Abstract
Aims
Coastal sand dunes have a well-established abiotic gradient from beach to land and a corresponding spatial gradient of plant species representing succession in time. Here, we relate the distribution of plant-feeding nematodes with dominant plant species in the field to host specialization and impacts on plant species under controlled greenhouse conditions.
Methods
We assessed plant-feeding nematodes in soil and roots of six plant species that dominate the vegetation at successional positions along the gradient. In controlled conditions, we determined performance of all plant-feeding nematodes on each plant species and their effects on plant biomass.
Results
Specialist feeding type nematodes were confined to plant species in either foredunes or landward dunes. Generalist feeding type nematodes were found in highest numbers in the landward dunes. Most tested nematode species decreased root, but not shoot or rhizome biomass.
Conclusions
Host plant suitability determined occurrence of some plant-feeding nematodes in dunes, but abiotic and biotic soil conditions may play a role as well. Generalist feeding type nematodes were able to reproduce on all plant species. Feeding specialists, which are more protected by plant roots, might prefer host plants in the foredunes for the same reason as their host plants: to escape from natural enemies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is increasing awareness that soil biota play a role as drivers of patterns and spatio-temporal dynamics in natural vegetation (Bardgett and Wardle 2010). Individual plant species stimulate specific soil organisms, thereby creating distinct soil communities (Bezemer et al. 2010). Soil biota include an array of functional types of organisms, including decomposers, mutualistic symbionts, root feeders, and pathogens. Here, we examine how the distribution of plant-feeding nematodes (also named plant-parasitic or root-feeding nematodes) in a coastal successional vegetation gradient may relate to host specificity and to effects on plant biomass production. Plant-feeding nematodes are well known to cause yield reductions in crop systems (Neher 2010) and vary in degree of host plant specificity (Perry and Moens 2006; Jones et al. 2013). Coastal sand dunes have been used as a model for plant-soil biota interactions in general, and plant-nematode interactions in particular (Oremus and Otten 1981; Seliskar and Huettel 1993; Van der Putten et al. 2006; de la Peña et al. 2008).
Coastal sand dunes are characterized by a gradient in environmental conditions from highly dynamic, salty and poor in organic matter along the beach to more stable, low salt and higher organic matter landward (De Boer et al. 1998). The gradient in environmental conditions is reflected by a sequence of different dominant plant species (Huiskes 1979; Doing 1985). The foredunes along the beach are exposed to deposition of sand by wind and to salt spray from the sea, and plant species that naturally occur in these sites are adapted to withstand, or even prefer these extreme conditions (Hope-Simpson and Jefferies 1966; Huiskes 1979; Wilson and Sykes 1999). In North-Western Europe, the sand dune grass Ammophila arenaria is more vigorous in dynamic foredunes that are exposed to frequent deposition of wind-blown sand from the beach than in stable landward dunes where there is almost no sand deposition. Sand deposition is thought to provide these plants with an opportunity to temporarily escape from soil-borne pathogens and plant-feeding nematodes (Van der Putten et al. 1988). When sand burial ceases and A. arenaria dies off, other plant species that are insensitive to soil-borne plant pathogens that affect A. arenaria become dominant. Thus, soil-borne plant pathogens are supposed to promote succession in coastal sand dunes because of being host specific (Van der Putten et al. 1993).
In spite of the studies on occurrence of soil-borne pathogens and plant-feeding nematodes in coastal sand dunes (Rooij-van et al. 1995; Wall et al. 2002), host specificity has not received much attention yet. In part, it has been difficult to establish which species are potential pathogens (Rooij-van and der Goes 1995) and how they may be quantified. In the present study, we focus on plant-feeding nematodes, because they can be relatively easily quantified and cultured. Plant-feeding nematodes have been studied in relation to the ecology of both the European dune grass A. arenaria and the North-American congener Ammophila breviligulata. In both cases it has been argued that these nematodes were not the only control agents for the grasses, but that they may operate in combination with other organisms, such as fungal pathogens (Rooij-van and der Goes 1995; Little and Maun 1997).
Thus far most nematode species tested were either too low in abundance in the field to cause growth reduction (Rooij-van and der Goes 1995), or they appeared to be bottom-up controlled by the plant to a non-damaging level (Van der Stoel et al. 2006). However, in some cases nematodes can have substantial growth reducing effects on their host plant species. For example, in a large-scale field experiment evidence was provided that the root-knot nematode Meloidogyne maritima was able to reduce biomass production of the dune grass A. arenaria when it was added alone. When added in combination with other plant-feeding nematodes, M. maritima did not reduce growth of A. arenaria, probably because it was controlled by competition (Brinkman et al. 2005).
Several other studies on factors that may control plant-feeding nematodes in coastal dunes have pointed at multi-factor controls, including microbes, arbuscular mycorrhizal fungi, and predators influencing the abundance of plant-feeding nematodes in the root zone of A. arenaria (de la Peña et al. 2006b; Hol et al. 2007; Piśkiewicz et al. 2008). However, except for work on the foredune cyst nematode Heterodera arenaria (Van der Stoel and van der Putten 2006) there has been relatively little attention for the role of host plant specificity as a factor that may determine the presence and abundance of plant-feeding nematodes in coastal dunes. We combined field surveys with inoculation studies under controlled conditions in order to single out the role of host plant specificity, or suitability.
