Abstract
Aims
The principal objective was to evaluate the interference by the invasive species, H. pilosella, on native grassland species at the physiological performance level. We hypothesised that the invasive species is able to alter the nitrogen uptake of native plant species, and can modify community functioning.
Methods
This study was performed under field conditions in the Magellanic Steppe (Argentina). We compared stable isotope signatures, nutrient content and several functional physiological traits in four grassland species with and without H. pilosella interference.
Results
We found significant interference effects from the invasive species on native species, mostly through changes in nitrogen uptake. The variation in the natural abundance of foliar δ15N suggests that the native plants switched nitrogen sources due to interference with the exotic species. A linear relationship between chlorophyll and proline content that disappears when species are under H. pilosella interference, suggests major changes in the N allocation of native species. Grassland species under interference with exotic species exhibit lower photochemical efficiency and higher water use efficiency. Canonical discriminant analysis evidenced the existence of a different set of functional traits between invasive and native plants, and also among native species with and without H. pilosella interference.
Conclusions
Our results support the hypothesis that H. pilosella exerts intense interference with native species through shifting the N sources available for native species, a lower leaf N content, and increasing water stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biological invasions occur when natural communities are threatened by an increase in distribution and abundance of exotic species that cause significant changes in the ecosystem structure and functioning (Müller-Schärer et al. 2004). The invasion of natural ecosystems by alien species leads to a reduction of biodiversity and modifies the function and economic sustainability of ecosystems (D’Antonio and Vitousek 1992; Levine et al. 2003; Asner and Vitousek 2005).
Invasive species present structural and functional traits that enable a great capacity to compete for limited resources, such as water and nutrients, and can also cause significant changes in soil composition and in nutrient cycles (Kourtev et al. 2002; Levine et al. 2003). Many studies have shown that the invasive success of certain introduced plants and their ecological impacts on both native communities and ecosystem functioning depends on plant traits, environmental characteristics of the receptive area, and biological interactions with native organisms (Ordonez et al. 2010; Ehrenfeld 2010; Bottollier-Curtet et al. 2013).
Most studies have attempted to assess patterns of invasion on different spatial scales (Day and Buckley 2011); there are nevertheless only a handful of studies which deal with physiological responses caused by interference and competition between native and alien species under natural conditions (see Funk and Vitousek 2007; Gilgen et al. 2010; Rascher et al. 2012). These studies provide, in part, a key to the understanding of the mechanisms behind the invaders’ success in new communities. Particularly, in resource-poor environments, plants possess a set of traits associated with resource conservation, such as a long leaf lifespan, a high concentration of defence compounds, low tissue-nutrient content or thicker leaves (Vitousek 1982; Coley et al. 1985). In a comparative study, Funk and Vitousek (2007) have shown that invasive species can outperform natives on short-timescale functions such as photosynthetic activity; in contrast, invasive and native species were similarly efficient in a time-integrated scale (as shown by stable isotope tracers) and invasive species may persist under conditions of continued low resource availability. There is therefore a current need to study the effect of the invasion at physiological levels on different spatial and time scales. Competition experiments in grassland ecosystems usually point out that exotic species have higher competitive ability than the native species (see Bottollier-Curtet et al. 2013). Nevertheless, the results of competition have been shown to depend strongly on the environmental conditions (Daehler 2003; Radford et al. 2006). For instance, Davidson et al. (2011) showed that native grassland species record a smaller decline in fitness than invasive exotic species only when resources are limited or conditions stressful.
Invasion processes in plant communities affect photosynthesis, primary productivity, herbivore forage quality, leaf litter and soil organic matter decomposition and, ultimately, plant-mediated nutrient and carbon cycling pathways and rates (Shaver et al. 2001; Aerts et al. 2006; Dorrepaal et al. 2007). It is therefore critical to understand the sensitivity of native sub-Antarctic plant communities to the invasion of alien species, as these ecosystems accumulate large stocks of soil carbon due to their slower organic matter decomposition caused by high precipitation and low temperatures (Collantes et al. 1999).
The herb Hieracium pilosella L. (Mouse-ear hawkweed, Asteraceae) recently (around 20 years ago) invaded the northern Tierra del Fuego rangelands (Livraghi et al. 1998; Cipriotti et al. 2010; Rauber et al. 2013). This species is a known invader of grasslands in different parts of the world, where it rapidly forms mono-specific patches due to vegetative, sexual, and apomictic reproduction (Bishop and Davy 1994). H. pilosella usually ends up forming large patches, replacing native flora, and decreasing palatable forage biomass and secondary productivity (Johnstone et al. 1999; Makepeace et al. 1985; Treskonova 1991). H. pilosella is already a strong grassland invader in North America (Bishop and Davy 1994), New Zealand (Meurk et al. 2002), and Chile (Domínguez 2004).
It has been shown that H. pilosella has a more labile litter with a higher decomposition rate than the dominant native species in the Fuegian steppe (Braun 2009). In New Zealand, H. pilosella patches grow leaving a halo, which has been explained as a nutrient depletion effect that leaves bare soil (McIntosh et al. 1995). It is thus evident that one direct impact of the invasion by H. pilosella involves changes in nutrient cycling; however, studies focusing on leaf nutrient and carbon dynamics of this invasive species are still largely lacking. To the best of our knowledge, only Radford et al. (2006) have studied the effect of nutrient stress and performance of invasive Hieracium lepidulum. They concluded that this species grows in habitats of intermediate fertility and stress, since it is tolerant to ammonium as an alternative source of nitrogen while other species are not.
