Abstract
Background
Plants must acquire at least 14 mineral nutrients from the soil to complete their life cycles. Insufficient availability or extreme high levels of the nutrients significantly affect plant growth and development. Plants have evolved a series of mechanisms to adapt to unsuitable growth conditions where nutrient levels are too low or too high. microRNAs (miRNAs), a class of small RNAs, are known to mediate post-transcriptional regulation by transcript cleavage or translational inhibition. Besides regulating plant growth and development, miRNAs are well documented to regulate plant adaptation to adverse environmental conditions including nutrient stresses.
Scope
In this review, we focus on recent progress in our understanding of how miRNAs are involved in plant response to stresses resulting from deficiency in nutrients, such as nitrogen, phosphorus, sulfur, copper and iron, as well as toxicities from heavy metal ions.
Conclusions
Accumulated evidence indicates that miRNAs play critical roles in sensing the abundance of nutrients, controlling nutrient uptake and phloem-mediated long-distance transport, and nutrient homeostasis. miRNAs act as systemic signals to coordinate these physiological activities helping plants respond to and survive nutrient stresses and toxicities. Knowledge about how miRNAs are involved in plant responses to nutrient stresses promise to provide novel strategies to develop crops with improved nutrient use efficiency which could be grown in soils with either excessive or insufficient availability of nutrients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants require at least 17 elements for normal growth and successful reproduction. Of these 17 essential elements, 14 are mineral nutrient elements (except for the non-minerals carbon, hydrogen, and oxygen), which have to be taken up from soil by the root system. Upon uptake by plant roots, the tissue and cellular concentrations of these nutrient elements need to be controlled within a narrow physiological range to ensure optimal growth and development. However, in agricultural systems or natural environments, nutrient composition is found to vary and could range from an extreme lack of nutrients, to optimal amounts of nutrients, and to excess nutrients in the soil. Nutrient stress, defined as sub-optimal availability of essential nutrients or excessive and toxic levels of essential and non-essential macronutrients, is becoming one of the most important environmental stresses that impede plant growth and development, and therefore, reduce crop yield (Alam 1999; Clarkson and Hanson 1980). Research has revealed that plants have evolved sophisticated mechanisms to sense their nutrient levels and adapt to fluctuations of nutrient availability (Ohkama-Ohtsu and Wasaki 2010; Schachtman and Shin 2007). With the completion of genome sequences of several plant species and the development of new genomics tools, a large number of regulatory components involved in responses to nutrient stress have been identified (Chiou and Lin 2011; Kobayashi and Nishizawa 2012; Rubio et al. 2009; Schroeder et al. 2013; Tsay et al. 2011; Wang and Wu 2013). These components, including nutrient transporter proteins, transcription factors, riboregulators, and ubiquitin-related proteins constitute a complex regulatory network enabling plants to sense and adapt to various nutrient stresses by reprogramming a wide variety of biochemical, physiological, morphological, and metabolic processes.
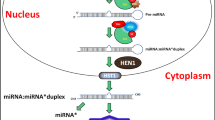
In the past few years, miRNAs have been found to be involved in plant responses to nutrient stresses by mediating post-transcriptional regulation of various effector genes (Chiou 2007; Kehr 2012; Kuo and Chiou 2011; Scheible et al. 2011). miRNAs are endogenous, single-stranded, and non-coding small RNAs with a length of about 19 to 24 nucleotides. They are generated from a single-stranded RNA precursor with a hairpin secondary structure, and are known to mediate endogenous gene silencing at the post-transcriptional level by targeting cognate mRNAs for transcript degradation or translational repression (Jones-Rhoades et al. 2006; Voinnet 2009). In plants, miRNAs are mostly transcribed from independent MIR genes through RNA polymerase II activity to form 5’ capped, spliced, and 3’ poly (A) tailed primary miRNAs (pri-miRNAs) (Xie et al. 2005). Pri-miRNAs are cut by DICER-LIKE 1 (DCL1) proteins to form precursor miRNAs and then processed by the same DCL1 proteins to release miRNA/miRNA* duplexes. One of the duplex strands (the miRNA) is incorporated into an AGO protein, the catalytic component of the RNA-induced silencing complex (RISC); this guides the RISC to bind to the target transcripts by sequence complementarity, while its counterpart from the duplex called miRNA* is often degraded after release of the mature strand (Jones-Rhoades et al. 2006; Voinnet 2009). miRNAs are actively involved in controlling plant growth and development (Jones-Rhoades et al. 2006; Meng et al. 2010; Wu 2013), and in plant response to various biotic and abiotic stresses including nutrient stress (Khraiwesh et al. 2012; Kruszka et al. 2012; Sunkar et al. 2012). Since the report of Arabidopsis miR395, a miRNA responsive to sulfate deficiency (Jones-Rhoades and Bartel 2004), many miRNA species have been identified that are responsive to nutrient stresses such as phosphorus (P) deficiency, copper (Cu) limitation, nitrogen (N) starvation, iron (Fe) deprivation, and stresses from excess metal ions (e.g., Fe, Cu, Mn, Cd, and Hg) (Abdel-Ghany and Pilon 2008; Bari et al. 2006; Chiou et al. 2006; Fujii et al. 2005; Kawashima et al. 2009; Kong and Yang 2010; Liang et al. 2010; Yamasaki et al. 2007; Yang and Chen 2013). In this review, we summarize the miRNAs responsive to these nutrient stresses and discuss their regulatory roles in plant adaptation to these nutrient stresses.