Our study had three aims. First, we investigated the occurrence of plant-feeding nematodes in soil and roots of six plant species that dominate the vegetation gradient from beach to land across coastal dunes in the Netherlands. Second, we assessed the level of specialization of the nematodes to these plants under controlled conditions. Specialist nematodes are often endoparasites that enter plants roots and either feed on inner cell layers or create special feeding sites. Generalists are often ecto- or semi-endoparasites that feed on the outer cell layers of the root (Perry and Moens 2006; Jones et al. 2013). Third, we determined the effects of the different nematode species on plant biomass production under controlled conditions. In general, endoparasitic nematodes are more associated with crop losses than are ectoparasites (Jones et al. 2013). These three aims enabled us to relate nematode distribution in the root zone of the dominant plant species across the vegetation gradient to their host specialization and their impacts on the plant species.
We tested the hypothesis that nematodes typical of the most dynamic parts of the dunes were the most specialized to their host plants. The assumption underlying this hypothesis is that extreme abiotic conditions may not only drive specialization of plants, but also of the plant-feeding nematodes. We also tested the hypothesis that nematodes from landward dunes would be able to reproduce on a wider range of plant species in the vegetation gradient. The underlying assumption for this hypothesis is that generalist nematodes may not develop populations on the early successional plant species because of the extreme conditions near the beach. The data used for the present study originate from a series of separate experiments. A small fraction of the data are already published (Van der Stoel and van der Putten 2006), but are also included in the present work (see methods) in order to present a comprehensive study on linking occurrence, specificity, and effectiveness of plant-feeding nematodes in a natural ecosystem.
Materials and methods
Field survey
Soil samples were taken from the root layer of six monocotyledonous plant species that grow on coastal dunes at Voorne, the Netherlands. The plant species that we sampled are, in successional order from the beach towards the inner dunes: Elytrigia juncea (L.) Nevski subsp. boreoatlantica (Simonet & Guin.) N.Hyl. (sand couch), Ammophila arenaria (L.) Link (marram grass; vigorous and degenerated stage), Festuca arenaria Osbeck (sand fescue), Carex arenaria L. (sand sedge), Elytrigia atherica (Link) Kerguélen (sea couch) and Calamagrostis epigejos (L.) Roth (wood small-reed). All sampled plant species occur naturally along the coast of North-Western Europe. They are perennial, grow in relatively large mono-specific stands, and they all have an optimum abundance in a specific vegetation zone (Doing 1985; Van der Laan et al. 1997). Sampling in a long-lived monospecific stand was supposed to provide a good indication of which nematodes could be supported by that particular plant species. The samples were taken along four approximately 150 m long transects from the foredune to the inner dunes at each of two sites: Oostvoorne ‘car beach’ (dune with approximately 5 cm annual sand accretion; 51°9′ N 04°06′ E) and ‘Haringvlietdam’ (dune with approximately 20 cm annual sand accretion; 51°52′ N 04°04′ E). To ascertain detection of all nematode species, including those that may have low densities at certain times of the year (Van der Stoel et al. 2002), soil samples were collected on four dates (April, June, August and October 1996). Each replicate consisted of two combined samples of about 1.5 L soil collected within 2 m distance from a depth of 0.10–0.15 m underneath the soil surface. When the newly deposited upper soil layer did not yet contain roots, this layer was removed until the first roots appeared. The soil sample was then collected from the rooted sublayer and stored at 4 °C until processing.
Roots were separated from the soil by sieving through 1.0 and 0.5 cm mesh sizes, after which the soil was gently homogenized. Nematodes were extracted from a sample of 0.25 L soil (density ca. 1.4 kg/L soil) by Oostenbrink elutriation (Oostenbrink 1960), followed by decantation onto a stack of one 75 μm and three 45 μm sieves. To collect free-living nematodes, the debris on the sieves was transferred to a double cotton milk filter (Hygia rapid, Hartmann AG, Heidenheim, Germany) held by a tray in a dish with a layer of tap water. The nematodes were allowed to pass through the filter for 24 h and were then stored at 4 °C until counting. When roots of a different plant species were present in the sample, these were removed prior to extraction. Nematodes were extracted from the roots by the funnel spray method for 48 h (Oostenbrink 1960). For identification and counting, 5 out of 100 ml suspension was used from the soil extracts and 5 out of 50 ml from the root extracts. Only plant-feeding nematodes, which were the focus of the study, were counted and identified to genus or species level according to Bongers (1988).
Inoculation experiments
All the plant species that were sampled in the field survey were tested for host suitability. Seeds of the different plant species were collected at the ‘Haringvlietdam’ field site (see Field survey). The seeds were germinated on moist glass beads at 25/15 °C at a 16/8 h light/dark regime. The seedlings were planted in 1.5 L pots filled with 1500 g sterilized (25 kGray) beach sand with a moisture content of 10 % (w w−1). They were grown in a greenhouse with daytime temperature of 21 ± 2 °C and night temperature of 19 ± 2 °C. The plants were provided with extra light to ensure a minimum photosynthetic photon fluence rate of 200 μmol m−2 s−1 over the waveband 400–700 nm at daytime. Four seedlings were planted per pot, except when adding Meloidogyne duytsi, Meloidogyne maritima and Pratylenchus spp. (a mixture of P. dunensis and P. brzeskii) three seedlings were planted per pot (for no specific reason, except that we planted more than one seedling per pot in order to produce more biomass).