Nitrogen resources are known to exhibit spatial and temporal variability and are one of the key factors that control the outcome of plant-plant interactions and that trigger changes in community composition (Wilson and Tilman 1991; Clark et al. 2007). In particular, N-source utilisation is a dynamic process which can vary on a small scale even within one plant community (Stahl et al. 2011). The origin of plant nitrogen (e.g. atmospherically derived, organic derived or soil derived) can be tracked by investigating the concentrations of the stable isotope 15N, when δ15N signatures of the potential sources are clearly distinguishable from one another. Plants that take up nitrogen from sources with a certain 15N signature will normally obtain a signature that closely resembles that of the source (Robinson 2001). In this context, the natural abundance of foliar δ15N signatures has been used to assess the impact of exotic species on the N cycle of native species (Rascher et al. 2012; Gilgen et al. 2010).
The main goal of this study was to evaluate the effect of plant interference between native and exotic invader species on key physiological traits related to water, light, and nutrient uptake in Fuegian grassland communities. We compared plant traits between patches with and without interference with H. pilosella under field conditions and addressed the following questions: (i) Does interference with H. pilosella alter nutrient utilisation in Fuegian native species? (ii) Does interference with H. pilosella alter photochemical and water-use efficiency of native species? and (iii) Does the invasive species have similar or dissimilar functional traits to the native species?
Materials and methods
Site description
The Fuegian steppe covers about 5000 km2 from the Strait of Magellan to approximately 54°S in Tierra del Fuego Island. In a regional context, the area can be viewed as a transition between arid Patagonia and humid sub-Antarctic climates. The climate of the Magellanic steppe is chilly, windy and cloudy; the average temperature during the coldest month (July) is 0 °C, and during the warmest month (January) this is 10 °C. Mean annual precipitation is evenly distributed throughout the year, ranging from 300 to 450 mm per year (NE to SW gradient, Koremblit and Forte Lay 1991). The water balance has a noticeable deficit in December and January, to a great extent due to the high wind speeds (30 km/h), producing high potential evaporation rates (Walter and Box 1983). Vegetation has been classified as a humid grass steppe (León et al. 1998; Collantes et al. 2005).
We conducted our study in the Estancia Cullen, close to San Sebastian Bay in the NE part of Tierra del Fuego Island (52°53′S 68°27′O). The main land use is extensive sheep breeding; vegetation consists of a grassland co-dominated by an open tussock layer of Festuca gracillima (Rothm.) interspersed in a matrix of short grasses, such as Poa spiciformis (Steud) Hauman and Parodi and Hordeum pubiflorum (Hooker f.) among other graminoids, and some prostrate forbs, such as Acaena pinnatifida Ruiz and Pavón, Perezia recurvata (Vahl) Less. and Trifolium repens L. (a non-invasive N-fixer exotic species appreciated by sheep).
Study species
H. pilosella (Mouse-ear hawkweed) is a small yellow-flowered herb belonging to the Asteraceae family, native to Europe and Eastern Asia. It is a perennial plant, with a basal rosette of leaves, and glandular hairs covering all vegetative organs. The flowering stem sprouts from the centre of the basal rosette and the flower-heads are born singly onto the scape. H. pilosella grows in dense patches, covering the soil surface and excluding other species. It produces stolons that generate a new rosette at their extremity, and can develop into a new clone forming dense patches in an open space. The species may also produce seeds, sexually or asexually generated (Krahulcova and Krahulec 1999) that are mostly dispersed by wind. It is also an allelopathic plant (Makepeace et al. 1985; Petrovic et al. 1999; Scott et al. 2001). H. pilosella is avoided by sheep livestock due to its prostrate growth-form, highly pubescent leaves, and high foliar concentrations of secondary metabolites (Bishop and Davy 1994; Rose et al. 1995). However, according to the observed distribution and composition of the patches, it appears that sheep grazing of the native species under the current study conditions remains differentially unaffected by the plant-plant interferences. Invasive patches offer no protection to native plant species because of the extreme prostrate growth form of H. pilosella, thus the location of native species is constrained to the border of invading patches. On the other hand, the invading patches (1–2 m diameter) are interspersed in a matrix of tussock and short grasses separated by 5–10 m at least, so sheep can easily forage on native species. In addition, dietary studies performed in the same paddock reflect the occurrence of H. pilosella in sheep faeces.
H. pilosella is widely distributed across the entire Magellanic steppe in Tierra del Fuego, but with a low mean regional cover, suggesting that the invasion is in its initial stages (Cipriotti et al. 2010). Nevertheless, it is already possible to find hot spots of invasion related to a previous history of massive soil disturbances (e.g., the building of roads or oil pipelines, shrub removal, and pasture sowing) that were able to create bare ground patches, which probably favoured the colonization by this alien species (Cipriotti et al. 2010).
To evaluate the interference effects of H. pilosella, we selected species that are able to outcompete H. pilosella (Scott and Sutherland 1993). We selected four non-invasive foraging species (three native and one naturalized, all of which are commonly consumed by sheep). The four study species (hereafter referred to as “native species”) were: the tussock grass F. gracillima, selected due to being the most abundant and conspicuous in the community and thus representative of the tussock grassland; the short grass P. spiciformis (Poaceae), representative of the lawn and livestock’s favourite (the most abundant and important grass forage source in these rangelands); the prostrated forb, A. pinnatifida (Rosaceae), chosen since it is the most abundant and important forb forage source and for its different life-form; and the non-invasive and naturalized legume T. repens (Fabaceae), also chosen thanks to its different life-form strategy.