miRNAs responsive to N
N is a major component of amino acids, proteins, nucleic acids, coenzymes, and a myriad of plant secondary metabolites, and thus plays an essential role in plant growth and development. Although N is available in many different forms in the soil, plant roots predominantly take up N in the form of nitrate and ammonium. The availability of N to plant roots is often an important limitation for plant growth and crop yield (McAllister et al. 2012; Richardson et al. 2009; Xu et al. 2012). Plants have evolved multiple strategies, including morphological, physiological, and biochemical adaptations to respond to variations of N availability in the soil (Kant et al. 2011a; Kraiser et al. 2011; Schachtman and Shin 2007; Tsay et al. 2011). Recently, the regulatory roles of miRNAs in response to nitrate and N-deficiency have been explored. miR167 and miR393 regulate root growth in response to N (Gifford et al. 2008; Vidal et al. 2010). miR169 is involved in plant adaptation to N deficiency (Zhao et al. 2011). Based on analyses of microarray hybridisation, high-throughput small RNA sequencing, qRT-PCR, northern blot and in situ hybridization, many other miRNAs have also been found to be responsive to N starvation in plants. The N-deficiency-responsive miRNAs belonging to at least 27 families are summarized in Table 1.
miR167/ARF8 module regulates lateral root growth in response to N
miR167 is a conserved miRNA in plants, with four and ten members in Arabidopsis and rice, respectively (http://www.mirbase.org). Two auxin response factors, ARF6 and ARF8 are targeted by miR167 in Arabidopsis (Jones-Rhoades and Bartel 2004). Recently, a cell-specific expression profile in nitrate-treated Arabidopsis roots was analyzed by microarray hybridization, and ARF8 was found to be induced in pericycle and lateral root cap cells (Gifford et al. 2008). qRT-PCR and GUS-fusion analysis confirmed the induction of ARF8 and the repression of miR167 by nitrate in the same cell types. Consistently, an ARF8-GUS fusion with a mutated miR167-binding site showed loss of nitrate regulation, supporting the hypothesis that repression of miR167 leads to ARF8 accumulation in the pericycle cells upon nitrate treatment. Transgenic plants overexpressing miR167 and arf8 null mutant both exhibited a complete loss of the regulation of lateral root emergence by nitrate. Thus, the miR167/ARF8 module mediates lateral root growth in response to nitrate, or more specifically to N metabolites downstream of nitrate reduction and assimilation (Gifford et al. 2008) (Fig. 1). In addition, miR167 was found to be responsive to N deficiency in Arabidopsis and in maize (Liang et al. 2012; Xu et al. 2011; Zhao et al. 2012) implying that miR167 species from both monocots and dicots share a conserved role in regulating plant adaptation to N deficiency.
