinsed with deionized water and germinated on wet paper in Petri dishes in the dark at room temperature for 96 h. The seedlings were then transferred to the greenhouse in a transient hydroponic device consisting of conical tubes whose cut bottom was submerged in a bubbled Hoagland’s nutrient solution (¼ strength for macronutrients, full strength for micronutrients), and left until the cotyledons were fully expanded (about 72 h). Thereafter, the seedlings were transplanted into 7 L plastic containers filled with 5 L of Hoagland’s nutrient solution (¼ strength for macronutrients, full strength for micronutrients) supplied with either 2.5 or 20 nM Cd(NO3)2, at pH 6.0. The free ionic fraction of Cd in the nutrient solution was estimated at 83 % (Visual MINTEQ 3.0, http://vminteq.lwr.kth.se/) for both Cd concentrations. The nutrient solution was aerated by bubbling and was partially renewed every 4 h by a computer-controlled system, at turnover rates adjusted to maintain the pH of the solution between 6 and 7, electrical conductivity above 400 μS cm−1 and the concentration of Cd to 2.5 ± 0.1 nM or to 20 ± 1 nM. These physical-chemical parameters were monitored twice a week using a portable device (WTW Multi 3430 Multiparameter) for pH and conductivity and by GF-AAS to quantify total Cd in the nutrient solution. Turnover rates gradually increased from 6 % day−1 during the first week to 60 % day−1 during the final week of harvest (stage 4). The air temperature used to calculate the growing degree-days was recorded throughout the experiment, together with air humidity and photosynthetic active radiation with a Campbell CR32X data logger. The air temperature varied between 9 °C and 31 °C and the photosynthetic active radiation reached 1300 μmoles cm−2 s−1 on sunny days.
Photosynthetic activity
Leaf photosynthetic activity was assessed at a single stage (sampling 4), from the net CO2 assimilation rate of the 17th fully expanded leaf. Measurements were made using a LI-6400 portable gas exchange system (LI-COR Inc., NE, USA). The net CO2 assimilation rate was obtained at 400 ppm of CO2 and an incident PAR level set at 400 μmol m−2 s−1. The air temperature and relative humidity inside the leaf chamber were maintained at 28 °C and 40 %, respectively.
Root architecture
The root branching index and the length of the apical unbranched zone (LAUZ) of first order lateral roots were determined at a single stage (sampling 1) on 10 extra plants (5 per Cd treatment). Three first order lateral roots were collected from each plant and scanned using a double light scanner (EPSON Expression 10000XL). The images were analyzed with the WinRHIZO software (WinRHIZO pro V.2005a) to determine root length and the number of apices, thereby enabling estimation of the number of laterals per root length unit (i.e. the root branching index) and the length of the apical unbranched zone.
Xylem sap collection and analyses
At harvest, xylem sap was collected from all plants after decapitation of shoots. Collection started at 10 am (± 10 min). Plant shoots were cut just above the cotyledons and the first drop of the stem exudate was discarded. Afterwards, all the drops were collected with a micropipette until 1.5 mL was obtained. The sap was stored in pre-weighted microtubes bathed in ice. At the end of collection, the microtubes were weighed again to determine the exact volume of sap assuming a density of 1 g mL−1. Then, after centrifugation at 16,000 g for 10 min, the supernatants were collected and stored at −20 °C until analysis. The exact duration of sap collection was recorded to calculate the sap exudation rate. The diameter of the stem section was also measured using a calliper. The concentrations of Cd, Cu and Zn in the xylem sap were determined by ICP-MS (Agilent 7700) in a Cofrac certified laboratory (http://www6.bordeaux-aquitaine.inra.fr/usrave) and the concentrations of Ca by flame-AAS (Solaar M6, Thermo Electron). Amino acid composition was determined by reversed phase liquid chromatography, following a method adapted from Chuche et al. (2014). Briefly, 75 μL of xylem sap were freeze-dried, resuspended in 40 μL of 0.1 M HCl and derivatized using o-phthaldialdehyde (OPA) and 9-fluorenylmethyloxycarbonyl (FMOC) before being separated on a ZORBAX Eclipse AAA C18 column at 40 °C, using an Agilent 1100 series with a diode array and fluorescence detectors. The protocol followed the Agilent ZORBAX Eclipse AAA application with phosphate buffer (40 mM, pH 7.8) and acetonitrile/methanol/water mix (45:45:10, v/v/v) as mobile phases.
The free ionic fraction (Cd2+) of Cd in xylem sap was measured at a single stage (sampling 4) on 10 extra plants (5 per Cd treatment), using the method described by Schneider (2006). This method is based on the Cd/Ca exchange properties of a cation exchange resin (Amberlite IR-120) converted to the Ca form. Briefly, 2 mg of Ca resin were added to 1 mL of sap in a 5 mL polyethylene tube. A known amount of radioactive 109Cd2+ was immediately added to the suspension as Cd2+ tracer, and the tube was shaken for 24 h at 20 ± 1 °C on a roller. At equilibrium, the quantity of 109Cd2+ sorbed onto the resin is controlled by the concentration of free Cd2+ in the sap solution and by the concentration of cations in competition, mainly Ca2+. The free ionic Cd fraction was determined from the radioactivity remaining in the solution after contact with the resin, from the total Ca concentration in the solution before and after contact, and from the resin Vanselow selectivity coefficient for Cd/Ca exchange (vKCd/Ca = 0.661) (Schneider, 2006).