Experimental design
The field sampling was conducted during December, corresponding to the early summer season. H. pilosella grows in dense monospecific patches, thus we considered that the interaction with the other species would mainly take place in the border of the patches.
Ten patches of H. pilosella were randomly selected; patches were 1–2 m in diameter and 5–25 m distant from each other. In the border of the selected patches we marked 3 ramets of each of the four native species and of H. pilosella. Due to the difficulty in differentiating individual plants of the study species, we identified ramets; these were defined following Harper (1977), as the unit of clonal growth that may follow an independent life if severed from the parent plant. In total, 30 ramets were selected of each interacting species growing in the border of the patches and 30 H. pilosella ramets. For controls in H. pilosella 30 ramets in the centre of the patches, were selected. For controls for each native study species, we selected another 30 ramets without interference with H. pilosella; in this case the control patches were intermixed into Festuca-Acaena and Poa-Trifolium (we used a total of 20 patches, 10 for each intermixed patch). We averaged together the 3 ramets within each patch for each species and the patch was treated as a block (5 species × 2 interference levels, N = 10) to take into account possible microsite heterogeneity effects.
Field measurements
In each of the selected ramets, we determined chlorophyll fluorescence kinetics of leaves in situ at midday (12:00–14:00 h solar time) by means of the pulse-amplitude modulation technique using a portable fluorometer (mini-PAM, Walz, Germany). The maximum quantum yield of PSII was determined from the ratio of variable to maximal fluorescence, i.e. Fv/Fm=(Fm−Fo)/Fm, where Fo and Fm are initial and maximal fluorescence of dark-adapted leaves after 15 min, a period found to be sufficient to allow complete reoxidation of the PSII reaction centres.
We calculated intrinsic water use efficiency (WUEi) as the ratio of net photosynthesis to stomatal conductance (WUEi=A/gs), using a portable compact CO2/H2O system (LCi-portable photosynthesis ADC system, UK). Due to the difficulty of estimating leaf surface in species such as P. spiciformis and A. pinnatifida, data on net photosynthesis and leaf conductance are not shown. Gas exchange measurements were carried out during the morning (9:00–10:00 h solar time) when maximum photosynthetic activity takes place.
For chlorophyll fluorescence and gas exchange measurements, we selected three leaves per species within each patch (30 shoots × 5 species × 2 interference levels) and then averaged the results for each patch (N = 10 per species and interference level). All measurements were conducted on the current year’s fully expanded leaves from the same cohort.
Laboratory analysis
We collected sun-exposed, fully expanded leaf samples from each study patch and species (2 g of pooled leaves × 5 species × 10 patches × 2 interference levels, N = 10) and separated them into two subsets. One subset was frozen in liquid nitrogen upon arrival at the laboratory and was kept at –24 °C until analysis of photosynthetic pigments and proline content. The other subset was kept refrigerated and was employed to calculate relative water content following Saura-Mas and Lloret (2007): RWC (%) = ((Mf−Md)/( Mt−Md)) × 100; where Mf is fresh mass, Mt turgid mass, and Md dry mass. Fresh mass of leaves was determined on arrival to the laboratory, after which they were watered and kept refrigerated for 24 h. After having carefully removed water from the surface, we measured turgid mass (Mt). Leaf samples were then dried for 48 h at 70 °C, and weighed for the leaf dry mass (Md) measurement. The leaf dry matter content (LDMC) was calculated following Garnier et al. (2001) and Saura-Mas and Lloret (2007) as: LDMC (mg g−1) = Md/Mt.
We extracted photosynthetic pigments from the frozen-kept leaves with 100 % acetone and determined their concentrations spectrophotometrically following Lichtenthaler (1987). Chlorophyll a and b, total chlorophyll, and carotenoids content were calculated on a dry weight basis.
The free proline content is determined either as a measure of stress or as osmotic regulation in plants (Hare and Cress 1997). We measured proline concentration colorimetrically by the ninhydrin acid method of Bates et al. (1973). Proline concentration of the frozen leaves was calculated on a dry weight basis using L-proline from the standard curve. For details see Ain-Lhout et al. (2001).
Stable isotope analysis
We collected leaves from the refrigerated-kept subset and oven-dried them at 60 °C for 48 h. They were then milled to a fine powder for isotopic analysis. Leaf N and C concentrations and δ15N and δ13C signatures of the leaf material were determined in separate runs for N and C using an elemental analyser (Carlo Erba EA, Hekatech, Wegberg, Germany) for combustion, coupled with a continuous-flow isotope ratio mass spectrometer SIRA II (VG-Isotech, Middlewich, UK). Samples were standardised to IAEACH-4 and IAEA-CH-6 (International Atomic Energy Agency, Vienna, Austria). The precision of the repeated measurement was 0.05 ‰. Carbon isotope ratios are reported relative to Vienna Pee Dee Belemnite carbonate standard (VPDB):
where Rsample and Rstandard refer to the 13C/12C ratios of the plant sample and of the VPDB standard, respectively. In accordance with the classic two-step discrimination model (Farquhar et al. 1982), 13C/12C discrimination (Δ13C (‰)) was calculated as:
As study plants were in natural conditions, we assumed a δ13C value for atmospheric CO2 of −8 ‰. All the selected species have C3 photosynthetic pathway.