miR393 and target AFB3 mediate nitrate-induced changes in root system architecture
In order to identify nitrate-responsive miRNAs at a global scale, small RNAs were sequenced from control and nitrate-treated Arabidopsis seedlings. One of the identified small RNAs was miR393, which was found to be induced by nitrate (Vidal et al. 2010). The nitrate induction of miR393 was abolished in a nitrate-reductase null mutant and miR393 was shown to be induced by the nitrate reduction/assimilation products, ammonium and glutamate. Together, these results suggest that miR393 is induced by N signals produced after nitrate reduction and assimilation (Fig. 1). miR393 targets transcripts encoding the basic helix-loop-helix bHLH transcription factor bHLH77, and the auxin receptors TIR1, AFB1, AFB2 and AFB3 (Chen et al. 2011; Jones-Rhoades and Bartel 2004). However, only auxin receptor AFB3 was found to be induced by nitrate treatment and specifically cleaved via a miR393-mediated process (Vidal et al. 2010). Using AFB3 promoter-driven GUS reporter, it was shown that the expression of AFB3 was induced at root tips by nitrate coincidently with an increase in auxin response. Furthermore, auxin-responsive genes and auxin-related genes which are not affected by auxin treatment, such as ARF9 and ARF18 were also found to be regulated by nitrate. It appears that nitrate modulates auxin signaling at multiple levels via the miR393/AFB3 module (Vidal et al. 2010). In addition, primary root growth of afb3-1 and miR393-overexpressing plants was not inhibited by nitrate, suggesting that the miR393/AFB3 regulatory module plays a role in modulating primary root growth in response to nitrate (Vidal et al. 2010). Besides primary root growth, lateral root growth is also regulated by miR393/AFB3 module upon nitrate treatment. Together, these data demonstrated that miR393/AFB3 module regulates the nitrate induced changes in root system architecture possibly via the auxin signaling pathway (Vidal et al. 2010) (Fig. 1).
miR169 regulates plant response to N starvation
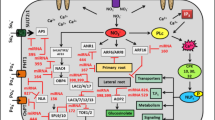
miR169 targets mRNA of transcription factors in the HAP2 family (also called nuclear factor Y-A subunits, NF-YA) in plants and plays various roles such as regulation of nodule differentiation and drought tolerance (Combier et al. 2006; Li et al. 2008). Recently, Arabidopsis miR169 was found to be significantly down-regulated by N deficiency, whereas, expression of its target genes NFYA2, NFYA3, NFYA5, and NFYA8 was induced (Hsieh et al. 2009; Liang et al. 2012; Pant et al. 2009; Zhao et al. 2011) (Fig. 2). Further study showed that miR169a is the only miR169 locus down-regulated in both roots and shoots of Arabidopsis by N starvation, and is the major locus important for the regulation of NFYA expression under N deficient conditions (Zhao et al. 2011). Transgenic plants overexpressing miR169a accumulated less N and were more sensitive to N deficiency as compared with wild-type plants (e.g., higher anthocyanin concentration, lower chlorophyll content, and early senescence phenotype). The hypersensitivity of miR169a-overexpresing plants was attributed to the variation in N acquisition, which was associated with impaired expression of nitrate transporter genes, AtNRT1.1 and AtNRT2.1. Interestingly, four CCAAT elements were found in the promoter regions of AtNRT1.1 and one was found in the promoter of AtNRT2.1, suggesting a direct regulation of AtNRT1.1 and AtNRT2.1 by NF-YA transcription factor. These results support the hypothesis that miR169 plays an important role in plant adaptation to fluctuations of N availability in the soil (Zhao et al. 2011) (Fig. 2). It is notable that miR169 was also found to be responsive to N deficiency in other species, including maize and soybean (Liang et al. 2012; Trevisan et al. 2012; Wang et al. 2013; Xu et al. 2011; Zhao et al. 2012, 2013b), suggesting a role for miR169 in N-deficiency adaptation is conserved among plant species.