Two weeks after planting, nematodes were inoculated close to the roots of each plant in the pot. Nematodes for the inoculation were extracted from cultures that were maintained on A. arenaria in the greenhouse, except for Heterodera arenaria, M. duytsi and M. maritima, of which egg-bearing females were collected from the field. The number of nematodes that was inoculated to each pot varied due to limited availability of some species: 200 individuals (Helicotylenchus pseudorobustus, Hemicycliophora conida, Mesocriconema xenoplax, Rotylenchus goodeyi, Neodolichorhynchus microphasmis and Telotylenchus ventralis (when added to E. juncea)); 400 (M. duytsi, M. maritima and Pratylenchus spp.); 476 (T. ventralis when added to the other plant species; inoculum was added twice with 1 week intercept due to low recovery); 1700 (juveniles of H. arenaria). Tap water was added in the same manner to the control plants. The inoculations with the different nematode species were not all performed at the same time. However, in every experiment with a particular nematode species all plant species were inoculated with that nematode species and a control without nematode addition was included. Five replicates per treatment were used, except for the test with H. arenaria when six replicates were used. The results of the test with H. arenaria have been published separately (expressed in numbers per amount of root biomass; Van der Stoel and van der Putten 2006), but we expressed the numbers per amount of soil and added them to the current overview for completeness. When relevant, we have indicated the source of the published data on H. arenaria in the Tables. Two times per week, the soil moisture content was re-set with demineralized water to 10 % (w w−1) by weighing. Hoagland nutrient solution was added weekly in an increasing amount to meet the demands of the growing plant (weeks 1 to 5: 25 ml half strength; weeks 5 and 6: 25 ml full strength (1H); weeks 7 and 8: 50 ml 1H and from 9 weeks onwards: 75 ml 1H; but for the experiment with H. arenaria weeks 1 to 6: 12.5 ml 1H, weeks 7 to 11: 25 ml 1H and weeks 12 and 13: 50 ml 1 H per pot).
The plants were harvested 10–14 weeks after nematode inoculation. The sand was washed from the roots using tap water and shoots were separated from the roots. The plant material was dried at 70 °C for 48 h and then weighed. Nematodes were extracted from the sand by decantation. Tap water was added to the soil slurry to obtain a volume of 5 L. The suspension was stirred, after which the water was poured onto a stack of one 75 μm and three 45 μm sieves. This procedure was performed four times. The extraction of the nematodes from the debris on the sieves was the same as described above for the field survey. For extraction of cysts (females) of H. arenaria, the suspension was poured onto a 180 μm sieve and the debris was collected on filter paper. The contents were air-dried and then visually inspected for cysts using a stereomicroscope. Due to very poor growth of C. arenaria in the inoculation experiment with H. arenaria, that part of the experiment was terminated at an early stage before female cysts could have developed (data not shown in Tables 2 and 4).
Data analysis
For the greenhouse experiments, data were only compared that originated from experiments that were performed at the same time. Final nematode numbers in the greenhouse experiment were ln-transformed to achieve homogeneity of variances, after which the results were analyzed by one-way ANOVA with plant species as factor. Biomass of plants was ln-transformed to achieve homogeneity of variances. Effects of plant species and nematode addition (yes/no) on plant biomass were analyzed by two-way ANOVA. When transformation did not result in homogeneous variances, the results were analyzed by non-parametric Scheirer-Ray-Hare test, which is a two-way extension of the Kruskal-Wallis test (Sokal and Rohlf 1995). Approximate P-values for the Scheirer-Ray-Hare test were obtained from Rohlf and Sokal (1981). The correlation between the successional range of plant species and numbers of co-occurring endoparasites and ecto- and semi-endoparasites, as well as Pf/Pi (=ratio of final to initial population size in the inoculation experiment) and nematode addition effects on shoot and root biomass, was tested with Spearman’s rank-order correlation using Statistica 10. Pf/Pi of H. arenaria was left out from the analysis as only numbers of first generation female cysts were determined and not the offspring that they contained.
