Abstract
Aim
Rhizobacteria can influence plant growth and metal accumulation. The aim of this study was to evaluate the effect of rhizobacterial inoculants on the Ni phytoextraction efficiency of the Ni-hyperaccumulator Alyssum pintodasilvae.
Method
In a preliminary screening 15 metal-tolerant bacterial strains were tested for their plant growth promoting (PGP) capacity or effect on Ni bioaccumulation. Strains were selected for their Ni tolerance, plant growth promoting traits and Ni solubilizing capacity. In a re-inoculation experiment five of the previously screened bacterial isolates were used to inoculate A. pintodasilvae in two contrasting Ni-rich soils (a serpentine (SP) soil and a sewage sludge-affected agricultural (LF) soil).
Results
Plant growth was greater in serpentine soil (where it grows naturally) than in the LF soil, probably due to Cd phytotoxicity. Rhizobacterial inoculants influenced plant growth and Ni uptake and accumulation, but the effect of the strains was dependent upon soil type. The increase in plant biomass and/or Ni accumulation significantly promoted shoot Ni removal.
Conclusion
One strain (Arthrobacter nicotinovorans SA40) was able to promote plant growth and phytoextraction of Ni in both soil types and could be a useful candidate for future field-based trials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the last three decades there has been increasing interest in developing plant-based technologies for the remediation of contaminated soils (Chaney et al. 1997; Mench et al. 2009). For trace metal-contaminated soils, phytoextraction has been proposed as a potentially cost-effective option, which is less invasive than conventional civil engineering techniques for soil clean-up (e.g. encapsulation, vitrification, soil washing) and can even restore soil structure and functions (Moreno-Jiménez et al. 2012; Vangronsveld et al. 2009). Phytoextraction cultivates plants to accumulate trace metals from contaminated soils and transport them to the shoots which can then be harvested. Metal-hyperaccumulating plants are ideal candidates due to their extraordinary capacity to absorb and accumulate metals in their harvestable parts (Baker et al. 1994). The metal accumulation levels of hyperaccumulating plants can be several magnitudes higher than common values for other plants, although they are often only able to accumulate one or two trace elements (Chaney et al. 2007; Van der Ent et al. 2013). Phytoextraction using hyperaccumulators has been described as a cost-effective method to mine Ni from naturally Ni-rich ultramafic soils (Ni phytomining), or to remediate Ni phytotoxic soils (Bani et al. 2007; Chaney et al. 2007; He et al. 2012). Ash from incineration of Alyssum murale biomass contains approximately 20 % Ni and can be used as an ore in electric furnace refining of Ni (Chaney et al. 2007).
To be effective phytoextractors, hyperaccumulators must be highly metal tolerant, able to accumulate large concentrations of the targeted trace elements in harvestable shoots, and have a reasonable biomass production so that metal removal from the site is cost-effective (Li et al. 2003; Vangronsveld et al. 2009). Agronomic management practices (such as fertilisation, liming or herbicide regimes) have been proposed as a means of maximising the performance and yields of hyperaccumulator crops (Kukier et al. 2004; Li et al. 2003). Biotechnological approaches have also been suggested and several authors have proposed incorporating plant-associated microorganisms (rhizosphere and endophytic bacteria, as well as mycorrhizal fungi) into phytoextraction systems (Abou-Shanab et al. 2006; Kidd et al. 2009; Ma et al. 2009; Rajkumar and Freitas 2008a; Sessitsch et al. 2013).
Some microorganisms present plant growth promoting traits. Plant Growth Promoting Rhizobacteria (PGPR) can enhance tolerance, growth and survival under the stress conditions of metal-rich soils (e.g. nutrient deficiency, phytotoxic concentrations of trace metals). Many PGPR facilitate plant growth through the production of plant growth regulators and phytohormones (i.e. indoleacetic acid (IAA), gibberellins or cytokinins), or via the release of essential nutrients (e.g. N2-fixers, phosphate-solubilisers, and siderophore-producers), or the induction of plant defence mechanisms (Glick 2003; Glick et al. 1998; Weyens et al. 2009a). Zaidi et al. (2006) reported that an IAA-producing Bacillus subtilis strain was able to promote the growth of Brassica juncea and thereby increased Ni extraction. Inoculation with the plant growth-promoting bacterium Psychrobacter sp. SRS8 stimulated growth and Ni accumulation in Ricinus communis and Helianthus annuus grown in Ni-contaminated soil (Ma et al. 2011). Furthermore, microorganisms can modify trace metal mobility and phytoavailability through the release of chelating agents (organic acids and siderophores), acidification or redox changes (Gadd 2010; Lebeau et al. 2008). Rhizobacteria increased soil Ni availability and hyperaccumulation of Ni in Alyssum murale (Abou-Shanab et al. 2003, 2006). Cd- and Pb-mobilising rhizosphere bacterial strains enhanced the uptake of metals in tomato (Jiang et al. 2008) and a Zn-mobiliser promoted Zn accumulation in Ricinus communis (Rajkumar and Freitas 2008b).
In many cases the effects of these plant-microbial associations have been shown to be plant-species specific (Becerra-Castro et al. 2012). However, few studies have evaluated their efficiency in relation to the properties of the growth substrate. It seems likely that their effects may be both plant- and substrate-dependent. This study aimed at evaluating the effect of selected rhizobacterial strains on plant biomass production and Ni phytoextraction by the Ni-hyperaccumulator Alyssum pintodasilvae in two contrasting soils. Firstly, 15 bacterial isolates were screened for their PGP capacities by growing Alyssum pintodasilvae in a simple perlite:sand mixture (2:1 v/v). Secondly, Alyssum pintodasilvae was grown in two soils, a naturally Ni-rich serpentine soil and a sewage sludge-amended agricultural soil with Ni and Cd as the main contaminants, which were inoculated with five selected bacterial isolates. The effects of bacterial inoculants on soil metal availability, plant growth, nutrient status, Ni accumulation and extraction were evaluated.
