Abstract
Aims
The aims were to identify the effects of interactions between litter decomposition and rhizosphere activity on soil respiration and on the temperature sensitivity of soil respiration in a subtropical forest in SW China.
Methods
Four treatments were established: control (CK), litter removal (NL), trenching (NR) and trenching together with litter removal (NRNL). Soil CO2 efflux, soil temperature, and soil water content were measured once a month over two years. Soil respiration was divided into four components: the decomposition of basic soil organic matter (SOM), litter respiration, root respiration, and the interaction effect between litter decomposition and rhizosphere activity. A two-factor regression equation was used to correct the value of soil CO2 efflux.
Results
We found a significant effect of the interaction between litter decomposition and rhizosphere activity (R INT) on total soil respiration, and R INT exhibited significant seasonal variation, accounting for 26 and 31 % of total soil respiration in the dry and rainy seasons, respectively. However, we found no significant interaction effect on the temperature sensitivity of soil respiration. The temperature sensitivity was significantly increased by trenching compared with the control, but was unchanged by litter removal.
Conclusions
Though the interaction between litter decomposition and rhizosphere activity had no effects on temperature sensitivity, it had a significant positive effect on soil respiration. Our results not only showed strong influence of rhizosphere activity on temperature sensitivity, but provided a viable way to identify the contribution of SOM to soil respiration, which could help researchers gain insights on the carbon cycle.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil is the largest organic carbon pool in terrestrial ecosystems (Lal 2004). Global soil respiration (R S), which has been estimated to be 98 ± 12 Pg C yr−1 in 2008 (Bond-Lamberty and Thomson 2010), is an important source of atmospheric CO2 (Raich and Schlesinger 1992; Friedlingstein et al. 2006). Soil respiration contains many components that are difficult to distinguish (Kuzyakov 2006). However, to obtain a better understanding of C-cycling on regional and global scales, previous studies have developed methods to quantify the components of R S (Hanson et al. 2000; Kuzyakov 2006; Subke et al. 2006). Soil respiration is usually divided into autotrophic respiration (R A) and heterotrophic respiration (R H) (Hanson et al. 2000; Subke et al. 2006). In forest ecosystems, trenching (or girdling) has been widely used to partition R A and R H (Hogberg et al. 2001; Subke et al. 2006; Li et al. 2010; Sayer and Tanner 2010), and the contribution of R H was 45–70 % of R S, which declined with increasing annual R S (Subke et al. 2006) . Since litter decomposition commonly makes a significant contribution to R S, litter removal has been used to quantify the contribution of aboveground litter decomposition (R AL) to R S (C AL) in forest ecosystems (Bowden et al. 1993; Sulzman et al. 2005; Sayer 2006; Wang et al. 2013). Combining the above, the decomposition of soil organic matter (SOM) can thus be calculated as follows: R SOM = R S–R A–R AL, thereby enabling estimates of the contribution of SOM decomposition to R S (C SOM) (Rey et al. 2002; Sulzman et al. 2005; Chang et al. 2008).
However, the above equation may underestimate the contribution of SOM to R S. For example, Rey et al. (2002) reported lower estimated values of C SOM compared with measured values based on experiments combining trenching with litter removal. This discrepancy is due to the priming effect caused by both rhizosphere activity (Fu and Cheng 2002; Kuzyakov 2002) and litter decomposition (Park et al. 2002; Kalbitz et al. 2007). Subke et al. (2004) indicated that since both litter decomposition and rhizosphere activity can promote SOM decomposition, they may positively interact. Therefore, to correctly partition the components of R S, it is important to quantify this interaction. However, few studies have investigated this interaction in forest ecosystems (see Subke et al. 2004).