Results
Ecto- and semi-endoparasites
In the field, ecto- and semi-endoparasitic nematodes were found on all the plant species that we sampled (Table 1). However, potential multiplication in the inoculation experiment showed different reproductive capacities than would be predicted from the densities in the field. In the field, H. conida and H. pseudorobustus mainly were associated with C. epigejos (Table 1). However, in the greenhouse reproduction of H. conida did not differ significantly among the six plant species, whereas reproduction of H. pseudorobustus was only intermediate on C. epigejos (Tables 2 and 3). Criconematidae (Criconemoides amorphus and M. xenoplax; the two species were not distinguished during counting) in the field were detected in low densities on all plant species except for E. juncea (Table 1). However, in the greenhouse experiment, M. xenoplax reproduced on all plant species, although less so on F. arenaria (Tables 2 and 3). In the field, R. goodeyi was detected on all plant species except for vigorous A. arenaria, although densities were higher in the inner dunes (Table 1). In the greenhouse, numbers remained low on all plant species (Tables 2 and 3). In the field, N. microphasmis and/or Geocenamus nanus (the two species were not distinguished during counting) were found on all plant species, although densities were higher in the inner dunes (Table 1). In the greenhouse experiment, N. microphamis reproduced on all plant species, although reproduction was lower on E. atherica. In the field, T. ventralis was found in low densities on all plant species (Table 1). In the greenhouse, reproduction was high on all plant species except C. arenaria (Tables 2 and 3).
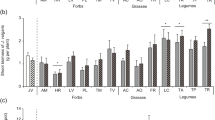
The density of ectoparasites and semi-endoparasites associated to the plant species in the dune succession gradient significantly increased from beach to land (Fig. 1a; Spearman R = 0.340, P < 0.001). When inoculated to the six plant species, Pf/Pi of the ecto- and semi-endoparasites was not significantly related to the successional order of the plant species in the coastal dunes (Fig. 1b; Spearman R = −0.046, P = 0.54).
a) Field density (Number of nematodes 100 g dry soil−1) of endoparasites and ecto- and semi-endoparasites that occurred with dominant plant species across a successional gradient in coastal dunes from the sea (left) land inward (right; n = 16); (v) and (d) indicate vigorous and degenerated stages of A. arenaria, respectively. b) The ratio of final to initial population size (Pf/Pi) of endoparasites (only Pratylenchus spp. and Meloidogyne spp.) and ecto- and semi-endoparasites when added to the same plant species and c) effect of nematode addition on shoot and d) root biomass of the plants (ln(+nema/-nema); n = 5, except for H. arenaria n = 6). Median ± quartile values are presented in all four panels; note the breaks on the y-axes of panels a) and b). P-values indicate significance of Spearman’s rank-order correlations
Endoparasites
The genera Heterodera, Meloidogyne and Pratylenchus were detected on all the plant species included in our study (Table 1). However, in the field survey that we present here, different nematode species had not yet been recognized. When tested in the greenhouse experiment, H. arenaria mainly reproduced on E. juncea, A. arenaria and on C. epigejos (Tables 2 and 3). Meloidogyne duytsi reproduced on the congeners E. juncea and E. atherica, whereas M. maritima reproduced only on A. arenaria. The Pratylenchus spp. that we tested, presumably a mixture of P. brzeskii and P. dunensis, produced high numbers on E. juncea and A. arenaria, whereas numbers remained low on the other plant species.
In contrast to the ectoparasites and semi-endoparasites, the density of endoparasites in the field was low (as is usual in the coastal dunes) and did not significantly correlate with the succession of plant species in the dunes (Spearman R = 0.074, P = 0.25). However, when added to the same range of plant species, Pf/Pi of the endoparasites tended to decrease along the successional gradient from the sea landward (Fig. 1b; Spearman R = −0.179, P = 0.09). This was mainly due to the relatively high reproduction of Pratylenchus spp. on E. juncea and A. arenaria (Fig. 1b).
Effects on plants
In the greenhouse experiment, all nematode species except Pratylenchus spp. significantly decreased root biomass of the tested plant species. However, pairwise comparisons between control plants and inoculated plants in most cases were not significantly different, except for T. ventralis and, to a lesser extent, H. arenaria (Tables 4 and 5). Most nematode species that we added to the plant species did not have a significant effect on shoot and rhizome biomass. Only T. ventralis significantly decreased shoot biomass of A. arenaria and C. epigejos, and rhizome biomass of E. atherica and C. epigejos (Tables 4 and 5). Considering the effects of the nematodes on plant biomass production, the ecto- and semi-endoparasitic plant-feeding nematodes had a more negative effect on shoot biomass of plant species from the landward part of the succession gradient than on plant species from foredunes (Spearman R = −0.228, P = 0.002). In contrast, the effects of the endoparasites on shoot biomass did not show a significant trend (Spearman R = 0.111, P = 0.23). Neither endoparasites (Spearman R = 0.144, P = 0.28), nor ecto- and semi-endoparasites (Spearman R = −0.022, P = 0.77) showed a relationship between position of the plant species in the successional gradient and effect on root biomass.
Discussion
In line with our hypothesis, we found that specialist plant-feeding nematodes were mostly confined to plant species that occur in the dynamic foredunes along the beach. On the other hand, generalist plant-feeding nematodes were detected with plants throughout the whole dune gradient, but densities were lower in the dynamic foredunes than in the stabilized dunes. During our survey, several of the plant-feeding nematode species turned out to be undescribed (Karssen et al. 1998b, 2000; de la Peña et al. 2006a). The inoculation experiments confirmed the high level of specialization of plant-feeding nematodes that thus far have only been found in coastal dunes. Here, we discuss our results in relation to current knowledge on occurrence and specialization of plant-feeding nematodes in coastal dunes. We discuss the various mechanisms other than host specialization, for example biotic interactions and abiotic environmental factors, that may influence the observed distribution of plant-feeding nematodes in the dunes.