Materials and methods
Screening of rhizobacterial isolates for promoting plant growth and Ni accumulation
Bacterial strains were previously isolated by Becerra-Castro et al. (2011) from the rhizosphere soil of two Ni-hyperaccumulating subspecies of Alyssum serpyllifolium Desf. (Brassicaceae): A. serpyllifolium subsp. lusitanicum Dudley and P. Silva (commonly referred to as A. pintodasilvae) and A. serpyllifolium subsp. malacitanum Rivas Goday (A. malacitanum). Both subspecies are endemic to the Iberian Peninsula (Asensi et al. 2004; Brooks et al. 1981; Menezes de Sequeira 1969). Alyssum pintodasilvae is found in the serpentinitic region of Trás-os-Montes in NE Portugal (Morais (M) and Samil (S)) and in the vicinity of Melide (L) in NW Spain, and A. malacitanum grows in the serpentinitic area of Sierra Bermeja (SB), Málaga in S Spain. The isolates were previously screened for Ni resistance, the ability to produce organic acids, and for various plant growth promoting (PGP) characteristics: phosphate solubilisation capacity, siderophore production and indoleacetic acid (IAA) production. In addition, they were characterised genotypically by BOX-PCR fingerprinting and comparative sequence analysis of partial 16S rRNA gene (Becerra-Castro et al. 2011). Isolate nomenclature (L, S, M or SB) indicates the serpentine site from which they originate. For this study 15 rhizobacterial strains were chosen, strains were selected according to their phenotypic traits (Table 1).
Seeds of A. pintodasilvae were collected from Trás-os-Montes (NE Portugal), surface-sterilized in 10 % sodium hypochlorite solution and then rinsed in sterile deionised water. Seeds were germinated on a 2:1 perlite:quartz sand mixture (2:1 v/v) in a growth chamber under controlled conditions (temperature 22–25 ºC, PPFD of 190 μmol m−2 s−1, under a 16/8 h light/dark cycle). Seeds were watered daily with deionised water until germination and thereafter with a Ni-rich serpentine-like macro-nutrient solution which consisted of 2 mM MgSO4, 0.8 mM Ca(NO3)2, 2.5 mM KNO3, 0.1 mM K2HPO4, 20 μM FeEDDHA, 10 μM H3BO3, 2 μM MnCl2, 1 μM ZnSO4, 0.5 μM CuSO4, 0.2 μM Na2MoO4 and 300 μM NiSO4 (based on Chaney et al. (2008)). One-month-old A. pintodasilvae seedlings were transferred into pots with the same perlite:quartz sand substrate. Three weeks after transferring into pots, seedlings were inoculated with one of the 15 bacterial strains. Fresh cultures of bacterial strains were grown in 869 medium (Mergeay et al. 1985) for 3 days, harvested by centrifugation (6,000 rpm, 15 min) and re-suspended in 10 mM MgSO4 to an OD660 of 1.0 (about 108 cells per mL). Each plant pot was inoculated with 9 mL of each bacterial suspension. The same amount of sterile 10 mM MgSO4 was added to non-inoculated plants. Eight replicates were prepared for each inoculation treatment. After inoculation, plants were watered with the Ni-rich nutrient solution (as above). Seven weeks after inoculation, plants were harvested and rinsed in deionised water to remove any adhering particles. Shoots and roots were separated, dried for 48 h at 40 °C and weighed to determine dry biomass. Plant aerial biomass was digested in a 2:1 HNO3:HCl mixture and the concentration of K, Ca, Mg, Fe, Cd, Cu, Mn, Ni, P, Pb and Zn were measured by ICP-OES (Vista Pro; Varian Inc., Australia). Data were expressed in mg kg−1 dry weight (DW) plant material. Shoot Ni removal was calculated as the product of the shoot Ni concentration and shoot DW yield.
Effect of selected rhizobacterial inoculants on Ni phytoextraction by A. pintodasilvae in two contrasting soils
Soil was collected from the serpentinitic region of Trás-os-Montes (SP) in Portugal (where A. pintodasilvae is a native species) and from the Louis Fargue (LF) field experiment in Villenave d’Ornon, Gironde, France (Boisson et al. 1998; Mench et al. 2006). The LF soil was treated with sewage sludge between 1976 and 1980 (total sludge input of 300 t DM ha−1) which showed high Ni and Cd concentrations (Mench et al. 2006; Weissenhorn et al. 1995). Soils were air-dried, sieved through a 2-mm stainless steel sieve and homogenised. Soil pH was measured in H2O using a 1:2.5 soil:solution ratio. Total C and N were analysed by combustion with a CHN analyser (Model CHN-1000, LECO Corp., St Joseph, MI). Exchangeable cations were extracted with 1 M NH4Cl. Calcium and Mg were determined by atomic absorption spectrometry (AAS; Perkin-Elmer 2380, Norwalk, CT). Available P was determined by Olsen’s NaHCO3 method (Olsen et al. 1954). Soils were digested in a 2:1 mixture of concentrated HNO3:HCl and pseudo-total concentrations of metals were analysed by AAS. Soil Ni availability was evaluated after extraction with 10 mM Sr(NO3)2 (Everhart et al. 2006). For pot preparation, the soils were mixed with perlite in the ratio of 10:1 (v/v) to improve aeration and drainage. Plastic pots (500 mL) were filled with either SP or LF soil (36 pots per soil), and one 4-week-old seedling of A. pintodasilvae (germinated under the same conditions as described above) was transplanted into each pot.