Nitrogen concentration and δ15N were measured against the ammonium sulphate standard (IAEA, N2). N isotope ratios are reported per mil (‰) relative to atmospheric N2 (Shearer and Kohl 1993) as:
where Rsample presents the isotope ratio (15N/14N) in sample foliage, and Ratmos is 15N/14N for atmosphere N2 as standard. Standard deviation of 10 repeated samples was < 0.2 % for δ15N.
Statistical analysis
In order to assess the effect of H. pilosella interference on the native species’ functional performance, we conducted a two-way ANOVA to analyse the effect of species and interference level in plant attributes (for LDMC, RWC, pigment and proline concentrations, N, C/N, Δ13C, δ15N, Fv/Fm, and A/gs). When necessary, individual comparisons between species were performed with the Tukey test. To assess the effect of H. pilosella at the species level, we conducted a one-way ANOVA for each individual species. The same analysis was performed for comparisons of monospecific patches of H. pilosella in the centre and at the border of patches.
Pearson correlations were made to test pairwise relationships between plant trait variables. As a more integrated approach, we conducted a multivariate analysis of variance (MANOVA) and a canonical discriminant analysis (CDA) to evaluate the global effect of the interference with H. pilosella within the community, and took into account the measured variables (LDMC, RWC, pigment and proline concentrations, N, C/N, Δ13C, δ15N, Fv/Fm and A/gs).
The assumption of normality was tested with the Kolmogorov-Smirnov test. All statistical analyses were performed with the SPSS 18 software package (Chicago, IL, USA).
Results
Effects of the interference with H. pilosella on native grassland traits
The results of two-way ANOVA (Table 1) show that the effect of H. pilosella interference was significant for the following functional traits: δ15N, C/N ratio, leaf proline content, and WUEi. The effect of H. pilosella interference was species-specific (species x interference level) for δ15N, LDMC, Fv/Fm, and for leaf proline and chlorophyll and carotenoids content. There were significant differences among species for all functional traits.
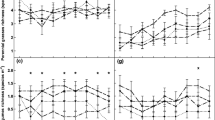
Nitrogen content tended to decrease under interference with H. pilosella in all species except F. gracillima, although this trend was not statistically significant (Fig. 1a). Leaf N concentrations varied three- to four-fold among the species, independently of the interference level, where the lowest was in F. gracillima and the highest in T. repens (as expected for an N-fixing species) (P < 0.001, Fig. 1). An expected opposite pattern was observed in the C/N ratio, with the lowest values in T. repens (Fig. 1b). Under interference with H. pilosella, there was a general trend of significantly higher C/N ratios (P < 0.05, Table 1, Fig. 1b). Natural abundance of N15 (δ15N) in T. repens was close to 0 ‰, as expected for a legume. There was a significant increase in δ15N values in plants subjected to interference by H. pilosella (P = 0.011, Table 1, Fig. 1c). Stable carbon isotope discrimination (Δ13C) showed significant differences between species (P = 0.002), but the effect of H. pilosella interference was not significant (Table 1, Fig. 1d).
Leaf proline content exhibited significant differences between species (P < 0.001), interference level (P < 0.001), and in the interference level x species effect (P < 0.001, Table 1, Fig. 2a). Under H. pilosella interference, the overall pool of native species showed a significantly lower leaf proline content in relation to control plants (13.04 ± 0.87 and 53.01 ± 8.94 μmol g−1, for interfered and control plants, respectively).
Free proline content (a) and Chlorophyll versus proline content (b) in leaves of grassland species with (grey bars and open markers) and without (black bars and filled markers) H. pilosella interference. Significant differences in response to interference within each species are marked with asterisks (* P < 0.005; ** P < 0.001)
Total chlorophyll content showed significant differences among species (P < 0.001) and in the species x interference level effect (P = 0.002, Table 1, Fig. 3a). All species except T. repens showed an effect of the interference with H. pilosella on chlorophyll content. A similar variable pattern was found for leaf carotenoid content, with significant differences between species (P < 0.001) and in the species x interference level effect (P < 0.001, Table 1). Total leaf chlorophyll was significantly correlated with proline content only in control plants (R2 = 0.79, P < 0.01, Fig. 2b) but not in the patches with H. pilosella interference.
Average and standard deviations for chlorophyll content (a) and maximum quantum yield (B, Fv/Fm) for leaves of grassland species with (grey bars) and without (black bars) H. pilosella interference. Significant differences in response to interference within each species are marked with asterisks (* P < 0.05; ** P < 0.001)
Maximal photochemical efficiency (Fv/Fm) showed significant differences among species (P < 0.03) and in the species x interference level (P < 0.028, Table 1, Fig. 3b).
Water use efficiency (WUEi) exhibited a significant general increase when plants interfered with H. pilosella (P < 0.027); and among species (P < 0.001), but the effect of species x interference level was not significant (Table 1, Fig. 4a).
Average and standard deviations for intrinsic water use efficiency (A, WUEi, calculated as A/gs) and relative water content (B, RWC) for leaves of grassland species with (grey bars) and without (black bars) H. pilosella interference. Significant differences in response to competition within each species are marked with an asterisk (*P < 0.05)
Leaf RWC only presented significant differences among species (P < 0.001, Table 1, Fig. 4b). LDMC presented significant differences both among species (P < 0.001) and in the effect between species and interference level (P < 0.001, Table 1).
The comparison of functional plant traits between centre (controls: no interspecific interference) and border (interference with native species) patches of H. pilosella revealed a significant increase in Δ13C (P < 0.01), F v /Fm (P < 0.01) and RWC (P < 0.02, Table 2).