Using experimental data collected from a subtropical montane cloud forest in SW China, this study tested the hypothesis that litter decomposition and rhizosphere activity has a positive interaction effect on soil respiration. In this forest, SOM mainly comes from roots and aboveground litter. Since roots release both low- and high-weight substances, such as sugars, amino acids, enzymes, and mucilage (Nguyen 2003), and litter decomposition percolates dissolved organic carbon into the mineral soil (Kalbitz et al. 2000), the trenching and litter removal treatments prevented labile input. Temperature-quality hypothesis suggests that old, low-quality SOM causes higher temperature sensitivity (Q 10) due to the higher activation energy required for the decomposition of low-quality SOM (Bosatta and Agren 1999); previous studies have supported this hypothesis (Davidson and Janssens 2006; Conant et al. 2008; Wetterstedt et al. 2010; Suseela et al. 2013). Therefore, we also hypothesized negative effects of litter decomposition and rhizosphere activity on the temperature sensitivity of R S.
Materials and methods
Site description
This experiment was conducted at the Ailaoshan Station for Subtropical Forest Ecosystem Studies (24°32′N, 101°01′E; 2,480 m above sea level) of the Chinese Ecological Research Network, which is located in Jingdong County, Yunnan Province. Over the past 10 years (2002–2011), the annual mean air temperature was 11.3 °C, with a minimum monthly mean temperature of 5.7 °C in January and a maximum monthly mean temperature of 15.6 °C in July. The average annual rainfall was 1,778 mm, with 86.0 % falling in the rainy season (May–October) (Fig. 1). The forest is influenced by the southwest monsoon and is exposed to frequent and intense wind and mist events throughout the year. The forest is described as a subtropical cloud forest, given the abundant moisture and persistent cloud cover (Song et al. 2012; Zhang et al. 2012). The dominant tree species in the forest are Vaccinium duclouxii, Lithocarpus chintungensis, and Schima noronhae, along with Sinarundinaria nitida in the shrub layer, and the litterfall is 864 g m−2 year−1. The soils are Alfisols, which have a pH value of 4.5, soil organic carbon 304 g kg−1, and total nitrogen 18 g kg−1 in the humus horizon (Chan et al. 2006).
Experiment design
Three plots (10 × 10 m) were selected in the forest, and four subplots were established in each plot: control (CK), litter removal (NL), trenching (NR) and trenching with litter removal (NRNL). Cover structures (i.e., a bamboo framework covered with 1-mm nylon mesh, 1 × 1 m) were established in the NL subplots at a height of 1.2 m above the ground to prevent new litter dropping. Visible litter in the subplots was removed at the beginning of this experiment. In the NR subplots, PVC pipes (diameter 630 mm, height 500 mm) were used for trenching. A circular trench (width about 300 mm) was dug to 500 mm, to form a cylinder of soil contained by the PVC pipe; soil was backfilled by its original layers with topsoil over subsoil (see Fig. S1). NRNL plots were established by combining the NL and NR treatments. In the center of each subplot, one PVC connector was permanently inserted into the soil to a depth of 20 mm at the beginning of the experiment, and a PVC top-closed pipe (diameter 200 mm, height 200 mm) was mounted on the connector, constituting a respiration chamber when we measured soil CO2 efflux.
Data collection
The experimental setup was finished on 15 January 2010 and measurements began on 7 February 2010, continuing for two years. Soil CO2 efflux was measured monthly using a gas analyzer (LI-840; Li-cor, Lincoln, NE, USA) between 9:00 and 11:00 (Beijing Time) to avoid diurnal fluctuations. Soil temperature (T, °C) was measured at 50 mm depth with a digital thermometer (6310; Spectrum, Illinois, USA) and soil water content (W, %) was measured by time domain reflectometry (MP-KIT; Beijing Channel, Beijing, China).
Soil CO2 efflux (R) was calculated as follows:
where R is the soil CO2 efflux (μmol m−2 s−1); M is the CO2 molar mass; V 0, P 0 and T 0 are constants (22.4 L · mol−1, 1013.25 hPa, and 273.15 K, respectively); Ta is air temperature (K); H is the height of the respiration chamber (m); and dc/dt is the slope of CO2 concentration variation with time over the measurement period.