Five bacterial isolates were used for this study, these were selected to represent different phenotypic traits and also according to the results obtained in the preliminary screening described above. The selected strains were identified (by partial sequencing of 16S rDNA) as Microbacterium hydrocarbonoxydans SA17, Arthrobacter nicotinovorans SA40, Arthrobacter nitroguajacolicus LA44, Microbacterium sp. SA5b and Streptomyces lincolnensis SBA50. Strains SA5b, SA17 and SA40 significantly increased shoot DW yield of A. pintodasilvae in the preliminary screening, while root DW yield was highest in plants inoculated with strain LA44. Strain SBA50 was the only strain found to negatively affect plant biomass and was therefore used for comparative purposes. Phenotypic traits such as the production of organic acids or siderophores have been implicated in soil metal mobilisation and can influence metal uptake and bioaccumulation. The strains SA5b, SA17 and LA44 are organic acid-producers, SA17 and SA40 produce siderophores, LA44 and SA40 are IAA-producers, and SA40 is able to solubilise inorganic phosphate (Table 1; Becerra-Castro et al. 2011). In addition, the metabolites produced by strains SA5b, SA17 and SBA50 can solubilise Ni from serpentine soil (Becerra-Castro et al. 2011). Bacterial inoculants were prepared as mentioned above and 3 weeks after transferring into pots each plant was inoculated with 2 mL of bacterial suspension. The same amount of sterile 10 mM MgSO4 was added to non-inoculated pots. Six replicates were prepared for each inoculation treatment. Plants were grown in an environmentally controlled growth chamber for 5 months. At harvest, plants were rinsed in deionised water to remove any adhering soil particles. Shoots and roots were separated, dried for 48 h at 40 °C and weighed to determine dry biomass. Plant tissues were digested in a 2:1 HNO3:HCl mixture and Ca, Cu, Fe, K, Mg, Mn, Ni, P and Zn were measured by ICP-OES (Vista Pro; Varian Inc., Australia). Data were expressed in mg kg−1 dry weight (DW) plant material. The ability of A. pintodasilvae to bioconcentrate Ni in its aboveground biomass from either LF or SP soil (Bioconcentration Factor, BCF) was calculated as the ratio of the shoot Ni concentration and the pseudo-total Ni concentration in the soil. The effect of soil type and/or microbial inoculation on the overall Ni phytoextraction efficiency was assessed by taking into account plant growth, and was calculated as the product of the shoot DW yield and the shoot Ni concentration in relation to the total soil Ni content.
Statistical analyses
Significant effects of bacterial strains on biomass production, nutrient and metal content in both inoculation experiments were determined using ANOVA followed by the “post-hoc” Dunnett test whenever data were normally distributed, or using the Mann–Whitney test for non-parametric data when homogeneity of variance and normality could not be met.
Results
Influence of rhizobacteria on the growth and shoot ionome of Alyssum pintodasilvae grown in a perlite:sand substrate
Plant biomass production
The effects of the bacterial inoculants on the growth of A. pintodasilvae depended on the strains. Figure 1 shows the mean plant tissue dry weights (shoots and roots) in non-inoculated and inoculated plants. Five strains (SA5b, SA17, SA40, SBA5 and SBA82) significantly improved shoot biomass production (Fig. 1a). Shoot biomass increased by 1.7- to 2.3-fold compared to non-inoculated plants. Root biomass was only significantly increased in the case of SA5b, which increased root dry weight yield by 1.7-fold. These growth-promoting strains were originally isolated from the rhizosphere soil of two populations of Alyssum pintodasilvae (L and S) and one population of Alyssum malacitanum (SB). Plants inoculated with the siderophore-producer SA17 (Microbacterium hydrocarbonoxydans) showed the highest shoot DW yield, whereas those inoculated with the IAA-producer LA44 (Arthrobacter nitroguajacolicus) showed the highest root biomass. Strain SBA50 (Streptomyces lincolnensis) was the only strain which negatively affected the growth of A. pintodasilvae (both shoot and root biomass were reduced by approximately 60 % compared to non-inoculated plants; Fig. 1; p < 0.05).
Shoot ionome and shoot Ni removal
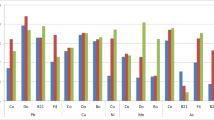
Although there was no clear generalised effect amongst bacterial inoculants and/or specific macro- or micro-nutrients, several strains significantly influenced the plant nutritional status (Fig. 1S, Supplementary material). Shoot concentrations (in mg kg−1) of Ca, Fe, K, Mg, Mn and P in non-inoculated plants were on average 31,900, 176, 53,000, 7,400, 184 and 8,400, respectively. Several strains (but not only those strains which improved biomass production) led to a significant increase in shoot Ca concentration (LA1, SA17, SA37, SBA82 and SBA86), K (LA80, SA5b and SA26), Mg (LA80), and Mn (LA1, LA10, LA80, SA37, SBA50, SBA82 and SBA86). One strain, identified as Arthrobacter oxydans SBA82 and which is able to solubilise inorganic phosphate, also tended to increase shoot P concentration, while the siderophore-producing strain SBA86 (Arthrobacter nitroguajacolicus) tended to increase shoot Fe content.
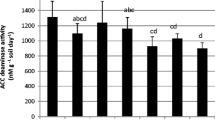
The mean shoot Ni concentration in non-inoculated plants was 6.2 ±1.1 g kg−1, and in general, inoculation of plants did not lead to a significant change in their shoot Ni concentration (values varied from 4.0 ± 0.4 to 9.3 ± 0.8 g Ni kg−1 (Fig. 2a). However, three inoculants significantly increased shoot Ni concentration, reaching values up to 1.5-fold higher than in controls: LA1 (9.1 ±0.7 g Ni kg−1), MA72 (8.6 ±0.8 g Ni kg−1) and SBA82 (9.3 ±0.8 g Ni kg−1). For strains SA40 and SBA82 the increase in plant biomass and/or shoot Ni concentration led to a significant increase in shoot Ni removal compared to that obtained with non-inoculated plants (Fig. 2b): mean Ni removal of control plants was 78 ±17 μg plant−1 compared to 161 ±16 and 157 ±32 μg plant−1 in SA40- and SBA82-inoculated plants, respectively (p < 0.05). Conversely, the negative effect of SBA50 on both growth and Ni accumulation significantly reduced (by 48 %) shoot Ni removal (Fig. 2b; p < 0.05).
Effect of selected rhizobacterial inoculants on the Ni phytoextraction efficiency of A. pintodasilvae grown in Ni-rich soils
To test these plant-microbial associations under contrasting soil conditions a reduced number of bacterial isolates were selected (four strains which stimulated plant growth and one which had a negative effect on growth).
Soil physicochemical characteristics and Ni phytoavailability
The serpentine (SP) soil presented a neutral pH (pHH2O 7.0), high concentrations (in mg kg−1 soil) of total Ni (3569), Co (154) and Cr (2587), and a predominance of Mg in the exchange complex. The LF agricultural soil had a pH close to neutrality (pHH2O 6.9), a significantly higher concentration of available P and a higher CEC (in this case dominated by Ca) compared to the SP soil (Table 2). The problematic trace metals in the LF soil were Ni (153 mg kg−1) and Cd (65 mg kg−1): the concentrations of both metals are higher than the maximum permitted by the EC in soils receiving sewage sludge (75 mg Ni kg−1 and 3 mg Cd kg−1) (Ewers 1991).