Community-level effects of H. pilosella interference
CDA results confirmed the existence of differences in the set of studied functional traits between H. pilosella, the native species growing without exotic interference (controls), and the native plants growing with H. pilosella interference (Fig. 5). The first CDA function separates the invasive from the native species well, while the second CDA function separates the interference with H. pilosella from native species well (Fig. 5). Nitrogen content and LDMC were the traits most strongly related to the first CDA function (coefficients 1.159 and 1.777, respectively), while proline content was better related to the second CDA function (coefficient -1.220). These results were also confirmed by the MANOVA, which showed significant differences between interference level when all the measured variables for the pooled native species were taken into account (F = 6.621, P < 0.001).
Observations in the 2D discriminant space resulting from CDA (Canonical discriminant analysis). Each plant species is represented with a different marker. The open and filled markers in black represent the interference level (open markers: interfered with H. pilosella; filled markers: non-interfered); the invasive species is indicated in grey. As before, open markers indicate interference with the invasive species (i.e. measures were taken in the centre of invaded patches, thereby reflecting the occurrence of intra-specific interference) and filled markers indicate interference with native species (i.e. measures were taken in the border of invaded patches, thereby reflecting the presence of inter-specific interference)
Discussion
The results of this study show that H. pilosella is interfering intensively (probably through competition) with native grassland species, thereby reducing their functional performance. H. pilosella interference induced changes that mainly involved the nitrogen balance in the native grassland species lowering leaf proline content, changing leaf pigments and N sources, which, in turn, led to lower photochemical efficiency. But more interestingly, the response to H. pilosella interference was species-specific.
Using δ15N as an ecological tracer, we were able to show for the first time how H. pilosella can change conditions at the ground level that lead to competition both at the species and community level, through effects in the N cycle of the native species. Our results agree with the effects of H. pilosella recorded in New Zealand (McIntosh et al. 1995), which showed that this invasive species has a strong potential for the progressive modification of grassland soils through intense competition for resources.
Although there were no significant changes in N content in leaves of native plants growing with or without H. pilosella interference, there was a general significant increase in C/N (Fig. 1b), as shown by the two-way ANOVA (Table 1). Moreover, the effect of H. pilosella involved a change in the native species’ N cycle, since it modified the nitrogen forms used by native plants. The δ15N values of F. gracillima, A. pinnatifida, and P. spiciformis were always positive and were significantly increased under interference with the invasive species; this trend was significant when considering all species, and also remained significant at the species level in the case of F. gracillima and P. spiciformis (Table 1, Fig. 1c). The δ15N values of T. repens were close to 0 ‰ in all cases; as a legume this species should have a direct access to atmospheric N2 (Robinson 2001).
In Tierra del Fuego grasslands, the presence of a halo around H. pilosella patches has not been observed (field observation), as occurs in New Zealand (McIntosh et al. 1995). It is nonetheless probable that the difference in δ15N, in invaded versus non-invaded patches found in this study, reflects changes in the N source as a possible response mechanism to interference or competition with H. pilosella. Two possible mechanisms have been described to explain changes in foliar δ15N towards more positive δ15N signatures; Averill and Finzi (2011) and Schulze et al. (1998) suggested a shift to a higher proportion of organic nitrogen uptake, especially from animal origin, as for example with the increase in herbivore fertilization of nutrient-poor soil. On the other side, Gilgen et al. (2010) found a significant increase of δ15N due to the preferences of nitrate vs. ammonium in response to drought. In our study, however, no evidence of water stress was found, since native plants always showed RWC values over 75 % and no strong effect of H. pilosella was found on the plant water used by native species (Fig. 4a and b). On the other hand, herbivory by sheep and guanacos constitutes a major component of this grassland ecosystem, which is consistent with the high δ15N values found in the native species.
Under interference with H. pilosella, native plants would use a higher proportion of organic N (supported by a higher δ15N signature), with an overall reduction in N absorption (Fig. 1b and c). In high latitudes or altitude organic soils, where N mineralization rates are low and organic N is potentially important for plant nutrition, the allelopathic compounds of H. pilosella can affect the soil microbial biomass (Miller and Bowman 2003). Knicker et al. (2000) observed that soil processes under H. pilosella competition were profoundly different from those occurring in native grassland soils. They attributed this difference to a reduction in net N mineralization related to polyphenols and lignins released by H. pilosella (Saggar et al. 1999). It has been demonstrated that some invasive species can alter the functional composition of the microbial community, such as the balance between bacteria and fungi in the soil (Scharfy et al. 2010), and this might affect not only the C:N ratio in soil, but also the availability of different N forms.
The reduction of N mineralization caused by allelopathic compounds of H. pilosella may oblige grassland species to shift to other N sources, probably organic forms such as amino acid N. Grassland species from high altitude or latitude sites can uptake amino acids as a nitrogen source, but this process is more expensive than nitrogen from inorganic origins (Miller and Bowman 2003; Averill and Finzi 2011). Hence, competition with the invasive species probably produces changes in the N regime that result in alterations in the relative proportion of NH4 +, NO3 −, and amino acids in the soil. Combined, these effects will likely impact grassland species that must shift to more expensive N sources. Such shifts in resource use as a result of competition are an implicit assumption in studies of resource partitioning but have rarely been documented in the field. Rascher et al. (2012) evidenced these changes in native dune species under competition with the alien species Acacia longifolia.