Plant sampling and analyses
After the xylem sap was collected, the intact root systems of de-topped plants were first bathed in a 5 L desorption solution (5 mM CaCl2) at 4 °C for 10 min to eliminate the Cd sorbed onto the root apoplast (Buckley et al. 2010). The plants were then divided into roots, stem and leaves. The leaves were cut just below the leaf blade (so that the petioles were part of the stem compartment) and their surface area was measured with an area meter (LI-3100C) to determine the total leaf area of the plant. Shoots (stem and leaves) were washed in two successive baths of deionized water. All the plant samples were dried at 50 °C to constant weight, weighed and milled (Retsch PM 400) before wet digestion in a HNO3/H2O2 mix (1:1, v/v) (MARS-X, CEM corporation), according to the procedure described in Laporte et al. (2015). The concentrations of Cd, Cu, Fe, Mn and Zn in plant digests were determined by ICP-MS (Agilent 7700) by the central analytical service of the University of Basque Country (http://www.ehu.eus).
Estimation of the root Cd absorbing power (α)
Here, the objective was to estimate the mean root absorbing power for Cd (α) and to check whether it changed during vegetative growth or with exposure to Cd. At these low levels of Cd, the Michaelis-Menten kinetics of Cd uptake can be simplified by a linear model (Tynker and Nye 2000) so that the kinetics of Cd influx by plant roots is given by:
where Q Cd is the quantity of Cd2+ absorbed (μg), t is the thermal time (GDD), α is the root Cd absorbing power (L g−1 GDD−1), C s is the concentration of Cd2+ in nutrient solution (μg L−1) and W is the root DW (g). Assuming an exponential growth of roots over thermal time:
Equation (1) gives:
where rootRGR is the relative growth rate of roots (GDD−1) and W 0 is the initial root biomass (g). The root biomass data were first fitted to the experimental growth model (eq. 2) to estimate rootRGR. The variable QCd*rootRGR/Cs was then plotted against the root DW (W(t)), the slope allowing estimation of the root absorbing power α.
Statistical analyses
Non-linear regressions were performed to assess the RGR of roots and shoots from the relationships with thermal time of root and shoot dry weight (Fig. 1). Analyses of variance (ANOVA) and Tukey’s HSD test at 5 % were performed to identify significant differences between phenological stages and between Cd treatments. Analyses of covariance (ANCOVA) were used to test the effect of the level of exposure to Cd on the linear relationships relative to Cd uptake (Fig. 3), Cd partitioning between organs (Fig. 4) and Cd complexation in xylem sap (Fig. 5c). Principal component analysis (PCA) was used to study groups of correlations between the xylem sap variables. SYSTAT 11 Edition 2004 (SPSS Inc., 233S. Wacker Drive, Chicago, USA) software was used for statistical analyses.
Changes in shoot dry weight (a) and root dry weight (b) over thermal time (expressed in growing day-degrees GDD) in sunflower (cv. ES BIBA) grown in hydroponics at 2.5 and 20 nM Cd. The data presented are average values ± one standard deviation (shown as bars in the figure) calculated from five replicates per treatment. The solid lines in (a) and (b) represent the best fit obtained with an exponential model for shoot growth and root growth, respectively, with all the data (n = 40)
Results
Plant growth
No visual symptoms of chlorosis or necrosis were observed in sunflower, even at 20 nM Cd. The root architecture of plants collected at stage 1, characterized by the degree of root branching and by the length of the apical unbranched zone of the first order lateral roots (suppl. Fig. F1), did not differ significantly (P < 0.05) among Cd treatments. The photosynthetic activity measured in the top leaves of 25-day-old plants was the same at 2.5 and 20 nM Cd (suppl. Fig. F2). There was no difference (P < 0.05) in shoot biomass in plants grown at 2.5 and 20 nM Cd (Fig. 1a), in stem height or in total plant dry weight (Table 1). Conversely, their root biomass (P = 0.007), stem diameter (P = 0.001) and total leaf area (P = 0.027) varied with the level of exposure to Cd, and, on average, was higher at 20 nM Cd (Table 1, Fig. 1b). However, biomass partitioning between roots, stem and leaves remained fairly similar at 2.5 nM and 20 nM Cd. The biomass ratio of shoots to roots remained constant throughout vegetative growth: an average of 25 to 30 % of plant dry weight was allocated to roots and 70 to 75 % to shoots. This ratio is close to the ratio obtained by Laporte et al. (2015) in the same sunflower cultivar, also grown in hydroponics. In the shoots, the impact of the phenology on biomass partitioning mainly resulted in an increase, over time, of the relative stem DW, from 34 % of aboveground biomass at stage 1 to 54 % at stage 4. Figure 1 shows an increase over time in shoot and root DW that can be fitted using the exponential function: W(t) = W0 exp. (RGR*t), where RGR is the relative growth rate of roots or shoots, W0 their dry weight at transplanting, and t thermal time. For both shoots and roots, the model parameters did not differ significantly (P > 0.05) between the Cd treatments. As expected, the estimated RGR values for shoots (0.0108 GDD−1) and roots (0.0104 GDD−1) were similar, since their biomass ratio remained constant over time (see above).