Calculations
As soil temperature and soil water content affected soil respiration in forest ecosystems, previous studies have developed two-factor regression models to reflect the relationship of soil respiration with soil temperature and soil water content (Xu and Qi 2001; Qi et al. 2002). At the present site, soil temperature and soil water content showed similar seasonal variations (Fig. 2). Considering their interaction effect on soil CO2 effluxes, their product was taken as variable. Thus, a two-factor regression model was developed to reveal the relationship of soil CO2 efflux with soil temperature and soil water content, and given high regression coefficients of determination (R2 values) ranged from 0.76 to 0.93 (Fig. S2). The two-factor regression model equation was as follow:
where a and b are constants estimated from regression model (details see Fig. S2), T is soil temperature (°C), and W is soil water content (%).
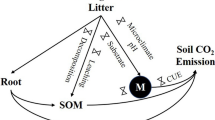
R S, R NL, R NR, and R NRNL represent the soil CO2 effluxes of CK, NL, NR, and NRNL, respectively. We divided soil respiration into four components: basic SOM respiration (R SOM), litter respiration (R L), root respiration (R R), and a component representing the interaction between litter decomposition and rhizosphere activity (R INT) (Fig. 3). The two-factor regression model showed that both T and W had positive effects on soil CO2 efflux (Fig. S2), while the treatments affected the soil microclimate (Sayer 2006; Sayer and Tanner 2010), especially W (Fig. 2). Therefore, to eliminate the biases due to soil microclimate change, effluxes should be compared under the same environmental conditions. Accordingly, the mean soil temperature and soil water content measured in control subplots were used to correct the values of R S, R NL, R NR, and R NRNL by Eq. (2). The same T and W variables, but different parameters (a and b) for each subplot, were used for this correction (Fig. S2). The correction did not change R S and R NL, but it reduced R NR and R NRNL (Fig. S3).
Soil respiration divided into four components: basic SOM decomposition (R SOM), litter respiration (R L), root respiration (R R), and interaction (R INT). R A is autotrophic respiration including R R and R INT, R AL is aboveground litter decomposition including R L and R INT, R H is heterotrophic respiration including R SOM and R L, and R NL is soil CO2 efflux after litter removal including R SOM and R R
We calculated the components as follows (details see Fig. 3):
Temperature sensitivity (Q 10) was calculated from R T+10/R T; R T was a regression model of efflux with soil temperature in field experiments. For example, an exponential equation (Q 10 = eb*T) was used to estimated Q 10 previously, which was derived from a one-factor regression model (R = R 0*eb*T). However, one-factor regression model could not well reveal the relationship of efflux with soil temperature. Therefore, as mentioned above, two-factor regression models were developed for better understanding of the relationship. Q 10 was not a constant; it changed with the variations of soil temperature and soil water (Davidson and Janssens 2006).
Base on the two-factor regression model (Eq. 2), we could get R T and R T+10.
where W in R T was the same to R T+10, because R T+10 meant efflux rate after increasing 10 °C soil temperature, and thereby soil water content should keep the same compared to R T. Therefore, we developed Q 10 equation as follow:
where a and b are as estimated by Eq. (2) for each subplot (Fig. S2). Eq (10) revealed the relationship of Q 10 with soil temperature and soil water content as reviewed by Davidson and Janssens (2006); Q 10 should be compared under the same environmental conditions for different treatments. Therefore, the mean soil temperature and soil water content measured in control subplots were used to calculate Q 10 values of all subplots for each measurement time.
Statistical analysis
All the data were subjected to tests for normality and homoscedasticity before ANOVA analyses. Univariate general linear modeling (GLM) was used to test the interaction effect of litter and root treatments. All differences were tested for statistical significance at the 95 % level. Statistical analyses were performed with SPSS 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Influence of interaction between litter and rhizosphere on soil respiration
The Univariate GLM analyses showed that both rhizosphere activity and litter decomposition had significant effects on R S, and there were significant interactions between them (Table 1A).