In our survey, several endoparasitic nematode genera were detected in the root zone of all the plant species that we sampled. The level of specificity was highest for nematodes that occurred with plants in the dynamic foredunes, especially E. juncea and A. arenaria. Cyst nematodes (Heterodera spp.) were detected with plants throughout the entire dune gradient, but were likely represented by different species. The sedentary endoparasite H. arenaria appeared to be confined to plants in the foredunes that are exposed to sand deposition and salt spray. Landward, H. arenaria became gradually replaced by H. hordecalis (Fig. 2; Clapp et al. 2000; Van der Stoel and van der Putten 2006). Also root knot nematodes (Meloidogyne spp.) were detected in the root zone of all studied plant species, but probably different plant species hosted different root knot nematode species. For example, M. duytsi was found predominantly, if not exclusively on E. juncea, M. maritima on A. arenaria, whereas non-identified Meloidogyne spp. other than M. duytsi and M. maritima were isolated from plant species with a more landward occurrence. The migratory endoparasites Pratylenchus spp. were found throughout the dune succession gradient as well, although it is more difficult to link their species names to host plant species. The migratory endoparasites P. brzeskii and P. dunensis have been described to occur on E. juncea and A. arenaria, whereas P. brzeskii also has been detected on Leymus arenarius (L.) Hochst. (Karssen et al. 2000; de la Peña et al. 2006a). From our own observations, Pratylenchus spp. also occur on the other plant species, however, their species names have not yet been established.
The host range of the endoparasitic nematode species ascertained from the field survey did not fully correspond to the host range as determined by the inoculation experiment. In the greenhouse, the level of host plant specificity increased in the following order: H. arenaria, P. brzeskii and/or P. dunensis, M. duytsi and M. maritima. We found that H. arenaria was capable to reproduce on both E. juncea and on A. arenaria, but also on C. epigejos, whereas in the field this nematode species only occurred on plant species in the foredunes (Van der Stoel and van der Putten 2006). Thus, host suitability is not the only factor that determines the occurrence of H. arenaria in the foredunes. It might be that H. arenaria prefers dynamic abiotic conditions, such as sand deposition and salt spray, but these abiotic factors have not been included in the greenhouse experiments. Alternatively, like the host plant A. arenaria, the dynamic foredune environment might enable the cyst nematode H. arenaria to escape from natural enemies (Piśkiewicz et al. 2008). The specificity of the migratory endoparasites P. dunensis and P. brzeskii to E. juncea and A. arenaria suggests that they are restricted to foredunes due to the ecology of their host plants (de la Peña et al. 2008).
To our knowledge, dune vegetation is the only natural habitat for both M. duytsi and M. maritima, and the host plant preference of these two root knot species is rather specific compared to many other species within this nematode genus (Jones et al. 2013). As M. duytsi in our inoculation experiment did not reproduce on A. arenaria, it is likely that the reported occurrence on A. arenaria in Karssen et al. (1998b) originated from intermingled growth of the roots of A. arenaria and E. juncea. Interestingly, in the field M. duytsi has been found on E. juncea, but in the greenhouse it was able to reproduce as well on the congener E. athericus. This suggests that the occurrence of M. duytsi in foredunes is due to preference of dynamic abiotic conditions, or escape from natural enemies under those conditions. The results of our inoculation experiment suggest that A. arenaria is the only host plant of M. maritima, although the distinction between hosts and non-hosts may be obscured by overall low numbers of juveniles and males at the end of the experiment. Previously, M. maritima had been detected on A. arenaria, L. arenarius and C. epigejos (Karssen et al. 1998a). However, it is likely that M. maritima was feeding only on roots of A. arenaria that can still be present as remnant plants that grow intermingled with the other plant species in landward dune sites.
The ectoparasitic and semi-endoparasitic nematodes in our tests were generalists: they were capable of reproducing on the full range of coastal dune plant species, although most of them reproduced less well on one of the plant species. Therefore, the prevalence of ectoparasites in the landward dunes is likely caused by other factors than the availability of specific host plants. The host range of H. pseudorobustus is wide and it has a cosmopolitan distribution (Verschoor et al. 2001a; Davis et al. 2004; Silva et al. 2008). The species is dominant in high-productive grasslands, but decreases after cessation of fertilization (Verschoor et al. 2001b). That may be an important reason why H. pseudorobustus was found in highest numbers in landward dunes, where nutrient availability may be higher than close to the beach. Rotylenchus goodeyi is usually found in low numbers in many different vegetation types and it seems to have a long generation time (Boag and Neilson 1996), as also was indicated by the low reproduction in our greenhouse specificity trial. The slow reproduction may be disadvantageous in the dynamic dunes close to the beach, where ample offspring might be necessary insurance against extreme abiotic conditions. Hemicycliophora conida, M. xenoplax and N. microphasmis all have been found on woody plant species (Loof 1984; Zoon et al. 1993; Nico et al. 2002) and may not be adapted to the dynamics of the foredunes. Telotylenchus ventralis was first described from the roots of rye, oats and rye-grass (Loof 1963) and thus is not specific for coastal dunes. In the field survey, densities of T. ventralis were low on all studied plant species, whereas in the greenhouse reproduction was high on the grasses, but low on C. arenaria. The species may not thrive well in the abiotic conditions of sand dunes. Further experiments are needed to verify how abiotic conditions like salinity, nutrient and organic matter content and sand accretion may affect the occurrence of the nematodes (Nkem et al. 2006; Erb and Lu 2013).