Plant Cd
Figure 2 shows that the amount of Cd taken up by sunflower was linearly related to the plant dry weight, with no (or low) intercept. This means that the average concentration of Cd in the plant tissue remained almost constant throughout vegetative growth, as also shown in Table 2. Figure 2 confirms that Cd uptake was higher at 20 nM than at 2.5 nM Cd. As a consequence, the mean concentration of Cd in the plant, estimated from the slope of the corresponding regression line, was higher at 20 nM (0.715 μg g−1 DW) than at 2.5 nM Cd (0.084 μg g−1). The average concentration of Cd in the plant thus increased 8.5 fold when the concentration of Cd2+ in nutrient solution (Cs) increased 8 fold (i.e. from 2.5 to 20 nM). Figure 3 shows the relationship between QCd*rootRGR/Cs and root DW. According to Eq. 2, this plot tests whether the root absorbing power for Cd (α) varied with the age of the plant and with the level of exposure to Cd. A linear relationship with no significant intercept was obtained for both 2.5 and 20 nM Cd. The difference in the slope between the two exposure levels (Fig. 3) was minor and weakly significant (p = 0.039) and it was hard to determine if it was the result of a physiological response. The average value of α estimated for the two exposure levels was 0.0113 L g−1 GDD−1, which, in the cultivar ES BIBA, whose specific root surface area averages 2741 cm2 g−1 DW (Laporte et al. 2015), corresponds to a root Cd absorbing power of 6.7 10−10 L cm−2 s−1 at 20 °C.
Amount of Cd taken up by sunflower (cv. ES BIBA) grown in hydroponics at 2.5 and 20 nM Cd as a function of plant dry weight. The solid line and the dashed line represent the linear regression between the two variables at 2.5 and 20 nM Cd, respectively. The intercept was not significant at 2.5 nM Cd (P = 0.098) but slightly significant at 20 nM Cd (P = 0.046)
Relationship between the variable QCd*root RGR/Cs and root dry weight in sunflower (cv. ES BIBA) grown in hydroponics at 2.5 and 20 nM Cd. QCd stands for the plant uptake of Cd, root RGR for the relative growth rate of roots (taken from Fig. 1) and Cs for the concentration of Cd2+ in the nutrient solution. An ANCOVA showed that the slope of the linear relationship between the two variables differed significantly (P = 0.039) between 2.5 and 20 nM Cd. The solid line represents the best linear fit obtained between the two variables at 2.5 nM Cd (n = 20) and the dashed line the best linear fit obtained at 20 nM Cd (n = 20)
Table 2 shows that the concentration of Cd in aboveground tissues tended to decrease with the growth of the plant, especially between stages 1 and 3. This trend was observed at both Cd exposures but was a little more pronounced at 20 nM Cd. Figure 4a shows that the amount of Cd recovered in plant shoots correlated well with the amount of Cd taken up by plant roots. The intercept of the linear relationship was, however, always significant (P < 0.05). This was due to the lack of linearity at early stages, when the rate of Cd translocation was higher than later on. Analysis of covariance showed that the intercept of the regression differed significantly (P = 0.024) between the Cd exposures, the one at 20 nM Cd (0.413) being higher than the one at 2.5 nM Cd (0.073). However, the slope did not depend on exposure, which means that the same proportion (i.e. 27–28 %) of the Cd taken up by plant roots during the vegetative growth (early stages excepted) was translocated to the shoots, regardless of the level of Cd exposure. Figure 4b shows that the amount of Cd recovered in plant leaves was not linearly correlated with the amount of Cd translocated to plant shoots. The relationships between the two parameters was described adequately using the power function: Cdleaves = a*Cdshoots^b. The fitted value of b was found to be lower than 1 at both levels of exposure to Cd and did not differ significantly (P > 0.05) between Cd treatments.
Amount of Cd translocated to plant shoots as a function of the amount of Cd taken up by the plant roots (a), and the amount of Cd allocated to plant leaves as a function of the amount of Cd translocated to plant shoots (b). In (a), the solid line represents the linear regression obtained at 2.5 nM Cd and the dashed line the linear regression obtained at 20 nM Cd. In (b), the solid line represents the best fit obtained with a power model at 2.5 nM Cd and the dashed line the best fit obtained at 20 nM Cd. An ANCOVA showed that, for the linear relationships shown in (a), the regressions differed significantly (P < 0.001) between 2.5 and 20 nM Cd
Plant Cu, Fe, Mn and Zn
The uptake of Cu, Fe, Mn and Zn was slightly higher when plants were exposed to 20 nM Cd (supplementary Table T1). This increase was significant (P < 0.05) at all phenological stages except stage 1 and ranged from 12 to 41 % for Cu, from 40 to 91 % for Fe, 25–64 % for Mn and from 15 to 63 % for Zn. The partitioning of Cu, Fe, Mn and Zn between plant organs was not affected by the level of Cd exposure (data not shown). As a consequence, the concentration of these four micronutrients in plant tissues was slightly higher at 20 nM Cd, both in roots and shoots (supplementary Table T2).