Changes in N uptake in the studied species under interference with H. pilosella were also evidenced by a significant decrease in leaf proline accumulation (Fig. 2a). In addition, there was a significant positive correlation between proline and chlorophyll content for all the native species in the absence H. pilosella interference, which then became altered under H. pilosella interference (Fig. 2a). We observed that the proline content depletion in response to H. pilosella interference was only present in the proline-accumulator species; this species also showed a decrease in chlorophylls content (Figs. 2a and 3a). The only legume in our study, T. repens, was the species showing the highest proline accumulation, probably due to its N fixing trait. Our results may indicate effects of allelopathic compounds of H. pilosella in N fixation, probably through an effect on soil microorganisms and nodulation in this legume (Batish et al. 2007; Heckman and Kluchinski 1995).
As a free amino acid, proline can play different functions in plant leaves; one of these functions is that of osmotic protector against low temperatures (Hare and Cress 1997). Together with the shifts found in δ15N, our results suggest that under H. pilosella interference, grassland native species might suffer a decrease in N-pools, which would affect proline levels in leaves, thereby making them more sensitive to low temperatures. This assumption is supported by a significant general increase in C/N ratio under H. pilosella interference (Fig. 1).
The H. pilosella interference revealed two possible functional strategies within the native species. P. spiciformis and T. repens are proline accumulators that did not show decreased Fv/Fm values under H. pilosella interference, but presented decreased proline values that, in turn, showed changes in N cycle uptake. Non-proline accumulator species F. gracillima and A. pinnatifida showed decreased Fv/Fm values under exotic species interference, and increases in leaf pigments and WUEi, which were significant for F. gracillima, the species that was most affected by H. pilosella (Figs. 2a, 3 and 4a).
F. gracillima has leaves that are harder and more resistant to wind and colder temperatures (Braun 2009) and has a higher investment in support tissues (Cingolani et al. 2005) in relation to the other grassland species. Under invasion conditions, this species exhibited an increase in water use efficiency (both intrinsic and integrated through Δ13C) and a decrease in Fv/Fm, which indicate changes in F. gracillima water economy and photochemical efficiency. Similarly, A. pinnatifida also suffered a significant decrease in Fv/Fm as an effect of competition. To compensate for the Fv/Fm decrease, both A. pinnatifida and F. gracillima displayed an increase in leaf chlorophyll and carotenoid content.
The integration of all physiological variables and species by CDA showed how H. pilosella exhibits a set of functional attributes that are completely different to those of the native grassland species (Fig. 5). Among the native species, about 80 % of the cases showed differences between those that had been interfered with and those with no interference by the invasive species; this provides evidence that grasslands modify their functional attributes to face interference with H. pilosella, which was further confirmed by the MANOVA. This shift in plant traits, such as changes in N sources, proline content or leaf characteristics, may decrease native plant performance from affecting community composition under the extreme environmental conditions of Tierra del Fuego. Our result showed that competition between H. pilosella with native species mainly arises from different physiological traits and this concurs with the study of Hejda and de Bello (2013) in Central Europe; these authors suggested that invading aliens tend to be functionally different from native species and are therefore more likely to occupy an empty niche in the invaded vegetation. Other authors also suggested that the invasive potential of an alien species to alter ecosystem properties is dependent on the functional groups of the invading and native species (Scharfy et al. 2011 and van Kleunen et al. 2010).
Our results support the idea that the competition between grassland species and the invader H. pilosella occurs through shifts in the allocation of nutrients and water. Plasticity in the uptake of different forms of N may be the underlying mechanism that explains the competition effects found in the native grassland species (Leffler et al. 2011). Under the current invasion scenario of H. pilosella in Tierra del Fuego, it could be expected that this invasive species will outperform native grassland species through a more competitive use of soil resources.
References
Aerts R, van Logtestijn RSP, Karlsson PS (2006) Nitrogen supply differentially affects litter decomposition rates and nitrogen dynamics of sub-arctic bog species. Oecologia 146:652–658
Ain-Lhout F, Zunzunegui M, Tirado R, Clavijo A, Díaz-Barradas MC, García Novo F (2001) Comparison of proline accumulation in two Mediterranean scrubs subject to natural and experimental water deficit. Plant Soil 230:175–183
Asner GP, Vitousek PM (2005) Remote analysis of biological invasion and biogeochemical change. PNAS 102:4383–4386
Averill C, Finzi A (2011) Increasing plant use of organic nitrogen with elevation is reflected in nitrogen uptake rates and ecosystem δ15N. Ecology 92:883–891