miRNA-mediated regulation of nutrient homeostasis under nutrient deficiency. N starvation represses the expression of miR169, and thus leads to the increase of its targets NFYA transcription factors, which may positively regulate the expression NRT1.1 and NRT2.1, and thus contribute to enhancement of nitrate uptake. The induction of miR399 by Pi deficiency is regulated by upstream transcription factors MYB2 and PHR1; activity of miR399 is controlled by IPS1 through target mimicry mechanism; miR399 targets PHO2, which encodes an E2 conjugase protein and is required for PHO1 degradation; PHO2 may repress the expression of IPS1 and other PSI genes; E3 ligase gene NLA is targeted by Pi-deficiency-activated miR827; Pi transport-related proteins PHF1 and PHT1.1 are likely to be targeted by PHO2 and NLA directly or indirectly. Sulfate deficiency leads to a SLIM1-dependent induction of miR395, which targets APS genes and SULTR2;1, and thus regulates sulfate assimilation and sulfate translocation by down-regulating APS genes and restricting expression of SULTR2;1 in root-xylem parenchyma cells; APS3 and SULTR2;1 are dually up-regulated by sulfate starvation. Copper deficiency leads to SPL7-dependent induction of miR397, miR398, miR408, and miR857, which down-regulate genes encoding Cu-containing proteins including Cu/Zn superoxide dismutase (CSD), CCS1, COX5b-1, laccases (LAC), and plantacyanin (PLC), and thus save Cu for essential Cu proteins (e.g. plastocyanin). Solid arrow heads indicate positive regulation, solid “T” heads indicate negative regulation, and dashed lines designate putative regulation
Other miRNAs responsive to N fluctuations
Many N-responsive miRNAs have been identified in plant species by various approaches (Table 1). Some pri-miR169s as well as pri-miR398a were found to be repressed by N starvation by qRT-PCR analysis in Arabidopsis (Pant et al. 2009). Using small RNA deep sequencing analysis, 9 miRNA families (miR169, miR171, miR395, miR397, miR398, miR399, miR408, miR827, and miR857) were found to be repressed and 5 miRNA families (miR160, miR780, miR826, miR842, and miR846) were found to be induced by N deficiency in Arabidopsis; moreover, 9 novel miRNAs were found to be responsive to N deficiency (Liang et al. 2012). Based on microarray analysis, 15 miRNA families were found to be differentially expressed in rice genotypes sensitive to low N conditions, suggesting that miRNAs play an important role in low N tolerance in rice (Nischal et al. 2012). Recently, N-responsive miRNAs in maize have been identified by several groups. Using two different microarray systems, 14 miRNA families were observed to be responsive to transient and/or chronic low N conditions in maize (Xu et al. 2011). Of these 14 miRNA families, four (miR169, miR172, miR397 and miR398) were commonly responsive to both transient low N and chronic low N treatments, and four (miR169, miR399, miR408, and miR528) were commonly responsive to low N in both leaves and roots (Xu et al. 2011). By small RNA northern blot analysis, five conserved families (miR169, miR395, miR528, miR827 and miR171) were found to be expressed differentially in maize under N deficiency (Zhao et al. 2012). Through analyses of microarray, qRT-PCR and in situ hybridization, six miRNAs (miR528, miR528*, miR169, miR169*, miR166 and miR408) were observed to be down-regulated by nitrate starvation in maize seedlings (Trevisan et al. 2012). In addition, some novel miRNAs including novel miR169 species were also found to be responsive to low N or N deficiency in plants (Liang et al. 2012; Zhao et al. 2012, 2013b). Although there are differences in N-responsive miRNAs reported by different groups, which may result from different treatments and methods used, miR169 and miR528 were commonly found to be responsive to N fluctuations in maize. Also, by small RNA deep sequencing, 150 known miRNAs as well as two novel miRNAs were found to be responsive to short-term and/or long-term low N in two soybean genotypes (low N sensitive and low N tolerant), and among these miRNAs, several conserved miRNA families (e.g., miR159, miR160, miR169, miR319, miR396, miR397, and miR408) were commonly responsive to short-term and/or long-term low N in both genotypes (Wang et al. 2013). However, some of the soybean N-responsive miRNAs are genotype-specific (Wang et al. 2013), which might be associated with the differences of the two genotypes in their response to low N stress. Taken together, the expression levels of many different miRNA families are altered by N starvation in plants, and some families including miR169, miR397, miR398 and miR408 are commonly responsive to N deficiency in diverse plant species (Table 1). Notably, some N-responsive miRNAs (e.g., miR169, miR395, miR397, miR398, miR399, miR827, miR408 and miR857) are also responsive to other nutrient stresses in plants (e.g., P, S, and Cu starvation) (see below for more detail), suggesting the involvement of miRNA-mediated crosstalk among N, P, S, and Cu nutrient stress signalling pathways (Fig. 2). The majority of the putative target genes of N-responsive miRNAs are involved in plant development, signal transduction, nutrient homeostasis, and oxidative stress tolerance (Table 1), suggesting that these miRNAs might be involved in coordinating multiple physiological responses upon N-starvation.