Xylem sap composition
The rate of xylem sap exudation increased up to stage 3, from 1 to 5–6 mL h−1, and then remained stable until the last sampling (suppl. Fig. F3). The sap flow rate was impacted by exposure to Cd (P = 0.027). It was slightly higher at 20 nM Cd at samplings 3 and 4, possibly as a result of a slight increase in stem diameter (Table 1). The concentrations of Cu and Zn decreased with an increase in the sap flow rate (suppl. Fig. F4). In the case of Cd, this decrease was not easy to discern but could be observed in the sap collected at stages 2, 3 and 4 (suppl. Fig. F5). To account for the variability in the metal composition of the sap caused by the changes in the sap exudation rate, we considered the rate of metal released into the xylem sap (expressed in ng h−1) which corresponds to the sap metal concentration multiplied by the sap flow rate. As expected, the rate of Cd release was affected by the level of exposure to Cd (P < 0.001). On average, the rate of Cd release into the sap was between 0.9 and 1.5 ng h−1 in plants exposed to 2.5 nM Cd, and was 8.8-fold higher in plants exposed to 20 nM Cd (stages 2, 3, 4 considered) (Fig. 5a). In contrast, the rates of Cu and Zn release were similar at 2.5 and 20 nM Cd (suppl. Fig. F4).
Rate of Cd loading in xylem sap collected 195, 244, 301 and 392 GDD after transplanting (i.e. at stages 1, 2, 3, 4) (a), free ionic fraction of Cd (f Cd) measured in xylem sap collected 392 GDD after transplanting (i.e. at stage 4) (b), and relationship between f Cd and the total concentration of Ca in xylem sap collected at stage 4 (c), from sunflower (cv. ES BIBA) grown in hydroponics at 2.5 and 20 nM Cd. An ANCOVA showed that the linear relationship between f Cd and Ca differed significantly (P = 0.013) at 2.5 and at 20 nM Cd. In (a) and (b), the data presented are average values ± one standard deviation (shown as bars in the figure) calculated from the five replicates per treatment. For each phenological stage, *, ** and *** indicate that the value in Cd loading rate (a) and f Cd (b) measured at 20 nM Cd differed from the one measured at 2.5 nM Cd, at the probability level of P < 0.05, P < 0.01 and P < 0.001, respectively. In (c), the solid line represents the linear regression obtained at 2.5 nM Cd (n = 5) and the dashed line the linear regression obtained at 20 nM Cd (n = 5)
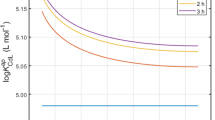
The free ionic fraction of Cd (f Cd) measured in the xylem sap collected at stage 4 did not differ significantly (P > 0.05) between the two levels of Cd exposure, being around 50 ± 25 % (Fig. 5b). Interestingly, the marked differences in f Cd observed between xylem saps were statistically correlated with the concentration of Ca (P < 0.001, r = 0.85) (Fig. 5c). Analysis of covariance showed that the slope of the regression between f Cd and Ca differed significantly (P = 0.013) at the two levels of exposure to Cd. For a given concentration of Ca, the free ionic fraction of Cd was larger at 20 nM Cd. Amino acid (AA) profiling of all xylem saps (supplementary Table T3) showed that glutamine was the main AA in the xylem, as expected from the literature (e.g. Kaiser and Lewis 1980). The PCA in Figure F6 (suppl. Data) shows that the concentration of most AA in the sap was not correlated with the sap exudation rate. The correlation ellipses relative to the two Cd exposure levels overlapped, indicating the amino acid composition of the xylem sap was similar at 2.5 and 20 nM Cd. In contrast, the correlation ellipses relative to the five phenological stages revealed a change in the AA composition of the sap, at least between early and late stages of vegetative growth.
Discussion
Effect of Cd exposure
The increase in the level of Cd exposure did not negatively affect the vegetative growth of sunflower, as evidenced by the similar growth kinetics of shoots observed at 2.5 and 20 nM Cd. The absence of leaf chlorosis, the unchanged CO2 assimilation rate and the concentration of glutamine in the xylem sap suggest that chlorophyll synthesis, photosynthetic performance and nitrogen assimilation, were not altered at 20 nM Cd. Root architecture and free proline level were also used as biomarkers of metal stress, but no differences were observed between 2.5 and 20 nM Cd, either in root branching and elongation or in the concentration of proline in the xylem sap. The fact the sunflower growth was not affected by 20 nM Cd2+ is consistent with results in the literature (e.g. Lopes et al. 2014; Nehnevajova et al. 2009; Stritsis and Claassen 2013), as the first visible symptoms of Cd toxicity were reported to appear at 0.1–1 μM Cd2+ in most sunflower cultivars. Unexpectedly, a slight plant growth promoting effect of Cd was suspected at 20 nM, from the higher root dry weight, stem diameter and leaf surface area observed at this concentration. This so-called hormetic effect of Cd has already been reported (Jia et al. 2013) but only in Cd hyperaccumulators for which a physiological role of Cd is sometimes proposed (Liu et al. 2008). A hormetic effect has also been described in the non-hyperaccumulator Miscanthus (Arduini et al. 2004) but, to our knowledge, this is the first time it is suggested for sunflower. Since root dry weight is one of the parameters that drive nutrient uptake (eq. 1), the increased uptake of Cu, Fe, Mn and Zn observed at 20 nM Cd can be interpreted as a consequence of the hormetic effect of Cd. In the case of Cu, Mn and Zn, this hypothesis is supported by the fact the same relationship was observed between root dry weight and root uptake of these elements at 2.5 and 20 nM Cd (Fig. F7).