Before planting, the Ni phytoavailability assessed by the Sr(NO3)2-extractable Ni concentration was similar in both soils (1.49 ±0.01 and 2.18 ±0.05 mg kg−1 soil in SP and LF, respectively) despite the differences in total Ni concentration (Table 3). After plant growth and in non-inoculated treatments, Sr(NO3)2-extractable Ni concentrations were reduced to 1.36 ±0.05 and 1.33 ±0.12 mg Ni kg−1 in SP and LF. Bacterial inoculants led to some numerical changes in Ni phytoavailability compared to non-inoculated treatments, but this depended on the soil type and was generally not statistically significant. After inoculation with strains LA44 and SBA50 a decrease in Ni phytoavailability was observed in SP, while strain SA40 led to significant decrease in LF (p < 0.05), compared to the respective non-inoculated samples (Table 3).
Plant biomass production
After 5 months, plants produced a significantly higher biomass when grown in the SP soil compared to the LF soil (up to 9- and 2-fold higher shoot and root biomass, respectively) (p < 0.001; Fig. 3). Depending on the soil type, the microbial inoculants influenced plant biomass production; however these differences did not reach statistical significance (Fig. 3). The mean shoot DW yield of non-inoculated plants grown in SP soil was 239 ±60 mg plant−1, and this increased to 344 ±49, 439 ±94, 496 ±187 and 390 ±47 mg plant−1 in plants inoculated with strains LA44, SA5b, SA17 and SA40, respectively. Similarly, root dry weight production in plants inoculated with SA5b was significantly higher than control plants (p < 0.05). The effect of the bacterial inoculants on the growth of Alyssum pintodasilvae differed in the LF soil, in this case one isolate significantly improved plant growth (strain SA40). The mean shoot DW yield increased from 61 ±13 mg (non-inoculated plants) to 95 ±9 mg DW plant−1 after inoculation with this strain (Arthrobacter nicotinovirans SA40). Strain SBA50 had no effect on the shoot DW yield of Alyssum pintodasilvae in the LF soil but significantly reduced root DW yield (Fig. 3).
Shoot ionome and shoot Ni removal
Plant shoot tissues showed similar concentrations of the nutrients, Ca, Fe and K, when grown in either soil (Table 4). Shoot Mg concentrations however were significantly lower in the LF plants than in the SP ones, while shoot P and Zn concentrations were significantly higher in LF compared to SP plants. In general, bacterial inoculants did not significantly influence the shoot ionome. Only a few significant differences were found, and these were mainly in the LF soil (Table 4). In this soil, some inoculants significantly increased the shoot content of Ca (SBA50), Mg (SA17) or P (SBA50). Conversely, in the same soil some inoculants led to a significant decrease in nutrient contents, such as Ca (SA5b), Fe (SA40), Mg (SA5b and SA40) or Zn (SA5b and SA40). The mean shoot Cd concentration of non-inoculated plants grown in the LF soil was 462 ±25 mg kg−1. All inoculants (except SBA50) led to a significantly lower accumulation of Cd in shoots compared to non-inoculated plants (Table 4). In SP soil, the only significant effect of inoculation on shoot ionome was an increase in the shoot K concentration after inoculation with strain SA40 (Table 4).
Nickel accumulation by plants was significantly affected by soil type (p < 0.001). This was most pronounced in shoot tissues where Ni concentrations were 8-fold lower in LF plants than SP plants, while root Ni concentrations were only 1.6-fold lower in LF plants than SP plants (Fig. 4). In the SP soil, non-inoculated A. pintodasilvae had a mean shoot Ni concentration of 6,892 ± 387 mg kg−1 DW, whereas the mean Ni concentration in the shoots of LF plants was 839 ±94 mg kg−1 DW. For the SP plants the highest shoot Ni concentrations were found in those plants inoculated with strain LA44: mean concentrations increased from 6,892 ± 387 mg Ni kg−1 to 11,282 ± 1,856 mg Ni kg−1 (representing an increase of 64 %) (p < 0.05; Fig. 4). Moreover, this increase was accompanied by a reduction in root Ni concentrations, which resulted in a significant increase in the shoot:root Ni concentration ratio (from 6 to 9.7, Fig. 4). For the LF plants there was no clear effect of inoculation on shoot Ni accumulation (Fig. 4).
The LF plants showed a BCF of up to 2.8-fold higher than the SP plants (Table 5). For the SP plants, Ni bioaccumulation was significantly higher in plants inoculated with strains LA44 and SA40 (showing BCF values up to 1.6-fold higher; p <0.05). For the LF plants no significant differences in BCF values were observed after inoculation.
Ni phytoextraction efficiency
The percentage of Ni phytoextracted by plants grown in the SP soil was significantly higher than total Ni phytoextracted in LF soil (p < 0.05), mean values ranged from 0.17 ± 0.04 to 0.48 ± 0.10 % and from 0.06 ± 0.02 to 0.21 ± 0.01 %, respectively (Fig. 5). In SP soil, the rhizobacterial inoculants LA44, SA5b, SA17 and SA40 significantly increased the phytoextracted Ni (not significant in case of SA17 (p = 0.096)), while strain SBA50 did not affect the phytoextracted Ni from the soil. In LF soil, only strain SA40 significantly improved phytoextracted Ni compared to non-inoculated plants (Fig. 5; p < 0.05).
Discussion
The effect of bacterial inoculants on plant growth and metal accumulation has previously been shown to be plant species-specific (Becerra-Castro et al. 2012). Here, plant-microbial associations were evaluated in relation to the growth substrate. In general, there was a higher variability in the measured parameters (e.g. DW yields, shoot element concentrations) in inoculated plants than non-inoculated plants, and some tendencies regarding plant growth or Ni accumulation after inoculation were not always significant. However, in the initial screening experiment, inoculation with five bacterial strains significantly promoted the growth of A. pintodasilvae. Moreover, in two cases this enhancement in shoot biomass production led to an increase in phytoextracted Ni. These PGP strains included members of the genus Arthrobacter (SA40 and SBA82), Microbacterium (SA5b and SA17) or Curtobacterium (SBA5). No individual phenotypic trait was consistently found amongst strains which promoted growth. Two strains that produced moderate to high levels of the phytohormone IAA (>25 mg L−1) also significantly increased plant growth (Fig. 1; SBA5 and SBA82). Beneficial effects of bacterial inoculants on the growth of metal-exposed plants have often been attributed to the production of this phytohormone (Dell’Amico et al. 2008; Shilev et al. 2006). However, some of the strains used in the screening which stimulated plant growth (such as SA5b or SA17) did not show the capacity to produce IAA, and there was no clear correlation between their PGP traits and the induced growth promotion (these strains were either able to produce organic acids or siderophores). In the initial screening method, nutrient supply (via Hoagland solution) was presumably adequate for plant growth, whereas in a soil system essential nutrients (such as P or Fe) may be limiting. Under nutrient deficiency the PGP traits of the bacterial inoculants are more likely to be activated. Thus, in a plant-microorganism-soil system the bacterial response may differ from that observed when using a simple perlite/sand growth substrate.