miRNAs responsive to phosphate deficiency
P is one of the mineral nutrients essential for plant survival and productivity. Not only it is a major structural constituent of fundamental macromolecules, such as nucleic acids and phospholipids, it is also involved in energy transfer, metabolic regulation and protein activation. However, most of the P in the soil is unavailable to plants because of adsorption by soil particles, precipitation, or conversion to organic forms (Raghothama and Karthikeyan 2005; Richardson et al. 2011; Vance et al. 2003). To maintain internal phosphate (Pi) homeostasis, plants have developed a series of adaptive strategies, including enhancing acquisition of Pi, coordinating allocation of Pi among different organs, and remobilizing Pi from old to young tissues (Chiou and Lin 2011; Richardson et al. 2011; Vance et al. 2003). Recently, miRNAs have been observed to act as regulatory factors during plant responses to Pi deficiency.
miR399 is involved in regulation of Pi homeostasis
miR399 is induced by Pi deficiency and regulates Pi homeostasis through Pi acquisition, allocation and remobilization by down-regulating its target gene UBC24 (also called PHO2) (Chiou et al. 2006; Fujii et al. 2005) (Fig. 2). UBC24 encodes an ubiquitin-conjugating E2 enzyme and contains five miR399 targeting sites in its 5’ UTR (Allen et al. 2005; Sunkar and Zhu 2004). Based on the evidence that UBC24 was down-regulated by Pi deficiency, miR399 was subsequently found to be up-regulated strongly and specifically by Pi deficiency (Chiou et al. 2006). Overexpression of miR399 in Arabidopsis increases uptake and allocation of Pi to the shoot, leading to over-accumulation of Pi in the shoot, which eventually causes Pi toxicity when grown under Pi-sufficient conditions (Aung et al. 2006; Bari et al. 2006; Chiou et al. 2006; Fujii et al. 2005). Similar phenotypes were also observed in transgenic rice (Oryza sativa) constitutively overexpressing osa-miR399f or osa-miR399j (Hu et al. 2011). In addition, heterologous overexpression of Arabidopsis miR399d in tomato led to increased Pi content in both shoots and roots, enhanced expression of Pi transporter genes, and improved proton exudation from roots (Gao et al. 2010).
The phenotypes of miR399-overexpressing plants and T-DNA knockout mutants of UBC24 are similar: both resemble that of a previously reported Pi over-accumulator, the pho2 mutant (Delhaize and Randall 1995). Results from two groups independently showed that the phenotype of pho2 is caused by a nonsense mutation in the UBC24 gene (Aung et al. 2006; Bari et al. 2006). In pho2 mutants or miR399 overexpression plants, Pi no longer represses certain Pi starvation-induced (PSI) genes including IPS1 (induced by phosphate starvation 1), and the Pi transporter genes PHT1;4, PHT1;8, and PHT1;9 (Aung et al. 2006; Bari et al. 2006; Pant et al. 2009), suggesting that loss of PHO2 mimics Pi-starvation responses, which contributes to shoot Pi over accumulation under Pi-sufficient conditions. Recently, PHO1, which functions in Pi loading to the xylem, was identified to be a downstream component of the PHO2 dependent regulatory pathway (Liu et al. 2012). The PHO2-mediated degradation of PHO1 on the endomembrane is required to maintain Pi homeostasis, although additional unknown factors regulated by PHO2 might exist to account for the Pi toxicity phenotype of pho2 (Liu et al. 2012).