The 8-fold increase in the level of Cd exposure led to an 8.5-fold increase in the total plant Cd. In other words, Cd uptake increased in direct proportion to the concentration of Cd2+ in the nutrient solution between 2.5 and 20 nM. In addition, the root Cd absorbing power (α) obtained at 20 nM Cd was very close to that obtained at 2.5 nM Cd (Fig. 3). Taken together, these results support different conclusions regarding the response of sunflower to low doses of Cd. First, Cd exposure at 20 nM did not trigger regulatory mechanisms to limit the Cd influx into the root symplast such as enhanced development of apoplastic barriers (Lux et al. 2011) or enhanced exudation of organic acids (Chiang et al. 2006). These root responses to Cd probably require much higher concentrations of Cd2+ (in the μM range) than those tested in our experiment. Second, they confirm that the protein-mediated transport of Cd through the root cell membrane was not saturated at 20 nM Cd. This was expected from the range of Km values (20–600 nM) reviewed by Lux et al. (2011) for non-hyperaccumulators, and from recent studies (Stritsis and Claassen 2013; Laporte et al. 2013), which showed that the influx of Cd into the root was a linear function of the concentration of Cd2+ in the nutrient solution up to 500 nM in sunflower.
Plants can tolerate an internal concentration of Cd up to a critical level that differs among plant species and organs. In sunflower, the critical level of Cd in leaves, i.e. the Cd concentration in leaves above which a decrease in overall plant growth is observed, has been estimated to be greater than 1 mg kg−1 DW (De Maria et al. 2013). In our experiments, the concentration of Cd in the leaves never exceeded 1 mg kg−1 DW, and is consequently consistent with the absence of effect on plant growth observed at 20 nM Cd. Cd detoxification requires reducing its undesirable interaction with cellular compounds by targeting cellular processes which aim at complexing and sequestrating Cd, particularly in the vacuole, so that most internalized Cd is not present as hydrated ion. These processes occur mostly in roots in non-hyperaccumulators like sunflower. They involve specific chelators like phytochelatins (PCs), specific enzymes like phytochelatins synthase (PCS) and specific transporters like ATP-binding cassette (ABC) transporters (which mediate the storage of the complex PC-Cd in the vacuole), whose synthesis is induced under Cd stress (Clemens 2006; Lin and Aarts 2012; Lux et al. 2011; Verbruggen et al. 2009). One can wonder whether some of the genes involved in Cd homeostasis (e.g. PCS genes) have been over-expressed in sunflower when exposed to 20 nM Cd. If this is the case, this study shows that overexpression did not alter the root-to-shoot partitioning of Cd. Indeed, the translocation of Cd did not decrease with an increase in the concentration of Cd, in contrast with the results of most studies conducted at higher Cd levels (μM range). The rates of Cd release into the xylem sap were in perfect agreement with this finding: they increased in direct proportion to the amount of Cd taken up by plant roots.
A specific investigation was conducted on xylem sap to see whether the speciation of Cd differed between 2.5 and 20 nM Cd. The underlying hypothesis was that the forms of Cd transported in the xylem could impact the partitioning of Cd between stem and leaves, for instance through the preferential fixation of the free ionic form (i.e. Cd2+) onto xylem vessels. The similar f Cd values observed at 2.5 and 20 nM Cd may at least partly explain why the partitioning of Cd between the stem and leaves was not affected by the level of Cd exposure (Fig. 4). On average, half the Cd was complexed by organic ligands in xylem sap. This contrasts with the results of previous studies based on simulated speciation data (e.g. Ueno et al. 2008 for A. halleri) which present Cd2+ as the major form of Cd (>80 %) in the sap. Interestingly, the f Cd values depended to a great extent on the concentration of Ca in the sap. The positive correlation observed between f Cd and total Ca underlines the potential role played by Ca2+ ions (together with H+) in saturating the complexing sites (mainly carboxylic sites) of Cd-binding compounds. The relationship between f Cd and Ca differed with the level of Cd exposure. The larger slope observed at 20 nM Cd suggests that the concentration of Cd-binding compounds in the sap, which consist mainly of the organic acids malate and citrate (Alvarez-Fernandez et al. 2014; Yoneyama et al. 2015), was lower at this Cd concentration. However, this result needs to be checked. The level of histidine and cysteine, also known to be involved in xylem transport of metals (Hazama et al. 2014), was monitored but remained low at both Cd exposure levels (supplementary Table T4).
Effect of phenology
This study focused on vegetative growth, i.e. the interval when maximum plant biomass is produced and the remobilization of photoassimilates is negligible. One goal was to test whether the root Cd absorbing power (α) declined over the period of vegetative growth in sunflower. The goodness of the fit of the experimental data to a constant root power model (Fig. 3) suggests that the root Cd absorbing power did not vary much over time. This could reflect preserved activity of root Cd transporters despite the ageing of root tissues induced by the phenology. The value of α estimated from our work (6.7 10−10 L cm−2 s−1 at 20 °C) was two orders of magnitude lower than that reported for the same sunflower cultivar by Laporte et al. (2013). The main difference between the two protocols was the duration of Cd exposure, which was 2 to 4 weeks in our study but only 20 min in Laporte et al. (2013). The low α can thus be interpreted as a consequence of a Cd2+ depletion around the root occurring in “long-term” experiments, as a result of a diffusion-limited transport of Cd2+ to the root surface (Degryse et al. 2012). The role of other “long-term” processes able to decrease the concentration of Cd2+ in nutrient solution, such as root exudation of organic compounds, should also be mentioned. It is worth noting that the root Cd absorbing power of sunflower (cv. Ikarus) assessed by Stritsis and Claassen (2013) in a similar “long-term” experiment (α = 2.3 10−9 L cm−2 s−1 at 25 °C) was on the same order of magnitude as that calculated at the same temperature in our study (α = 9.1 10−10 cm−2 s−1 at 25 °C).