Both serpentine soils and anthropogenic-contaminated soils have been suggested as suitable for Ni phytomining (Li et al. 2003). Serpentine soils develop from ultramafic parent material and are therefore frequently enriched in trace metals other than Ni, such as Co, Cr, Mn or Fe. In order to use hyperaccumulating plants to extract Ni from these soils they must be tolerant to these co-contaminants (Tappero et al. 2007). In the soil experiment, the soil type strongly affected the growth of A. pintodasilvae. High total Co and Cr concentrations in the SP soil did not negatively affect its growth or Ni bioaccumulation capacity. This is unsurprising since the SP soil was collected from a serpentine outcrop where this species is found growing naturally, and the soils are characterised by an elevated concentration of Co, Cr and Ni but low labile pools of Co and Cr. Alyssum pintodasilvae is adapted to serpentine soils, and to the unfavourable conditions that these present for plant growth and development, such as a high Ni phytoavailability, but also to poor fertility (deficiency in N, P and K), a high Mg:Ca quotient and low Fe solubility due to the near-neutral soil pH. In contrast, shoot biomass of A. pintodasilvae was up to 8-fold lower in the sewage sludge-amended soil (LF). The LF soil was co-contaminated with both Ni and Cd (Sr(NO3)2-extractable concentrations of Cd of 0.62 ±0.02 mg kg−1 soil was determined) and the poorer growth observed in this soil could have been due to the phytotoxicity of Cd. Cadmium is an element which is rarely found in appreciable concentrations in serpentine soils. Evidence of co-tolerance of hyperaccumulating Alyssum species to other metals (other than the hyperaccumulated metal) can be found in the literature. Elevated concentrations of Co or Zn had no effect on the plant’s ability to accumulate Ni in hydroponically-grown A. murale (Tappero et al. 2007). The authors concluded that A. murale could therefore be used to recover Ni from most metal-enriched soils containing these metal co-contaminants. Conversely, in hydroponics, the growth of the Ni hyperaccumulator Alyssum bertolonii was significantly reduced when the solution Cd concentration increased (0 to 10 μM CdSO4), and Cd was primarily accumulated in the root tissues (Barzanti et al. 2011). Cadmium is considered as a non-essential element for metabolic processes, and can negatively affect plant growth and development since it can replace essential elements that play a key role in active sites of enzymes or due to its high affinity for sulfhydryl groups (Vangronsveld and Clijsters 1994). Furthermore, compared to the SP soil, the LF soil presented significantly higher availability of nutrients such as P, and a CEC dominated by Ca (and not Mg). Calcium has been shown to depress both growth and nickel uptake by the Ni hyperaccumulator Alyssum bertolonii (Gabbrielli et al. 1990).
The shoot Ni concentrations of A. pintodasilvae were far above the criteria for Ni hyperaccumulators (>1,000 mg Ni kg−1) when grown in the serpentine (SP) soil, and were close to the threshold value when grown in the agricultural (LF) soil (Van der Ent et al. 2013). In both soils, shoot:root Ni transport ratios were above 1, confirming their ability to hyperaccumulate this element in the aboveground biomass. Soil Ni bioavailability (Sr(NO3)2-extractable Ni concentrations) was similar in both soils at the beginning of the experiment (and BCF values were even higher for LF plants). It is worth noting however that Sr(NO3)2-extractable concentrations of Ni were reduced after growth in the LF soil to a greater extent than in the SP soil, which could have contributed to the lower shoot Ni concentrations in the LF plants. Competitive interactions have also been shown to occur between Cd and Ni during the hyperaccumulation process (Assunção et al. 2008). In a hydroponic study, Ni uptake by Noccaea caerulescens was strongly suppressed in the presence of both Cd and Zn (Assunção et al. 2008). Antagonistic interactions such as these could explain the lower shoot Ni concentrations of A. pintodasilvae grown in the LF soil, since the phytoavailable concentration of Ni in the LF soil was not strongly in excess of that of Cd (while the opposite would be the case in SP soils).
Cabello-Conejo et al. (2013) found that the Ni phytoextraction efficiency of different Ni-hyperaccumulating Alyssum species grown in serpentine soil was, in decreasing order: A. murale > A. corsicum > A. malacitanum > A. pintodasilvae. Consequently, for considering A. pintodasilvae as a suitable candidate for Ni phytomining of serpentine soils, its biomass production and Ni extraction efficiency would need to be optimised. Similarly, in the case of the agricultural (LF) soil methods would need to be implemented to alleviate the Cd phytotoxicity symptoms as well as improve plant growth and biomass production. Plant growth promotion clearly plays a major role in the extraction and removal of trace elements since a simple improvement in biomass results in an increase in the overall shoot metal(loid) removal. Numerous studies have isolated and characterised rhizosphere or endophytic bacteria associated with trace element-tolerant or trace element-(hyper)accumulating plants as a means of identifying interesting strains for phytoextraction purposes (Rajkumar and Freitas 2008b; Weyens et al. 2009b). However, fewer studies have evaluated the application of these strains in contrasting soil types.