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Batish DR, Lavanya K, Singh HP, Kohli RK (2007) Phenolic allelochemicals released by Chenopodium murale affect the growth, nodulation and macromolecule content in chickpea and pea. Plant Growth Regul 51:119–128
Bishop GF, Davy AJ (1994) Hieracium pilosella L. (Pilosella officinarum F. Schultz and Schultz-Bip.). J Ecol 82:195–210
Bottollier-Curtet MB, Planty-Tabacchi AM, Tabacchi E (2013) Competition between young exotic invasive and native dominant plant species: implications for invasions within riparian areas. J Veg Sci 24:1033–1043
Braun K (2009) Efectos del pastoreo, el ambiente y las invasiones sobre procesos de la estepa fueguina. Dissertation Ph. D. Thesis, University of Buenos Aires, Argentina
Cingolani AM, Posse G, Collantes MB (2005) Plant functional traits, herbivore selectivity and response to sheep grazing in Patagonian steppe grasslands. J Appl Ecol 42:50–59
Cipriotti PA, Rauber RB, Collantes MB, Braun K, Escartín C (2010) Hieracium pilosella invasion in the Tierra del Fuego steppe, Southern Patagonia. Biol Invasions 12:2523–2535
Clark CM, Cleland EE, Collins SL, Fargione JE, Gough L, Gross KL et al (2007) Environmental and plant community determinants of species loss following nitrogen enrichment. Ecol Lett 10:596–607
Coley P, Bryant I, Chapin FS III (1985) Resource availability and plant antiherbivore defense. Science 230:895–899
Collantes MB, Anchorena J, Cingolani AM (1999) The steppes of Tierra del Fuego: floristic and growth form patterns controlled by soil fertility and moisture. Plant Ecol 140:61–75
Collantes MB, Braun K, Escartín C, Cingolani AM, Anchorena J (2005) Patrones de cambio de la vegetación de la estepa fueguina en relación al pastoreo. In: Oesterheld M, Aguiar MR, Ghersa C, Paruelo JM (eds) La heterogeneidad de la vegetación de los agroecosistemas. Ed. Facultad de Agronomía, Universidad de Buenos Aires, Buenos Aires
D’Antonio CM, Vitousek PM (1992) Biological invasions by exotic grasses, the grass/fire cycle, and global change. Annu Rev Ecol Syst 23:63–87
Daehler C (2003) Performance comparisons of co-occurring native and alien invasive plants: implication for conservation and restoration. Annu Rev Ecol Syst 34:183–211
Davidson AM, Jennions M, Nicotra AB (2011) Do invasive species show higher phenotypic plasticity than native species, and if so, is it adaptive? A meta-analysis. Ecol Lett 11:419–431
Day NJ, Buckley HL (2011) Invasion patterns across multiple scales by Hieracium species over 25 years in tussock grasslands of New Zealand’s South Island. Austral Ecol 36:559–570
Domínguez E (2004) Plantas exóticas presentes en el Parque Nacional Pali Aike, XII Región, Chile. Chloris Chilensis. Año 7. N° 2. www.chlorischile.cl
Dorrepaal E, Aerts R, Cornelissen JHC (2007) Changing leaf litter feedbacks on plant production across contrasting sub-arctic peatland species and growth forms. Oecologia 151:251–261
Ehrenfeld JG (2010) Ecosystem consequences of biological invasions. Annu Rev Ecol Syst 41:59–80
Farquhar GD, O’Leary MH, Berry JA (1982) On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust J Plant Physiol 9:121–137
Funk JL, Vitousek PM (2007) Resource-use efficiency and plant invasion in low-resource systems. Nature 446:1079–1081
Garnier E, Shipley B, Roumet C, Laurent G (2001) A standardized protocol for the determination of specific leaf area and dry matter content. Funct Ecol 15:688–695
Gilgen AK, Signarbieux C, Feller U, Buchmann N (2010) Competitive advantage of Rumex obtusifolius L. might increase in intensively managed temperate grasslands under drier climate. Agric Ecosyst Environ 135:15–23
Hare PD, Cress WA (1997) Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul 21:79–102
Harper JL (1977) Population biology of plants. Academic Press, London
Heckman JR, Kluchinski D (1995) Soybean nodulation and nitrogen fixation on soil amended with plant residues. Biol Fertil Soils 20:284–288
Hejda M, de Bello F (2013) Impact of plant invasions on functional diversity in the vegetation of Central Europe. J Veg Sci 24:890–897
Johnstone PD, Wilson JB, Bremner AG (1999) Change in Hieracium populations in Eastern Otago over the period 1982–1992. N Z J Ecol 23:31–38
Knicker H, Saggar S, Bäumler R, McIntosh PD, Kögel-Knabner I (2000) Soil organic matter transformations induced by Hieracium pilosella L. in tussock grassland of New Zealand. Biol Fertil Soils 32:194–201
Koremblit G, Forte Lay JA (1991) Contribución al estudio agroclimático de norte de Tierra del Fuego (Argentina). An Inst Patagonia Cien Naturales 2:125–134
Kourtev PS, Ehrenfeld JG, Haggblom M (2002) Exotic plant species alter the microbial community structure and function in the soil. Ecology 83:3152–3166
Krahulcova A, Krahulec F (1999) Chromosome numbers and reproductive systems in selected representatives of Hieracium subgen. pilosella in the Krkonose Mts (the Sudeten Mts). Preslia 71:217–234
Leffler AJ, Monaco TA, James JJ (2011) Nitrogen acquisition by annual and perennial grass seedlings: testing the roles of performance and plasticity to explain plant invasion. Plant Ecol 212:1601–1611
León RJC, Ran D, Collantes MB, Paruelo J, Soriano A (1998) Grandes unidades de vegetación de la Patagonia extra andina. Ecología Aust 8:125–144
Levine JM, Vilá M, D’Antonio CM, Dukes JS, Grigulis K, Lavorel S (2003) Mechanisms underlying the impacts of exotic plant invasions. Proc R Soc Lond B Biol Sci 270:775–781