R INT exhibited seasonal variation, increasing rapidly from the dry season to the rainy season (Fig. 4a). The average value in dry season (0.82 ± 0.28 μmol · m−2 · s−1) was significantly different from that in rainy season (2.55 ± 0.50 μmol · m−2 · s−1) (Fig. 4b).
Seasonal variability in soil respiration components a and their contributions to soil respiration c: open circles represent R SOM or C SOM, upward-facing triangles represent R INT or C INT, downward-facing triangles represent R R or C R, and squares represent R L or C L. Comparisons among components in dry season average, rainy season average, and annual mean b, d: black vertical bar represents R SOM or C SOM, dark gray represents R INT or C INT, gray represents R R or C R, and white represents R L or C L; all data passed the normality and homoscedasticity tests before one-way ANOVA analysis; different letters for the same measurement time indicate significant differences (as indicated by LSD’s post-hoc test); *means significant difference between dry and rainy seasons (independent t testes). Data are mean + SE (n = 3)
The contribution of R INT to R S (CINT) showed a similar pattern to its flux; i.e., higher contributions in the rainy season (Fig. 4c). However, C INT in dry season (27 ± 8 %) did not significantly differ to that in rainy season (31 ± 6 %). R INT contributed 30 ± 7 % to R S as the annual mean (Fig. 4d).
Single-factor regressions of R INT as a linear function of T only, and as a linear function of W only, showed that T and W explained 64 and 85 % of the variation in R INT, respectively. Linear regression of T with C INT and logarithmic regression of W with C INT showed that T and W explained 41 and 93 % of the variation in C INT, respectively (Fig. 5).
Comparisons to other components
R SOM showed similar patterns to those for R INT; i.e., higher fluxes in the rainy season. R L showed minor seasonal variations whereas R R was largely unchanged throughout the year (Fig. 4a). There was significant difference for R SOM between dry season and rainy season, however, no significant differences were observed for R R and R L (Fig. 4b).
The contribution of R SOM to R S (C SOM) showed a similar pattern to that of C INT, whereas the contribution of R R to R S (C R) showed the opposite pattern, with large contributions during the dry season. The contribution of R L to R S (C L) showed no seasonal variations. Values of C SOM, C R, and C L were respectively 40 ± 4 %, 24 ± 2 %, and 9 ± 3 % in the dry season, and 49 ± 2 %, 11 ± 2 %, and 9 ± 4 % in the rainy season, among which only C R had significant difference between dry season and rain season (Fig. 4d).
In dry season, R INT only had significant difference with R L, while in rainy season and annual time, it had significant differences with not only R L but also R SOM and R R (Fig. 4b). The pattern also applied for C INT that was significant different from C L in dry season, while with C L,C SOM and C R in both rainy season and annual time (Fig. 4d).
Temperature sensitivity
Q 10 showed similar seasonal patterns in CK and NL, with both lowest and highest values in dry season. The lowest values (1.35 ± 0.06 and 1.24 ± 0.03, respectively) appeared in March 2010 while the highest values (2.19 ± 0.07 and 2.06 ± 0.03, respectively) in January 2012. Similar seasonal patterns were also observed in NR and NRNL, with lowest values (1.62 ± 0.01 and 1.63 ± 0.01, respectively) in July 2011, and highest values (2.61 ± 0.15 and 2.71 ± 0.14, respectively) in January 2011 (Fig. 6a). Q 10 values of CK, NL, NR, and NRNL were significantly different between dry season and rainy season (Fig. 6b).