Five strains were selected for the bioaugmentation experiment in soils: four of these were selected for their positive influence on growth in the first screening experiment and based on their phenotypic characteristics. This allowed for evaluating the response of these bacterial inoculants under soil conditions, as well as studying the soil-specificity of bacterial-induced modifications in plant growth/Ni extraction efficiency. The rhizosphere isolate LA44 shows intense IAA-production, is an organic-acid producer and highly Ni-resistant. While SA5b, SA17 and SA40 present intermediate Ni resistance, and either produce organic acids (SA5b, SA17), siderophores (SA17, SA40) or solubilise inorganic phosphates (SA40). Strain SBA50 (highly Ni-resistant, no PGP trait), which had a negative effect on plant growth, was also included for comparative means. Bacterial-induced effects were found to be soil-specific: in the SP soil inoculation generally led to an enhanced plant growth and shoot Ni removal, whereas in the LF soil there was a general lack of a plant-growth promoting effect. The growth-promoting effect demonstrated in the first screening was also seen in inoculated plants grown in the SP soil (with strains SA5b, SA17 and SA40). However, strain SBA50 (Streptomyces lincolnensis), which reduced plant growth in the perlite/sand substrate did not significantly reduce biomass production in the SP soil. The two Microbacterium spp. (SA5b and SA17) which significantly improved Ni removal in the SP soil had no effect on plants grown in LF soil. However, strain SA40 (Arthrobacter sp.) improved shoot DW yields of plants grown in both soils (SP and LF). As mentioned above plant growth was greatly reduced in the LF soil compared to SP soil, possibly due to Cd phytotoxicity. Bacterial inoculants have been shown to reduce plant stress levels, for example, by producing the enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase which suppresses the production of stress ethylene in plants (Glick et al. 1998). The beneficial effect of strain SA40 on plant growth makes it very interesting for bioaugmentation of anthropogenic-contaminated soils. Moreover, the identification of a bacterial strain which has a growth-promoting effect in contrasting soil types is valuable for application in real-life scenarios, where edaphic properties are likely to vary greatly. At least in the case of the SP soil, the congruent results obtained between the initial screening experiment and the soil experiment, suggest that this screening method can be a useful tool for the rapid selection of interesting strains which can then be tested under more realistic conditions. Moreover, this screening method was more helpful in identifying potentially useful strains than the in vitro phenotypical characterisation of the strains since the effect of these inoculants cannot always be related to their PGP traits.
For strains SA5b, SA17 and SA40 the increase in shoot Ni removal was largely a consequence of the microbial-induced stimulation in plant biomass. For strain SA40, this was the case in both SP and LF soils. Inoculation with metal-resistant PGP bacteria has previously been shown to increase the biomass of several crops (e.g. Brassica juncea, Ricinus communis, Helianthus annuus) and other hyperaccumulators (e.g. Sedum alfredii) growing in metal-contaminated soils (Dell’Amico et al. 2008; Jiang et al. 2008; Mastretta et al. 2009; Zaidi et al. 2006). However, plant-associated microorganisms can also modify soil metal mobility, by acidification, chelation or ligand-induced solubilisation (Abou-Shanab et al. 2003, 2006). The literature generally cites two main groups of bacterially produced natural chelators: organic acids and siderophores. Here, strain LA44 (identified as Arthrobacter nitroguajacolicus) significantly enhanced shoot Ni concentrations in A. pintodasilvae in SP soil, which could presumably be a result of an enhanced Ni phytoavailability and hence plant uptake. Strain LA44 has been shown to be an efficient mobiliser of Ni from ultramafic rocks under in vitro conditions, and principally liberates Ni associated with Mn oxides through the exudation of oxalate (Becerra-Castro et al. 2013). Nickel shoot:root transport ratios were also significantly increased, suggesting this bacterial inoculant led to an increase in Ni translocation to aboveground plant parts. It is possible that strain LA44 enhances the replenishment of Ni labile phases in the soil thus increasing plant Ni uptake. The dynamic nature of these solution-solid phase interactions would explain why no corresponding increase in Sr(NO3)2-extractable Ni concentrations were observed after inoculating with this strain. Inoculating ultramafic soils with the actinobacterial Microbacterium arabinogalactanolyticum AY509224 increased soil Ni extractability (Abou-Shanab et al. 2003, 2006). Becerra-Castro et al. (2011) showed that culture filtrates of strains SA5b and SBA50 (also used in this study) increased Ni extraction from ultramafic soils. However, no corresponding increase in soil Ni phytoavailability or shoot Ni concentrations were observed in A pintodasilvae inoculated with these two strains. In fact, Sr(NO3)2-extractable Ni concentrations were reduced after plant growth and no differences were observed between inoculants, although this is likely to be due to root uptake.
In conclusion this study has identified candidate strains which could be useful for future field-based trials. Plant growth-promoting effects by associated bacteria can improve plant performance and also result in higher amounts of phytoextracted Ni. They also seem to be able to mobilise trace metals in soils, thereby increasing the phytoavailable fraction and plant uptake. It has been shown that Ni phytoextraction (or phytomining) can be optimised under field conditions using distinct agronomic practices (e.g. fertilisation regimes; Bani et al. 2007) but it remains to be seen whether or not plant-associated microorganisms can further improve the shoot Ni removal on a field scale. Further studies are also required to establish the optimal method of inoculation, regarding inoculum bacterial densities, plant stage and age for inoculation (e.g. inoculating seed or plants), timing of inoculation (bacterial growth phase) or the need for re-inoculation events, as well as the persistence and competition capacity of inoculant strains. Advances in these aspects could lead to more pronounced effects of these plant-associated bacteria and further improvements in phytoextraction efficiency.