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Livraghi E, Cabeza S, Kofalt R, Humano G, Mascó M, Montes L (1998) Documento de trabajo sobre Hieracium pilosella L. Informe Técnico INTA
Makepeace W, Dobson AT, Scott D (1985) Interference phenomena due to mouse-ear and king-devil hawkweed. N Z J Ecol 23:79–90
McIntosh PD, Loeseke M, Bechler K (1995) Soil changes under mouse-ear hawkweed (Hieracium pilosella). N Z J Ecol 19:29–34
Meurk CD, Walker S, Gibson RS, Espie P (2002) Changes in vegetation states in grazed and ungrazed Mackenzie Basin grasslands, New Zealand, 1990–2000. N Z J Ecol 26:95–106
Miller AE, Bowman WD (2003) Alpine plants show species-level differences in the uptake of organic and inorganic nitrogen. Plant Soil 250:283–292
Müller-Schärer H, Schaffner U, Steinger T (2004) Evolution in invasive plants: implications for biological control. Trends Ecol Evol 19:417–422
Ordonez A, Wright IJ, Olff H (2010) Functional differences between native and alien species: a global scale comparison. Funct Ecol 24:1353–1361
Petrovic SD, Löscher R, Gorunovic MS, Merfort I (1999) Flavonoid and phenolic acid patterns in seven Hieracium species. Biochem Syst Ecol 27:651–656
Radford IJ, Dickinson KJM, Lord JM (2006) Nutrient stress and performance of invasive Hieracium lepidulum and co-occurring species in New Zealand. Basic Appl Ecol 7:320–333
Rascher KG, Hellmann C, Máguas C, Werner C (2012) Community scale 15N isoscapes: tracing the spatial impact of an exotic N2-fixing invader. Ecol Lett 15:484–491
Rauber RB, Collantes MB, Cipriotti PA, Anchorena J (2013) Biotic and abiotic constraints to a plant invasion in vegetation communities of Tierra del Fuego. Austral Ecol 38:436–442
Robinson D (2001) δ15N as an integrator of the nitrogen cycle. Trends Ecol Evol 16:153–162
Rose AB, Platt KH, Frampton CM (1995) Vegetation change over 25 years in a New Zealand short-tussock grassland: Effects of sheep grazing and exotic invasions. N Z J Ecol 19:163–174
Saggar S, McIntosh PD, Hedley C, Knicker H (1999) Changes in soil microbial biomass, metabolic quotient, and organic matter turnover under Hieracium (H. pilosella L.). Biol Fertil Soils 30:232–238
Saura-Mas S, Lloret F (2007) Leaf and shoot water content and leaf dry matter content of Mediterranean woody species with different post-fire regenerative strategies. Ann Bot 99:545–554
Scharfy D, Güsewell S, Gessner MO, Olde Venterink H (2010) Invasion of Solidago gigantea in contrasting experimental plant communities: effects on soil microbes, nutrients and plant–soil feedbacks. J Ecol 98:1379–1388
Scharfy D, Funk A, Olde Venterink H, Güsewell S (2011) Invasive forbs differ functionally from native graminoids, but are similar to native forbs. New Phytol 189:818–828
Schulze ED, Williams RJ, Farquhar GD, Schulze W, Langridge J, Miller JM, Walker BH (1998) Carbon and nitrogen isotope discrimination and nitrogen nutrition of trees along a rainfall gradient in northern Australia. Aust J Plant Physiol 25:413–425
Scott D, Sutherland BL (1993) Interaction between some pasture species and two Hieracium species. N Z J Ecol 17:47–52
Scott NA, Saggar S, McIntosh PD (2001) Biogeochemical impact of Hieracium invasion in New Zealand’s grazed tussock grasslands: sustainability implications. Ecol Appl 11:1311–1322
Shaver GR, Bret-Harte MS, Jones MH, Johnstone J, Gough L, Laundre J, Chapin FS (2001) Species composition interacts with fertilizer to control long-term vegetation change in tundra productivity. Ecology 82:3163–3181
Shearer G, Kohl DH (1993) Natural 15N abundance as a method of estimating the contribution of biologically fixed nitrogen to N2-fixing systems: potential for non-legumes. Plant Soil 110:317–327
Stahl VM, Beyschlag W, Werner C (2011) Dynamic niche sharing in dry acidic grasslands – a 15N labeling experiment. Plant Soil 344:389–400
Treskonova M (1991) Changes in the structure of tall tussock grasslands and infestation by specie of Hieracium in the Mackenzie country, New Zealand. N Z J Ecol 15:65–78
van Kleunen M, Weber E, Fischer M (2010) A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol Lett 13:235–245
Vitousek P (1982) Nutrient cycling and nutrient use efficiency. Am Nat 119:553–572
Walter H, Box EO (1983) Climate of Patagonia. In: West NE (ed) Temperate deserts and semi deserts. Ecosystems of the World, Vol. 5. Elsevier Scientific Publishing Company, Amsterdam, pp 432–435
Wilson SD, Tilman D (1991) Components of plant competition along an experimental gradient of nitrogen availability. Ecology 72:1050–1063
Acknowledgments
This research was supported by a project from the Spanish Agency of International Cooperation (AECI) and partially by a CFI project from Argentina. The authors want to acknowledge Drs. Cristina Máguas, Francisco García Novo, Jon Jáuregui and two anonymous reviewers for their valuable comments to the manuscript. We also thank to Mr. Errol O’Byrne for his kindness and hospitality in the cooperation to the access and accommodation to the study site.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Harry Olde Venterink.
Rights and permissions
About this article
Cite this article
Díaz-Barradas, M.C., Zunzunegui, M., Álvarez-Cansino, L. et al. Species-specific effects of the invasive Hieracium pilosella in Magellanic steppe grasslands are driven by nitrogen cycle changes. Plant Soil 397, 175–187 (2015). https://doi.org/10.1007/s11104-015-2608-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2608-0