The occurrence of plant-feeding nematode species may not only depend on host plant suitability and abiotic conditions (Mateille et al. 2011), but also on interactions with other organisms (Erb and Lu 2013). Many nematode species that occur in the dunes can be suppressed by micro-organisms present in the root zone of A. arenaria (de la Peña et al. 2006b; Piśkiewicz et al. 2008; Costa et al. 2012). It is not known if micro-organisms from the root zone of other plant species are suppressing T. ventralis to the same extent. Nematodes may also be suppressed by competition with co-occurring species: for example, in a previous study reproduction of M. maritima was delayed and reduced when H. arenaria and P. penetrans were added to the same plants (Brinkman et al. 2005). However, the opposite occurred when addition of T. microphasmis facilitated reproduction of T. ventralis on the non-host plant C. arenaria (Brinkman et al. 2008). Therefore, in order to further understand why some nematodes do not occur with potentially suitable host plant species, or why they occur at relatively low numbers in the field, additional studies are needed on biotic controls of these nematodes in the various successional stages of dune soil development.
For the nematodes to play a role in succession, they either need to be specific to certain plant species, or vary in effect strengths. In the inoculation experiment, most nematode species decreased root biomass, but did not affect shoot and rhizome biomass of the plant species. However, within-plant species comparisons of inoculated and control plants mostly were not significant. Exceptions were T. ventralis and H. arenaria that decreased shoot, rhizome or root biomass of several plant species, conform effects reported in previous studies (Rooij-van and der Goes 1995; Brinkman et al. 2004). In general, ecto- and semi-endoparasites decreased shoot biomass of plant species from landward dunes more than from foredunes. This may appear contrary to the hypothesis that plant species from foredunes benefit from sand burial because they need to escape from natural enemies in lower soil layers. However, in the field plants are attacked by a mixture of plant-feeding nematode and pathogenic microorganisms that co-occur and possibly interact with each other (Rooij-van and der Goes 1995; Brinkman et al. 2005, 2008). Hitherto unknown interactions with other suppressive soil organisms may further limit the extrapolation of our results to field conditions (Van der Stoel and van der Putten 2006; Piśkiewicz et al. 2008).
In conclusion, our data are largely in support of our two hypotheses: specialist plant-feeding nematodes were confined to plant species in the dynamic foredunes, but they may also reproduce on plant species from the same genus that occur in landward dunes. Generalist plant-feeding nematodes indeed were found in higher numbers in the less extreme -landward- environment, but under controlled conditions they were able to reproduce on all plant species. Therefore, it appears that in coastal dunes specialized nematodes are largely controlled by host plants, whereas generalist nematodes are controlled by extreme abiotic factors. In addition, our study and that of others (e.g., Rooij-van and der Goes 1995; de la Peña et al. 2006b; Piśkiewicz et al. 2008) strongly suggests that other (abiotic, but also biotic) factors may play an additional role in nematode control in coastal sand dune soil.
Abbreviations
- Pf/Pi:
-
(ratio of final to initial population size)
References
Bardgett RD, Wardle DA (2010) Aboveground-belowground linkages: biotic interactions, ecosystem processes, and global change. Oxford University Press, Oxford
Bezemer TM, Fountain MT, Barea JM, Christensen S, Dekker SC, Duyts H, van Hal R, Harvey JA, Hedlund K, Maraun M, Mikola J, Mladenov AG, Robin C, de Ruiter PC, Scheu S, Setälä H, Šmilauer P, van der Putten WH (2010) Divergent composition but similar function of soil food webs of individual plants: plant species and community effects. Ecology 91:3027–3036
Boag B, Neilson R (1996) Distribution and ecology of Rotylenchus and Pararotylenchus (Nematoda: Hoplolaimidae) in Great Britain. Nematologica 42:96–108
Bongers T (1988) De nematoden van Nederland. Pirola, Schoorl, The Netherlands
Brinkman EP, van Veen JA, van der Putten WH (2004) Endoparasitic nematodes reduce multiplication of ectoparasitic nematodes, but do not prevent growth reduction of Ammophila arenaria (L.) Link (marram grass). Appl Soil Ecol 27:65–75
Brinkman EP, Duyts H, van der Putten WH (2005) Consequences of variation in species diversity in a community of root-feeding herbivores for nematode dynamics and host plant biomass. Oikos 110:417–427