The second goal was to examine the impact of phenology on the root-to-shoot translocation of Cd during vegetative growth. The linear relationships observed between plant Cd and shoot Cd (Fig. 4a) suggests that the root-to-shoot partitioning of Cd reached a steady state at which a constant fraction (about 27 %) of the Cd taken up was allocated aboveground. This is without considering the early stages (i.e. before the emergence of the 6th leaf) during which the rate of Cd translocation appeared to be more intense, as underlined by the positive intercept of the regressions shown in Fig. 4a. This transitory enhanced translocation of Cd might be the result of incomplete development of apoplastic barriers in the endodermis (Enstone et al. 2002), which may have facilitated the loading of Cd into the xylem. Before endodermis differentiation, the stele is indeed not isolated from the cortex cell layers of the root so that the apoplastic movement of Cd to the xylem and its translocation to the shoots is less restricted. In addition, the development of apoplastic barriers along the root depends on the root environment. In hydroponics, where the root elongation rate is often high, the barriers develop less close to the root apex (Redjala et al. 2011) and the apoplastic movement of Cd to the xylem is suspected to be more pronounced. As a consequence, the more intense translocation of Cd to the shoots at early stages is likely to be exacerbated in hydroponics. We assume that the facilitated translocation of Cd in early growth stages was responsible for the decrease in the concentration of Cd in the shoot over time (Table 2), i.e. for the dilution of Cd in the aboveground parts of the plant over time. The higher intercept of the regression line observed at 20 nM Cd is consistent with a greater uptake of Cd during the early growth stages and may explain why the dilution of Cd was more intense at this level of exposure. It can also be interpreted as a consequence of the time required by sunflower to acclimate to the Cd stress, as reported by Küpper et al. (2009) in the Cd/Zn hyperaccumulator Noccaea caerulescens. This is assuming that the molecular processes involved in the root sequestration of Cd were induced at 20 nM Cd.
According to Nguyen et al. (2015), the dilution of metals in aboveground tissues can also be explained by a decrease in the root-to-shoot ratio in biomass over time. This was however impossible to demonstrate in our experiment in which the shoot dry matter increased in direct proportion to the root dry matter over the period of plant growth (Table 1). In any case, the dilution of Cd aboveground was not due to a decrease in the rate of Cd uptake over time, unlike the results reported by Chen et al. (2008) in lettuce. Neither was it due to an increase in the time course of the relative contribution of structural tissues (e.g. stem, vein) to aboveground biomass, in contrast to that described for the dilution of nitrogen (Plénet and Lemaire 1999).
The third goal was to investigate if phenology affected the aboveground partitioning of Cd during the vegetative stage. As shown in Fig. 4b, the fraction of aboveground Cd allocated to the leaves tended to decrease with plant growth. The relationship between Cd in the shoot and Cd in the leaves was described by a power function with a power of around 0.8 (0.747 at 2.5 nM Cd and 0.812 at 20 nM Cd). Interestingly, the relationship between shoot DW and leaf DW was also adequately described by a power function with a power of around 0.8 (0.805 at 2.5 nM Cd and 0.831 at 20 nM Cd, data not shown). This suggests that the fraction of aboveground Cd allocated to the leaves decreased over time together with the relative contribution of leaves to aboveground biomass. This result is an indicator of coupling between the allocation of biomass and of Cd in sunflower. One consequence is that the dilution of Cd in stem and leaves was of similar intensity over time (Table 2).
Implications for the management of agricultural Cd-contaminated soils
The results of this work show that the growth of the sunflower cultivar ES BIBA is not limited by exposure to levels of Cd commonly encountered in agricultural soils. The main concern in the cultivation of sunflower on agricultural Cd-contaminated soils is thus not linked to the decline in grain yield due to the metal toxicity, but to the production of oil cakes with a concentration of Cd above the European limit for feed. Our work also demonstrated that there is no apparent change in the regulation of Cd uptake and translocation between organs at Cd2+ concentration as high as 20 nM, during vegetative growth. Thus, in the agricultural context, the concentration of Cd in aboveground sunflower tissues increases almost in direct proportion to the concentration of Cd2+ in the porewater, namely the availability of Cd in the soil. This study therefore underlines the importance of managing the availability of soil Cd to control the level of the metal in sunflower oil cake. This is assuming that most of the Cd present in grains at maturity has been remobilized from vegetative tissues and that the efficiency of Cd remobilization is not affected by the level of Cd exposure. These are two questions for which further studies are required. Another challenging question concerns the critical stages of sunflower development during which Cd availability should be controlled more carefully. We can infer from this work that one critical stage is during the period of exponential growth, i.e. when plant uptake of Cd reaches maximum. According to works conducted on cereals, there may also be a need to manage the availability of Cd in the soil during grain filling, notably during the period of rapid accumulation of grain biomass (Harris and Taylor 2013; Rodda and Reid 2011).