Seasonal variation of Q 10 values of the four treatments, calculated using Eq. (10) for each measurement time point a (mean + SE, n = 3). Comparisons among treatments in dry season average, rainy season average and annual mean b (mean + SE, n = 3): all data passed the normality and homoscedasticity tests before one-way ANOVA analysis; different letters for the same measurement time indicate significant differences (as indicated by LSD’s post-hoc test); *means significant difference between dry and rainy seasons (independent t testes, p < 0.05)
Univariate GLM analyses showed no significant effect of interaction between litter decomposition and rhizosphere activity on Q 10 values, but a significant effect of rhizosphere activity on Q 10 (Table 1B). One way ANOVA analyses showed Q 10 values were not different between CK and NL (p > 0.05), but they were significantly higher in NR and NRNL than in CK, in dry season, rainy season, and annual time (Fig. 6b).
Discussion
As shown previously, intact rhizosphere activity is directly linked to litter decomposition (Subke et al. 2004; Subke et al. 2011). In the present study, the interaction between rhizosphere activity and litter decomposition had a significant impact on soil respiration (Table 1A). In terms of annual mean, R INT accounted for 30 % of R S (Fig. 4b). Clearly, our work indicates that R INT plays an important role in determining the dynamics of R S. The mechanism linking litter and rhizosphere is possibly microbial activity (Li et al. 2004; Subke et al. 2004; Feng et al. 2009). Litter and roots provide easily decomposable carbon for microbial growth, which leads to a positive feedback on SOM decomposition (Dighton et al. 1987; Chapela et al. 2001). In addition, we found that R INT and C INT were controlled by soil moisture (Fig. 5), and the lower values we observed in the dry season are perhaps due to competition for soil moisture between roots and rhizosphere microbes (Kuzyakov 2002).
As it shown in Fig. 3, R INT was in the realm of autotrophic respiration if soil respiration was divided into autotrophic and heterotrophic respiration, and was also in the realm of aboveground litter decomposition (R AL) if that was divided into aboveground and belowground respiration. Conclusively, the contribution of SOM to R S which was estimated from above two partition methods as mentioned in introduction would be underestimated.
R INT may include two components: SOM decomposition primed by both litter and rhizosphere activities, and litter decomposition primed by rhizosphere activity alone. Previous studies have shown that rhizosphere activity (Fu and Cheng 2002; Kuzyakov 2002) and litter decomposition (Park et al. 2002; Kalbitz et al. 2007) can cause priming effects on SOM decomposition. Over short timescales (in our case, 2 years) and in small areas (in our case, 1 m2), litter removal treatments cannot be expected to affect forest function (Sayer 2006), and nor can they be expected to influence R R, because R R is driven primarily by photosynthesis (Kuzyakov and Cheng 2001; Wang et al. 2010). However, rhizosphere activity can promote litter decomposition (Subke et al. 2004; Kuzyakov et al. 2007; Subke et al. 2011).
The lack of significant differences in Q 10 values between the CK and NL treatments, combined with the significant differences between the CK and NR (and NRNL) treatments (Fig. 6b), indicate that roots have a strong effect on the Q 10 of soil respiration; this inference was supported by the results of Univariate GLM (Table 1B). The results obtained for the NR treatment are consistent with temperature-quality hypothesis, as shown previously (Fierer et al. 2005; Knorr et al. 2005; Davidson and Janssens 2006; Conant et al. 2008; Hartley and Ineson 2008; Suseela et al. 2013), although some contrary results have also been reported (Giardina and Ryan 2000; Reichstein et al. 2000). Other studies have reported similar temperature dependences for different kinds of SOM (Cox et al. 2000; Fang et al. 2005; Jones et al. 2005), similar to our results for NL. The contrasting results we obtained for NR and NL may be explained in terms of labile carbon. The NL treatment allowed root rhizosphere activity, thereby permitting labile carbon as input into the soil. Although the NR treatment allowed litter input, little labile carbon made its way into the soil because trenching prevented the process of litter decomposition. This explanation is further supported by the fact that we observed no significant interaction between the litter and roots treatments (Table 1B). The influence of interactions between litter and rhizosphere activities on soil respiration have received little attention to date (Subke et al. 2004; Subke et al. 2011), and our study not only demonstrates the importance of such interactions, but also the need for further research to quantify these types of interactions in different ecosystems over longer timescales.