Brinkman EP, Duyts H, van der Putten WH (2008) Interactions between root-feeding nematodes depend on plant species identity. Soil Biol Biochem 40:2186–2193. doi:10.1016/j.soilbio.2008.01.023
Clapp JP, van der Stoel CD, van der Putten WH (2000) Rapid identification of cyst (Heterodera spp., Globodera spp.) and root-knot (Meloidogyne spp.) nematodes on the basis of ITS2 sequence variation detected by PCR-single-strand conformational polymorphism (PCR-SSCP) in cultures and field samples. Mol Ecol 9:1223–1232
Costa SR, Kerry BR, Bardgett RD, Davies KG (2012) Interactions between nematodes and their microbial enemies in coastal sand dunes. Oecologia 170:1053–1066. doi:10.1007/s00442-012-2359-z
Davis LT, Bell NL, Watson RN, Rohan TC (2004) Host range assessment of Helicotylenchus pseudorobustus (Tylenchida : Hoplolaimidae) on pasture species. J Nematol 36:487–492
De Boer W, Klein Gunnewiek PJA, Woldendorp JW (1998) Suppression of hyphal growth of soil-borne fungi by dune soils from vigorous and declining stands of Ammophila arenaria. New Phytol 138:107–116
de la Peña E, Moens M, van Aelst A, Karssen G (2006a) Description of Pratylenchus dunensis sp n. (Nematoda : Pratylenchidae), a root-lesion nematode associated with the dune grass Ammophila arenaria (L.) Link. Nematology 8:79–88. doi:10.1163/156854106776179917
de la Peña E, Rodríguez Echeverría S, van der Putten WH, Freitas H, Moens M (2006b) Mechanism of control of root-feeding nematodes by mycorrhizal fungi in the dune grass Ammophila arenaria. New Phytol 169:829–840
de la Peña E, Vandegehuchte M, Bonte D, Moens M (2008) Analysis of the specificity of three root-feeders towards grasses in coastal dunes. Plant Soil 310:113–120. doi:10.1007/s11104-008-9636-y
Doing H (1985) Coastal fore-dune zonation and succession in various parts of the world. Vegetatio 61:65–75. doi:10.1007/bf00039811
Erb M, Lu J (2013) Soil abiotic factors influence interactions between belowground herbivores and plant roots. J Exp Bot 64:1295–1303. doi:10.1093/jxb/ert007
Hol WHG, de la Peña E, Moens M, Cook R (2007) Interaction between a fungal endophyte and root herbivores of Ammophila arenaria. Basic Appl Ecol 8:500–509. doi:10.1016/j.baae.2006.09.013
Hope-Simpson JF, Jefferies RL (1966) Observations relating to vigour and debility in marram grass (Ammophila arenaria (L.) Link). J Ecol 54:271–275
Huiskes AHL (1979) Biological flora of the British isles: Ammophila arenaria (L.) Link (Psamma arenaria (L.) Roem. et Schult.: Calamagrostis arenaria (L.) Roth). J Ecol 67:363–382
Jones JT, Haegeman A, Danchin EGJ, Gaur HS, Helder J, Jones MGK, Kikuchi T, Manzanilla-Lopez R, Palomares-Rius JE, Wesemael WML, Perry RN (2013) Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol 14:946–961. doi:10.1111/mpp.12057
Karssen G, Van Aelst A, Cook R (1998a) Redescription of the root-knot nematode Meloidogyne maritima Jepson, 1987 (Nematoda : Heteroderidae), a parasite of Ammophila arenaria (L.) Link. Nematologica 44:241–253
Karssen G, Van Aelst A, van der Putten WH (1998b) Meloidogyne duytsi n. sp. (Nematoda : Heteroderidae), a root- knot nematode from Dutch coastal foredunes. Fundam Appl Nematol 21:299–306
Karssen G, Waeyenberge L, Moens M (2000) Pratylenchus brzeskii sp nov (Nematoda : Pratylenchidae), a root-lesion nematode from European coastal dunes. Ann Zool 50:255–261
Little LR, Maun MA (1997) Relationships among plant-parasitic nematodes, mycorrhizal fungi and the dominant vegetation of a sand dune system. Ecoscience 4:67–74
Loof PAA (1963) A new species of Telotylenchus (Nematoda: Tylenchida). Nematologica 9:76–80
Loof PAA (1984) Hemicycliophora species from Iran (Nematoda: Criconematoidea). Nematologica 30:22–41
Mateille T, Tavoillot J, Martiny B, Fargette M, Chapuis E, Baudouin M, Dmowska E, Bouamer S (2011) Plant-associated nematode communities in West-palearctic coastal foredunes may relate to climate and sediment origins. Appl Soil Ecol 49:81–93. doi:10.1016/j.apsoil.2011.06.012
Neher DA (2010) Ecology of plant and free-living nematodes in natural and agricultural soil. In: VanAlfen NK, Bruening G, Leach JE (eds) Annual Review of Phytopathology, Vol 48, vol 48. Annual Review of Phytopathology. pp 371–394. doi:10.1146/annurev-phyto-073009-114439
Nico AI, Rapoport HF, Jiménez-Díaz RM, Castillo P (2002) Incidence and population density of plant-parasitic nematodes associated with olive planting stocks at nurseries in Southern Spain. Plant Dis 86:1075–1079
Nkem JN, Virginia RA, Barrett JE, Wall DH, Li G (2006) Salt tolerance and survival thresholds for two species of Antarctic soil nematodes. Polar Biol 29:643–651. doi:10.1007/s00300-005-0101-6