References
Abou-Shanab RA, Angle JS, Delorme TA, Chaney RL, van Berkum P, Moawad H, Ghanem K, Ghozlan HA (2003) Rhizobacterial effects on nickel extraction from soil and uptake by Alyssum murale. New Phytol 158:219–224. doi:10.1046/j.1469-8137.2003.00721.x
Abou-Shanab R, Angle J, Chaney R (2006) Bacterial inoculants affecting nickel uptake by Alyssum murale from low, moderate and high Ni soils. Soil Biol Biochem 38:2882–2889. doi:10.1016/j.soilbio.2006.04.045
Asensi A, Rodríguez N, Díez-Garretas B, Amils R, de la Fuente V (2004) Nickel hyperaccumulation of some subspecies of Alyssum serpyllifolium (Brassicaceae) from ultramafic soils of Iberian Peninsula. In: Boyd RS, Baker AJM, Proctor J (eds) Ultramafic Soils: Their Soils, Vegetation and Fauna. Science Reviews, St. Albans, UK, pp 263–266
Assunção AL, Bleeker P, Bookum W, Vooijs R, Schat H (2008) Intraspecific variation of metal preference patterns for hyperaccumulation in Thlaspi caerulescens: evidence from binary metal exposures. Plant Soil 303:289–299. doi:10.1007/s11104-007-9508-x
Baker AJM, McGrath SP, Sidoli CMD, Reeves RD (1994) The possibility of in situ heavy metal decontamination of polluted soils using crops of metal-accumulating plants. Resour Conserv Recycl 11:41–49
Bani A, Echevarria G, Sulçe S, Morel J, Mullai A (2007) In-situ phytoextraction of Ni by a native population of Alyssum murale on an ultramafic site (Albania). Plant Soil 293:79–89. doi:10.1007/s11104-007-9245-1
Barzanti R, Colzi I, Arnetoli M, Gallo A, Pignattelli S, Gabbrielli R, Gonnelli C (2011) Cadmium phytoextraction potential of different Alyssum species. J Hazard Mater 196:66–72. doi:10.1016/j.jhazmat.2011.08.075
Becerra-Castro C, Prieto-Fernández A, Álvarez-Lopez V, Monterroso C, Cabello-Conejo M, Acea M, Kidd P (2011) Nickel solubilizing capacity and characterization of rhizobacteria isolated from hyperaccumulating and non-hyperaccumulating subspecies of Alyssum serpyllifolium. Int J Phytoremediat 13:229–244
Becerra-Castro C, Monterroso C, Prieto-Fernández A, Rodríguez-Lamas L, Loureiro-Viñas M, Acea MJ, Kidd PS (2012) Pseudometallophytes colonising Pb/Zn mine tailings: a description of the plant–microorganism–rhizosphere soil system and isolation of metal-tolerant bacteria. J Hazard Mater 217–218:350–359. doi:10.1016/j.jhazmat.2012.03.039
Becerra-Castro C, Kidd P, Kuffner M, Prieto-Fernandez A, Hann S, Monterroso C, Sessitsch A, Wenzel W, Puschenreiter M (2013) Bacterially induced weathering of ultramafic rock and its implications for phytoextraction. Appl Environ Microbiol 79:5094–5103. doi:10.1128/aem.00402-13
Boisson J, Mench M, Sappin-Didier V, Solda P, Vangronsveld J (1998) Short-term in situ immobilization of Cd and Ni by beringite and steel shots application to long-term sludged plots. Agronomie 18:347–359
Brooks RR, Shaw S, Marfil AA (1981) Some observations on the ecology, metal uptake and nickel tolerance of Alyssum serpyllifolium subspecies from the Iberian peninsula. Plant Ecol 45:183–188. doi:10.1007/bf00054673
Cabello-Conejo M, Centofanti T, Kidd P, Prieto-Fernández Á, Chaney R (2013) Evaluation of plant growth regulators to increase nickel phytoextraction by Alyssum species. Int J Phytoremediat 15:365–375
Chaney RL, Malik M, Li YM, Brown SL, Brewer EP, Angle JS, Baker AJM (1997) Phytoremediation of soil metals. Curr Opin Biotechnol 8:279–284. doi:10.1016/s0958-1669(97)80004-3
Chaney RL, Angle JS, Broadhurst CL, Peters CA, Tappero RV, Sparks DL (2007) Improved understanding of hyperaccumulation yields commercial phytoextraction and phytomining technologies. J Environ Qual 36:1429–1443. doi:10.2134/jeq2006.0514
Chaney RL, Chen KY, Li YM, Angle JS, Baker AJM (2008) Effects of calcium on nickel tolerance and accumulation in Alyssum species and cabbage grown in nutrient solution. Plant Soil 311:131–140. doi:10.1007/s11104-008-9664-7
Dell’Amico E, Cavalca L, Andreoni V (2008) Improvement of Brassica napus growth under cadmium stress by cadmium-resistant rhizobacteria. Soil Biol Biochem 40:74–84. doi:10.1016/j.soilbio.2007.06.024
Everhart JL, McNear D Jr, Peltier E, van der Lelie D, Chaney RL, Sparks DL (2006) Assessing nickel bioavailability in smelter-contaminated soils. Sci Total Environ 367:732–744. doi:10.1016/j.scitotenv.2005.12.029
Ewers U (1991) Standards, guidelines and legislative regulations concerning metals and their compounds. In: Merian E (ed) Metals and their Compound in the Environment. VCH, Weinheim, pp 687–711
Gabbrielli P, Pandolfini T, Vergnano O, Palandri MR (1990) Comparison of two serpentine species with different nickel tolerance strategies. Plant Soil 122:271–277. doi:10.1007/BF02851985
Gadd GM (2010) Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiology 156:609–643. doi:10.1099/mic.0.037143-0
Glick BR (2003) Phytoremediation: synergistic use of plants and bacteria to clean up the environment. Biotechnol Adv 21:383–393. doi:10.1016/S0734-9750(03)00055-7
Glick BR, Penrose DM, Li J (1998) A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theor Biol 190:63–68. doi:10.1006/jtbi.1997.0532
He S, He Z, Yang Z, Baligar VC (2012) Mechanisms of nickel uptake and hyperaccumulation by plants and implications for soil remediation. In: Advances in Agronomy, Vol 117. Elsevier, USA
Jiang C-y, Sheng X-f, Qian M, Wang Q-y (2008) Isolation and characterization of a heavy metal-resistant Burkholderia sp. from heavy metal-contaminated paddy field soil and its potential in promoting plant growth and heavy metal accumulation in metal-polluted soil. Chemosphere 72:157–164. doi:10.1016/j.chemosphere.2008.02.006
Kidd P, Barceló J, Bernal MP, Navari-Izzo F, Poschenrieder C, Shilev S, Clemente R, Monterroso C (2009) Trace element behaviour at the root-soil interface: implications in phytoremediation. Environ Exp Bot 67:243–259
Kukier U, Peters CA, Chaney RL, Angle JS, Roseberg RJ (2004) The effect of pH on metal accumulation in two species. J Environ Qual 33:2090–2102. doi:10.2134/jeq2004.2090