This work also helps modelling the soil-to-plant transfer of Cd in agricultural soils. Indeed, for sunflower; we provide the first rules for simulating the uptake and the partitioning of Cd between organs at low levels of exposure to Cd. One can reasonably infer from this work that in this context: (i) uptake of Cd is directly proportional to the concentration of Cd2+ at the root surface, following a root power that remains constant throughout the vegetative stage, (ii) the root-to-shoot partitioning of Cd is not influenced by the level of Cd exposure and reaches an equilibrium at which a constant fraction of the Cd taken up is allocated to aboveground plant parts, and (iii) the aboveground partitioning of Cd between stem and leaves is not altered by the Cd exposure level and follows that of dry matter. These rules remain to be validated in soil where root growth is restrained and the root-to-shoot ratio in biomass varies with the age of the plant. It is worth noting that Lovy et al. (2013) extracted fairly similar rules for Cd uptake and partitioning between organs for the hyperaccumulator Noccaea caerulescens exposed to micromolar concentrations of Cd. This similarity between plants that differ considerably in their ability to tolerate and accumulate Cd can be interpreted as evidence for genericity. The above mentioned rules for sunflower may therefore be valid for other higher plant genera and species exposed to non-toxic levels of Cd. However, particular care should be taken to describe the partitioning of Cd at early stages, when the rules of Cd allocation between roots and shoots appear to differ from those that prevail later on.
Finally, this study raises the question of the dependence of the speciation of Cd in the xylem sap on the level of Cd exposure. Further research is required to test this dependency and to better understand how it could impact the partitioning of Cd between stem and leaves.
References
Alvarez-Fernandez A, Diaz-Benito P, Abadia A, Lopez-Millan AF, Abadia J (2014) Metal species involved in long distance metal transport in plants. Front Plant Sci 5:105. doi:10.3389/fpls.2014.00105
Arduini I, Masoni A, Mariotti M, Ercoli L (2004) Low cadmium application increases miscanthus growth and cadmium translocation. Environ Exp Bot 52:89–100
Balestri M, Ceccarini A, Forino LMC, Zelko I, Martinka M, Lux A, Castiglione MR (2014) Cadmium uptake, localization and stress-induced morphogenic response in the fern Pteris vittata. Planta 239:1055–1064
Buckley WT, Buckley KE, Huang JZ (2010) Root cadmium desorption methods and their evaluation with compartmental modeling. New Phytol 188:280–290
Chen WP, Li LQ, Chang AC, Wu LS, Kwon SI, Bottoms R (2008) Modeling uptake kinetics of cadmium by field-grown lettuce. Environ Pollut 152:147–152
Chiang PN, Wang MK, Chiu CY, Chou SY (2006) Effects of cadmium amendments on low-molecular-weight organic acid exudates in rhizosphere soils of tobacco and sunflower. Environ Toxicol 21:479–488
Christensen TH, Haung PM (1999) Solid phase cadmium and the reaction of aqueous cadmium with soil surfaces. In: McLaughlin MJ, Singh AK (eds) Cadmium in Soils and Plants. Kluwer Academic Publishers, Dordrecht
Chuche J, Desvignes E, Bonnard O, Thiery D (2014) Phenological synchrony between Scaphoideus titanus (Hemiptera: Cicadellidae) hatchings and grapevine bud break: could this explain the insect’s expansion? B Entomol Res 105:82–91
Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88:1707–1719
Clemens S, Aarts MGM, Thomine S, Verbruggen N (2013) Plant science: the key to preventing slow cadmium poisoning. Trends Plant Sci 18:92–99
Custos J-M, Moyne C, Treillon T, Sterckeman T (2014) Contribution of Cd-EDTA complexes to cadmium uptake by maize: a modelling approach. Plant Soil 374:497–512
Dauguet S, Lacoste F (2013) Les enseignements de 7 années de plan de surveillance des oléagineux. OCL 20:119–123. http://dx.doi.org/10.1684/ocl.2012.0476
De Maria S, Puschenreiter M, Rivelli AR (2013) Cadmium accumulation and physiological response of sunflower plants to Cd during the vegetative growing cycle. Plant Soil Environ 59:254–261
Degryse F, Shahbazi A, Verheyen L, Smolders S (2012) Diffusion limitations in root uptake of cadmium and zinc, but not nickel, and resulting bias in the Michaelis constant. Plant Physiol 160:1097–1109
di Toppi LS, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41:105–130
EFSA (2009) EFSA (European Food Safety Authority) (2009) Scientific Opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on cadmium in food. EFSA J 980:1–139
Enstone DE, Peterson CA, Ma FS (2002) Root endodermis and exodermis: structure, function, and responses to the environment. J Plant Growth Regul 21:335–351
Grant CA, Clarke JM, Duguid S, Chaney RL (2008) Selection and breeding of plant cultivars to minimize cadmium accumulation. Sci Total Environ 390:301–310
Harris NS, Taylor GJ (2013) Cadmium uptake and partitioning in durum wheat during grain filling. BMC Plant Biol 13:103. doi:10.1186/1471-2229-13-103
Hazama K, Nagata S, Fujimori T, Yanagisawa S, Yoneyama T (2014) Concentrations of metals and potential metal-binding compounds and speciation of Cd, Zn and Cu in phloem and xylem saps from castor bean plants (Ricinus communis) treated with four levels of cadmium. Physiol Plant. doi:10.1111/ppl.12309