Conclusion
We found a significant positive effect of the interaction between litter decomposition and rhizosphere activity on soil respiration. The annual mean average values for C INT, C SOM, C R, and C L were 30 %, 46 %, 15 %, and 9 %, respectively. R INT and C INT showed seasonal variations driven by soil water content. Our finding of an interaction effect on soil respiration (R INT) suggests a way to identify the contribution of SOM to soil respiration and thereby enables an improved understanding of the carbon cycle on the regional scale. Litter decomposition had no effect on the temperature sensitivity of soil respiration, whereas rhizosphere activity had a strong effect. Together, they had no significant interaction effect on temperature sensitivity. Our results provided solid results to prove the temperature-quality hypothesis and showed a viable way for further studies to gain more insights on global carbon cycle.
References
Bond-Lamberty B, Thomson A (2010) Temperature-associated increases in the global soil respiration record. Nature 464:579–582
Bosatta E, Agren GI (1999) Soil organic matter quality interpreted thermodynamically. Soil Biol Biochem 31:1889–1891
Bowden RD, Nadelhoffer KJ, Boone RD, Melillo JM, Garrison JB (1993) Contributions of aboveground litter, belowground litter, and root respiration to total soil respiration in a temperate mixed hardwood forest. Can J For Res-Rev Can Rech For 23:1402–1407
Chan OC, Yang X, Fu Y, Feng Z, Sha L, Casper P, Zou X (2006) 16S rRNA gene analyses of bacterial community structures in the soils of evergreen broad-leaved forests in south-west China. FEMS Microbiol Ecol 58:247–259
Chang SC, Tseng KH, Hsia YJ, Wang CP, Wu JT (2008) Soil respiration in a subtropical montane cloud forest in Taiwan. Agr Forest Meteorol 148:788–798
Chapela IH, Osher LJ, Horton TR, Henn MR (2001) Ectomycorrhizal fungi introduced with exotic pine plantations induce soil carbon depletion. Soil Biol Biochem 33:1733–1740
Conant RT, Steinweg JM, Haddix ML, Paul EA, Plante AF, Six J (2008) Experimental warming shows that decomposition temperature sensitivity increases with soil organic matter recalcitrance. Ecology 89:2384–2391
Cox PM, Betts RA, Jones CD, Spall SA, Totterdell IJ (2000) Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 408:184–187
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–173
Dighton J, Thomas ED, Latter PM (1987) Interactions between tree roots, mycorrhizas, a saprotrophic fungus and the decomposition of organic substrates in a microcosm. Biol Fert Soils 4:145–150
Fang CM, Smith P, Moncrieff JB, Smith JU (2005) Similar response of labile and resistant soil organic matter pools to changes in temperature. Nature 433:57–59
Feng WT, Zou XM, Schaefer D (2009) Above- and belowground carbon inputs affect seasonal variations of soil microbial biomass in a subtropical monsoon forest of southwest China. Soil Biol Biochem 41:978–983
Fierer N, Craine JM, McLauchlan K, Schimel JP (2005) Litter quality and the temperature sensitivity of decomposition. Ecology 86:320–326
Friedlingstein P, Cox P, Betts R, Bopp L, Von Bloh W, Brovkin V, Cadule P, Doney S, Eby M, Fung I, Bala G, John J, Jones C, Joos F, Kato T, Kawamiya M, Knorr W, Lindsay K, Matthews HD, Raddatz T, Rayner P, Reick C, Roeckner E, Schnitzler KG, Schnur R, Strassmann K, Weaver AJ, Yoshikawa C, Zeng N (2006) Climate-carbon cycle feedback analysis: results from the C4MIP model intercomparison. J Clim 19:3337–3353
Fu SL, Cheng WX (2002) Rhizosphere priming effects on the decomposition of soil organic matter in C4 and C3 grassland soils. Plant Soil 238:289–294