Oostenbrink M (1960) Estimating nematode populations by some selected methods. In: Sasser JN, Jenkins WR (eds) Nematology. The University of North Carolina Press, Chapel Hill, pp 85–102
Oremus PAI, Otten H (1981) Factors affecting growth and nodulation of Hippophaë rhamnoides L. ssp. rhamnoides in soils from two successional stages of dune formation. Plant Soil 63:317–331
Perry RN, Moens M (2006) Plant nematology. CABI Publishing, Wallingford
Piśkiewicz AM, Duyts H, van der Putten WH (2008) Multiple species-specific controls of root-feeding nematodes in natural soils. Soil Biol Biochem 40:2729–2735. doi:10.1016/j.soilbio.2008.07.006
Rohlf FJ, Sokal RR (1981) Statistical tables, 2nd edn. W.H. Freeman and Company, San Fransisco
Rooij-van D, der Goes PCEM (1995) The role of plant-parasitic nematodes and soil-borne fungi in the decline of Ammophila arenaria (L.) Link. New Phytol 129:661–669
Rooij-van D, der Goes PCEM, van der Putten WH, van Dijk C (1995) Analysis of nematodes and soil-borne fungi from Ammophila arenaria (Marram Grass) in Dutch coastal foredunes by multivariate techniques. Eur J Plant Pathol 101:149–162
Seliskar DM, Huettel RN (1993) Nematode involvement in the dieout of Ammophila breviligulata (Poaceae) on the mid-Atlantic coastal dunes of the United States. J Coast Res 9:97–103
Silva RA, Oliveira CMG, Inomoto MM (2008) Fauna of plant-parasitic nematodes in natural and cultivated areas of the Amazon forest, Mato Grosso State, Brazil. Trop Plant Pathol 33:204–211
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research, 3rd edn. W.H. Freeman and Company, New York
Van der Laan D, van Tongeren OFR, van der Putten WH, Veenbaas G (1997) Vegetation development in coastal foredunes in relation to methods of establishing marram grass (Ammophila arenaria). J Coastal Conserv 3:179–190
Van der Putten WH, van Dijk C, Troelstra SR (1988) Biotic soil factors affecting the growth and development of Ammophila arenaria. Oecologia 76:313–320
Van der Putten WH, van Dijk C, Peters BAM (1993) Plant-specific soil-borne diseases contribute to succession in foredune vegetation. Nature 362:53–56
Van der Putten WH, Cook R, Costa S, Davies KG, Fargette M, Freitas H, Hol WHG, Kerry BR, Maher N, Mateille T, Moens M, de la Peña E, Piśkiewicz AM, Raeymaekers ADW, Rodríguez-Echeverría S, van der Wurff AWG (2006) Nematode interactions in nature: models for sustainable control of nematode pests of crop plants? Adv Agron 89:227–260
Van der Stoel CD, van der Putten WH (2006) Pathogenicity and host range of Heterodera arenaria in coastal foredunes. Nematology 8:255–263
Van der Stoel CD, van der Putten WH, Duyts H (2002) Development of a negative plant-soil feedback in the expansion zone of the clonal grass Ammophila arenaria following root formation and nematode colonization. J Ecol 90:978–988
Van der Stoel CD, Duyts H, van der Putten WH (2006) Population dynamics of a host-specific root-feeding cyst nematode and resource quantity in the root zone of a clonal grass. Oikos 112:651–659
Verschoor BC, de Goede RGM, de Hoop JW, de Vries FW (2001a) Seasonal dynamics and vertical distribution of plant-feeding nematode communities in grasslands. Pedobiologia 45:213–233. doi:10.1078/0031-4056-00081
Verschoor BC, de Goede RGM, de Vries FW, Brussaard L (2001b) Changes in the composition of the plant-feeding nematode community in grasslands after cessation of fertiliser application. Appl Soil Ecol 17:1–17. doi:10.1016/s0929-1393(00)00135-9
Wall JW, Skene KR, Neilson R (2002) Nematode community and trophic structure along a sand dune succession. Biol Fertil Soils 35:293–301
Wilson JB, Sykes MT (1999) Is zonation on coastal sand dunes determined primarily by sand burial or by salt spray? A test in New Zealand dunes. Ecol Lett 2:233–236
Zoon FC, Troelstra SR, Maas PWT (1993) Ecology of the plant-feeding nematode fauna associated with sea buckthorn (Hippophaë rhamnoides L. ssp. rhamnoides) in different stages of dune succession. Fundam Appl Nematol 16:247–258
Acknowledgments
We thank Sven-Erik Burger and André Kamp for assistance with the greenhouse experiments. The former Water and Civil Board ‘De Brielse Dijkring’ kindly permitted to sample their terrains. This is NIOO-KNAW publication 5821.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Rights and permissions
About this article
Cite this article
Brinkman, E.P., Duyts, H., Karssen, G. et al. Plant-feeding nematodes in coastal sand dunes: occurrence, host specificity and effects on plant growth. Plant Soil 397, 17–30 (2015). https://doi.org/10.1007/s11104-015-2447-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2447-z