Lebeau T, Braud A, Jézéquel K (2008) Performance of bioaugmentation-assisted phytoextraction applied to metal contaminated soils: a review. Environ Pollut 153:497–522. doi:10.1016/j.envpol.2007.09.015
Li Y-M, Chaney R, Brewer E, Roseberg R, Angle JS, Baker A, Reeves R, Nelkin J (2003) Development of a technology for commercial phytoextraction of nickel: economic and technical considerations. Plant Soil 249:107–115. doi:10.1023/a:1022527330401
Ma Y, Rajkumar M, Freitas H (2009) Improvement of plant growth and nickel uptake by nickel resistant-plant-growth promoting bacteria. J Hazard Mater 166:1154–1161. doi:10.1016/j.jhazmat.2008.12.018
Ma Y, Prasad MNV, Rajkumar M, Freitas H (2011) Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv 29:248–258. doi:10.1016/j.biotechadv.2010.12.001
Mastretta C, Taghavi S, van der Lelie D, Mengoni A, Galardi F, Gonnelli C, Barac T, Boulet J, Weyens N, Vangronsveld J (2009) Endophytic bacteria from seeds of Nicotiana tabacum can reduce cadmium phytotoxicity. Int J Phytoremediat 11:251–267
Mench M, Renella G, Gelsomino A, Landi L, Nannipieri P (2006) Biochemical parameters and bacterial species richness in soils contaminated by sludge-borne metals and remediated with inorganic soil amendments. Environ Pollut 144:24–31. doi:10.1016/j.envpol.2006.01.014
Mench M, Schwitzguebel JP, Schroeder P, Bert V, Gawronski S, Gupta S (2009) Assessment of successful experiments and limitations of phytotechnologies: contaminant uptake, detoxification and sequestration, and consequences for food safety. Environ Sci Pollut Res 16:876–900. doi:10.1007/s11356-009-0252-z
Menezes de Sequeira E (1969) Toxicity and movement of heavy metals in serpentinic soils (North-Eastern Portugal). Agron Lusit 30:115–154
Mergeay M, Nies D, Schlegel HG, Gerits J, Charles P, Van Gijsegem F (1985) Alcaligenes eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy metals. J Bacteriol 162:328–334
Moreno-Jiménez E, Esteban E, Carpena-Ruiz RO, Lobo MC, Penalosa JM (2012) Phytostabilisation with Mediterranean shrubs and liming improved soil quality in a pot experiment with a pyrite mine soil. J Hazard Mater 201:52–59
Olsen SR, Cole CV, Watanabe FS (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA, Washington
Rajkumar M, Freitas H (2008a) Effects of inoculation of plant-growth promoting bacteria on Ni uptake by Indian mustard. Bioresour Technol 99:3491–3498. doi:10.1016/j.biortech.2007.07.046
Rajkumar M, Freitas H (2008b) Influence of metal resistant-plant growth-promoting bacteria on the growth of Ricinus communis in soil contaminated with heavy metals. Chemosphere 71:834–842. doi:10.1016/j.chemosphere.2007.11.038
Sessitsch A, Kuffner M, Kidd P, Vangronsveld J, Wenzel WW, Fallmann K, Puschenreiter M (2013) The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol Biochem 60:182–194. doi:10.1016/j.soilbio.2013.01.012
Shilev S, Fernández A, Benlloch M, Sancho E (2006) Sunflower growth and tolerance to arsenic is increased by the rhizospheric bacteria Pseudomonas fluorescens. In: Morel JL, Echevarria G, Goncharova N (eds) Phytoremediation of Metal-Contaminated Soils. Springer, Netherlands, pp 315–326. doi:10.1007/1-4020-4688-X_12
Tappero R, Peltier E, Gräfe M, Heidel K, Ginder-Vogel M, Livi KJT, Rivers ML, Marcus MA, Chaney RL, Sparks DL (2007) Hyperaccumulator Alyssum murale relies on a different metal storage mechanism for cobalt than for nickel. New Phytol 175:641–654. doi:10.1111/j.1469-8137.2007.02134.x
Van der Ent A, Baker AJ, Reeves RD, Pollard AJ, Schat H (2013) Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil 362:319–334
Vangronsveld J and Clijsters H (1994) Toxic effects of metals. In: Farago ME (ed) Plants and the Chemical Elements. Biochemistry, Uptake,Tolerance and Toxicity. VCH, Weinheim, pp 149–177
Vangronsveld J, Herzig R, Weyens N, Boulet J, Adriaensen K, Ruttens A, Thewys T, Vassilev A, Meers E, Nehnevajova E, van der Lelie D, Mench M (2009) Phytoremediation of contaminated soils and groundwater: lessons from the field. Environ Sci Pollut Res Int 16:765–794. doi:10.1007/s11356-009-0213-6
Weissenhorn I, Mench M, Leyval C (1995) Bioavailability of heavy metals and arbuscular mycorrhiza in a sewage-sludge-amended sandy soil. Soil Biol Biochem 27:287–296. doi:10.1016/0038-0717(94)00179-5
Weyens N, van der Lelie D, Taghavi S, Newman L, Vangronsveld J (2009a) Exploiting plant-microbe partnerships to improve biomass production and remediation. Trends Biotechnol 27:591–598. doi:10.1016/j.tibtech.2009.07.006
Weyens N, van der Lelie D, Taghavi S, Vangronsveld J (2009b) Phytoremediation: plant-endophyte partnerships take the challenge. Curr Opin Biotechnol 20:248–254. doi:10.1016/j.copbio.2009.02.012
Zaidi S, Usmani S, Singh BR, Musarrat J (2006) Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere 64:991–997. doi:10.1016/j.chemosphere.2005.12.057
Acknowledgments
This work was supported by the Spanish Ministerio de Economía e Competitividad (CTM2012-39904-C02-01) and FEDER, the Fundación Mapfre (Ayudas a la Investigación 2012), and by the 7th Framework Program of the European Commission (FP7-KBBE-266124, GREENLAND).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Juan Barcelo.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. 1S
Concentrations of macro- and micro-nutrients in the shoots (mean ±SE) of Alyssum pintodasilvae grown in sand/perlite mixtures and inoculated with 15 rhizobacterial strains. Values of non-inoculated controls are indicated by a continuous line (±SE (broken lines)). Asterisks indicate significant differences from the control (p <0.05) (PNG 386 kb)
Rights and permissions
About this article
Cite this article
Cabello-Conejo, M.I., Becerra-Castro, C., Prieto-Fernández, A. et al. Rhizobacterial inoculants can improve nickel phytoextraction by the hyperaccumulator Alyssum pintodasilvae . Plant Soil 379, 35–50 (2014). https://doi.org/10.1007/s11104-014-2043-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2043-7