Helmke PA (1999) Chemistry of cadmium in soil solution. In: McLaughlin MJ, Singh BR (eds) Cadmium in soils and plants. Kluwer Academic Publishers, Dordrecht
Hough RL, Young SD, Crout NMJ (2003) Modelling of Cd, Cu, Ni, Pb and Zn uptake, by winter wheat and forage maize, from a sewage disposal farm. Soil Use Manag 19:19–27
Jia L, He XY, Chen W, Liu ZL, Huang YQ, Yu S (2013) Hormesis phenomena under Cd stress in a hyperaccumulator–Lonicera japonica Thunb. Ecotoxicology 22:476–485
Kaiser JJ, Lewis OAM (1980) Nitrate-Nitrogen assimilation in the leaves of Helianthus annuus L. New Phytol 85:235–241
Küpper H, Kochian LV (2009) Transcriptional regulation of metal transport genes and mineral nutrition during acclimatization to cadmium and zinc in the Cd/Zn hyperaccumulator, Thlaspi caerulescens (Ganges population). New Phytol 185:114–129
Laporte MA, Denaix L, Pagès L, Sterckeman T, Flénet F, Dauguet S, Nguyen C (2013) Longitudinal variation in cadmium influx in intact first order lateral roots of sunflower (Helianthus annuus. L). Plant Soil 372:581–595
Laporte MA, Sterckeman T, Dauguet S, Denaix L, Nguyen C (2015) Variability in cadmium and zinc shoot concentration in 14 cultivars of sunflower (Helianthus annuus L.) as related to metal uptake and partitioning. Environ Exp Bot 109:45–53
Lin YF, Aarts MGM (2012) The molecular mechanism of zinc and cadmium stress response in plants. Cell Mol Life Sci 69:3187–3206
Lin Z, Schneider A, Nguyen C, Sterckeman T (2014) Can ligand addition to soil enhance Cd phytoextraction? A mechanistic model study. Environ Sci Pollut Res 21:12811–12826
Liu MQ, Yanai JT, Jiang RF, Zhang F, McGrath SP, Zhao FJ (2008) Does cadmium play a physiological role in the hyperaccumulator Thlaspi caerulescens? Chemosphere 71:1276–1283
Lopes CA, Mazzafera P, Arruda MAZ (2014) A comparative ionomic approach focusing on cadmium effects in sunflowers (Helianthus annuus L.). Environ Exp Bot 107:180–186
Lovy L, Latt D, Sterckeman T (2013) Cadmium uptake and partitioning in the hyperaccumulator Noccaea caerulescens exposed to constant Cd concentrations throughout complete growth cycles. Plant Soil 362:345–354
Lux A, Martinka M, Vaculík M, White PJ (2011) Root responses to cadmium in the rhizosphere: a review. J Exp Bot 62:21–37
Nehnevajova E, Schmuelling T, Herzig T, Schwitzguebel JP (2009) Increased tolerance of sunflower mutant seedlings to Cd and Zn in hydroponic culture. Agrochimica 53:353–366
Nguyen C, Soulier AJ, Masson P, Bussière S, Cornu JY (2015) Accumulation of Cd, Cu and Zn in shoots of maize (Zea mays L.) exposed to 0.8 or 20 nM Cd during vegetative growth and the relation with xylem sap composition. Environ Sci Pollut Res. doi:10.1007/s11356-015-5782-y
Plénet D, Lemaire G (1999) Relationships between dynamics of nitrogen uptake and dry matter accumulation in maize crops. Determination of critical N concentration. Plant Soil 216:65–82
Redjala T, Zelko I, Sterckeman T, Legue V, Lux A (2011) Relationship between root structure and root cadmium uptake in maize. Environ Exp Bot 71:241–248
Rodda MS, Li G, Reid RJ (2011) The timing of grain Cd accumulation in rice plants: the relative importance of remobilisation within the plant and root Cd uptake post-flowering. Plant Soil 347:105–114
Sauvé S, Norvell WA, McBride M, Hendershot W (2000) Speciation and complexation of Cd in extracted soil solutions. Environ Sci Technol 34:291–296
Schneider A (2006) Adaptation of the ion exchange method for the determination of the free ionic fraction of cadmium in solution. J Environ Qual 35:394–401
Simon L (1998) Cadmium accumulation and distribution in sunflower plant. J Plant Nutr 21:341–352
Stritsis C, Claassen N (2013) Cadmium uptake kinetics and plants factors of shoot Cd concentration. Plant Soil 367:591–603
Tinker PB, Nye PH (2000) Solute movement in the rhizosphere. Oxford University Press, Oxford
Ueno D, Iwashita T, Zhao FJ, Ma JF (2008) Characterization of Cd translocation and identification of the Cd form in xylem sap of the Cd-hyperaccumulator Arabidopsis halleri. Plant Cell Physiol 49:540–548
Verbruggen N, Hermans C, Schat H (2009) Mechanisms to cope with arsenic or cadmium excess in plants. Curr Opin Plant Biol 12:1–9
Yoneyama T, Ishikawa S, Fujimaki S (2015) Route and regulation of zinc, cadmium and iron transport in rice plants (Oryza sativa L.) during vegetative growth and grain filling: metal transporters, metal speciation, grain Cd reduction and Zn and Fe biofortification. Int J Mol Sci 16:19111–19129
Acknowledgments
This work was supported by funding from ANR 2011 CESA 008 01. The authors are grateful for technical and human support provided by SCAB (PhD Juan Carlos Raposo) from SGIker of UPV/EHU.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jian Feng Ma.
Electronic supplementary material
ESM 1
(DOC 316 kb)
Rights and permissions
About this article
Cite this article
Cornu, J.Y., Bakoto, R., Bonnard, O. et al. Cadmium uptake and partitioning during the vegetative growth of sunflower exposed to low Cd2+ concentrations in hydroponics. Plant Soil 404, 263–275 (2016). https://doi.org/10.1007/s11104-016-2839-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2839-8