Giardina CP, Ryan MG (2000) Evidence that decomposition rates of organic carbon in mineral soil do not vary with temperature. Nature 404:858–861
Hanson PJ, Edwards NT, Garten CT, Andrews JA (2000) Separating root and soil microbial contributions to soil respiration: A review of methods and observations. Biogeochemistry 48:115–146
Hartley IP, Ineson P (2008) Substrate quality and the temperature sensitivity of soil organic matter decomposition. Soil Biol Biochem 40:1567–1574
Hogberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A, Hogberg MN, Nyberg G, Ottosson-Lofvenius M, Read DJ (2001) Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411:789–792
Jones C, McConnell C, Coleman K, Cox P, Falloon P, Jenkinson D, Powlson D (2005) Global climate change and soil carbon stocks; predictions from two contrasting models for the turnover of organic carbon in soil. Glob Chang Biol 11:154–166
Kalbitz K, Solinger S, Park JH, Michalzik B, Matzner E (2000) Controls on the dynamics of dissolved organic matter in soils: A review. Soil Sci 165:277–304
Kalbitz K, Meyer A, Yang R, Gerstberger P (2007) Response of dissolved organic matter in the forest floor to long-term manipulation of litter and throughfall inputs. Biogeochemistry 86:301–318
Knorr W, Prentice IC, House JI, Holland EA (2005) Long-term sensitivity of soil carbon turnover to warming. Nature 433:298–301
Kuzyakov Y (2002) Review: factors affecting rhizosphere priming effects. J Plant Nutr Soil Sci-Z Pflanzenernähr Bodenkd 165:382–396
Kuzyakov Y (2006) Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol Biochem 38:425–448
Kuzyakov Y, Cheng W (2001) Photosynthesis controls of rhizosphere respiration and organic matter decomposition. Soil Biol Biochem 33:1915–1925
Kuzyakov Y, Hill PW, Jones DL (2007) Root exudate components change litter decomposition in a simulated rhizosphere depending on temperature. Plant Soil 290:293–305
Lal R (2004) Soil carbon sequestration impacts on global climate change and food security. Science 304:1623–1627
Li YQ, Xu M, Sun OJ, Cui WC (2004) Effects of root and litter exclusion on soil CO2 efflux and microbial biomass in wet tropical forests. Soil Biol Biochem 36:2111–2114
Li XD, Fu H, Guo D, Wan CG (2010) Partitioning soil respiration and assessing the carbon balance in a Setaria italica (L.) Beauv. Cropland on the Loess Plateau, Northern China. Soil Biol Biochem 42:337–346
Nguyen C (2003) Rhizodeposition of organic C by plants: mechanisms and controls. Agronomie 23:375–396
Park JH, Kalbitz K, Matzner E (2002) Resource control on the production of dissolved organic carbon and nitrogen in a deciduous forest floor. Soil Biol Biochem 34:813–822
Qi Y, Xu M, Wu J (2002) Temperature sensitivity of soil respiration and its effects on ecosystem carbon budget: nonlinearity begets surprises. Ecol Model 153:131–142
Raich JW, Schlesinger WH (1992) The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus Ser B Chem Phys Meteorol 44:81–99
Reichstein M, Bednorz F, Broll G, Katterer T (2000) Temperature dependence of carbon mineralisation: conclusions from a long-term incubation of subalpine soil samples. Soil Biol Biochem 32:947–958
Rey A, Pegoraro E, Tedeschi V, De Parri I, Jarvis PG, Valentini R (2002) Annual variation in soil respiration and its components in a coppice oak forest in Central Italy. Glob Chang Biol 8:851–866
Sayer EJ (2006) Using experimental manipulation to assess the roles of leaf litter in the functioning of forest ecosystems. Biol Rev 81:1–31
Sayer EJ, Tanner EVJ (2010) A new approach to trenching experiments for measuring root-rhizosphere respiration in a lowland tropical forest. Soil Biol Biochem 42:347–352
Song L, Liu WY, Ma WZ, Qi JH (2012) Response of epiphytic bryophytes to simulated N deposition in a subtropical montane cloud forest in southwestern China. Oecologia 170:847–856
Subke JA, Hahn V, Battipaglia G, Linder S, Buchmann N, Cotrufo MF (2004) Feedback interactions between needle litter decomposition and rhizosphere activity. Oecologia 139:551–559
Subke JA, Inglima I, Francesca Cotrufo M (2006) Trends and methodological impacts in soil CO2 efflux partitioning: A metaanalytical review. Glob Chang Biol 12:921–943
Subke JA, Voke NR, Leronni V, Garnett MH, Ineson P (2011) Dynamics and pathways of autotrophic and heterotrophic soil CO2 efflux revealed by forest girdling. J Ecol 99:186–193
Sulzman EW, Brant JB, Bowden RD, Lajtha K (2005) Contribution of aboveground litter, belowground litter, and rhizosphere respiration to total soil CO2 efflux in an old growth coniferous forest. Biogeochemistry 73:231–256
Suseela V, Tharayil N, Xing B, Dukes JS (2013) Labile compounds in plant litter reduce the sensitivity of decomposition to warming and altered precipitation. New Phytol 200:122–133
Wang W, Peng SS, Fang JY (2010) Root respiration and its relation to nutrient contents in soil and root and EVI among 8 ecosystems, northern China. Plant Soil 333:391–401
Wang Q, He T, Wang S, Liu L (2013) Carbon input manipulation affects soil respiration and microbial community composition in a subtropical coniferous forest. Agr Forest Meteorol 178:152–160
Wetterstedt JAM, Persson T, Agren GI (2010) Temperature sensitivity and substrate quality in soil organic matter decomposition: results of an incubation study with three substrates. Glob Chang Biol 16:1806–1819
Xu M, Qi Y (2001) Soil-surface CO2 efflux and its spatial and temporal variations in a young ponderosa pine plantation in northern California. Glob Chang Biol 7:667–677
Zhang YJ, Meinzer FC, Qi JH, Goldstein G, Cao KF (2012) Midday stomatal conductance is more related to stem rather than leaf water status in subtropical deciduous and evergreen broadleaf trees. Plant Cell Environ 36:149–158
Acknowledgments
We thank Mr. Wenzheng Yang and Mr. Xin Luo for their careful observations and measurements. This study was supported by the Natural Science Foundation of Yunnan Province, China (2011FA025), the Development Program in Basic Science of China (2010CB833501-01-07), the Strategic Priority Research Program of the Chinese Academy of Sciences, (XDA05050601-01-05; XDA05050206), and the National Science Foundation of China (31290221). We also thank two anonymous reviewers for their constructive comments in improving this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Zucong Cai..
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
The process of trenching. (JPEG 809 kb)
Fig. S2
The relationship of soil CO2 efflux with soil temperature and soil water content for each subplot. Parameters (a, b), R2, and p values estimated from regression model (R = a * T * W + b). (JPEG 5390 kb)
Fig. S3
Seasonal variability in soil CO2 effluxes of the four treatments (both measured and modeled data). (JPEG 2653 kb)
Rights and permissions
About this article
Cite this article
Wu, C., Zhang, Y., Xu, X. et al. Influence of interactions between litter decomposition and rhizosphere activity on soil respiration and on the temperature sensitivity in a subtropical montane forest in SW China. Plant Soil 381, 215–224 (2014). https://doi.org/10.1007/s11104-014-2106-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2106-9