The regulation of miR399 expression and its actions have been well documented. The MYB transcription factors, PHR1 (phosphate starvation response 1) and PHL1 (PHR1-like 1), are critical regulators of Pi deficiency signaling involved in transcriptional activation of a broad range of PSI genes (Bustos et al. 2010; Rubio et al. 2001). The PHR1-binding site P1BS was found in the promoters of all six miR399 genes in Arabidopsis (Bari et al. 2006). Induction of both primary and mature miR399 by Pi deficiency was diminished in phr1 mutants indicating that PHR1 acts upstream to the induced increase in miR399 expression possibly through binding to P1BS in their promoters (Bari et al. 2006). In addition to P1BS, other Pi-responsive cis-elements, such as PHO, PHO-like, and P-responsive element, have also been shown to be enriched in the promoters of Pi-responsive miRNAs including miR399 (Zeng et al. 2010; Xu et al. 2013). Very recently, Arabidopsis miR399f was found to be regulated by a Pi-deficiency induced transcription factor MYB2, which binds directly to a MYB binding site in the miR399f promoter (Baek et al. 2013). Another important finding is that the action of miR399 is controlled by IPS1, a long non-coding RNA that contains a near-perfect binding site for miR399 (Franco-Zorrilla et al. 2007). IPS1 is not cleaved by miR399, but sequesters miR399 and leads to the reduced activity of miR399, and thus protects PHO2 transcript from cleavage (Franco-Zorrilla et al. 2007). This mechanism of non-coding RNA action is termed “target mimicry”; artificial target mimicry has been presented to be a promising and straight-forward approach to knocking down entire miRNA families (Todesco et al. 2010). Notably, the IPS1/miR399/PHO2 regulatory module seems to be conserved in plant species (Branscheid et al. 2010; Hu et al. 2011; Huang et al. 2011; Liu et al. 2010; Valdes-Lopez et al. 2008).
miR827 is involved in regulation of Pi homeostasis
miR827 was also found to be specifically induced by Pi deficiency and plays a critical role in regulating Pi homeostasis in a nitrate-dependent manner by down-regulating its target gene NLA (nitrogen limitation adaptation) in Arabidopsis (Hsieh et al. 2009; Kant et al. 2011b) (Fig. 2). NLA encodes a SPX (eucaryotic protein domain named after Syg1, Pho81 and XPR1)-domain E3 ligase protein and is involved in adaptive responses to low N conditions in Arabidopsis (Peng et al. 2007). Recently, two suppressors of the nla mutant, which restore the abrupt early-senescence phenotype of the nla mutant to that of the wild type, have been isolated and identified to be the Pi transport-related genes PHF1 (phosphate transporter facilitator 1) and PHT1;1 (Kant et al. 2011b). The early-senescence phenotype of nla mutant was found to be caused by excessive Pi accumulation under low nitrate conditions, suggesting an important role of NLA in maintaining Pi homeostasis in a nitrate-dependent manner (Kant et al. 2011b). Consistent with the negative regulation of NLA by miR827, miR827-overexpressing plants and nla mutant plants displayed a similar Pi toxicity phenotype, and surprisingly Pi content in mir827 mutant and in NLA-overexpressing plants was similar (Kant et al. 2011b). Like the nla mutant, the pho2 mutant also displays Pi toxicity in a nitrate-dependent manner (Kant et al. 2011b). However, the nla pho2 double mutant has no obvious additive accumulation of Pi compared with nla or pho2 mutants, which suggests that PHO2 and NLA may function in the same pathway to regulate Pi homeostasis by mechanisms mediated by miR399 and miR827 respectively (Kant et al. 2011b). Similar to the case in Arabidopsis, rice miR827 is also induced by Pi deficiency, and two SPX-MFS genes OsSPX-MFS1 and OsSPX-MFS2 are cleaved by miR827 (Lin et al. 2010). Interestingly, OsSPX-MFS1 and OsSPX-MFS2, which are implicated in Pi sensing or transport, showed inverse responses to Pi deficiency, suggesting a complex regulation of the two target genes by miR827 under Pi deficiency (Lin et al. 2010). Recently, it was revealed that osa-miR827 is regulated by OsPHR2, the ortholog of AtPHR1, and that osa-miR827-overexpressing plants or OsSPX-MFS1 mutants (mfs1) increase Pi accumulation in the leaves by reducing Pi remobilization from old to young leaves (Wang et al. 2012). This suggests that miR827/SPX-MFS1 module regulates Pi homeostasis in rice leaves.
Other miRNAs responsive to Pi deficiency
Using microarray, small RNA and degradome sequencing, qRT-PCR, and northern blot, many other Pi-deficiency responsive miRNAs have been identified in plant species including Arabidopsis (Hsieh et al. 2009; Lundmark et al. 2010; Pant et al. 2009), white lupin (Zhu et al. 2010), soybean (Sha et al. 2012; Zeng et al. 2010; Xu et al. 2013), common bean (Valdes-Lopez et al. 2010), tomato (Gu et al. 2010), wheat (Zhao et al. 2013a), barley (Hackenberg et al. 2013), and maize (Pei et al. 2013). Recently, Kuo and Chiou (2011) have summarized the Pi-responsive miRNAs. Notably, several miRNA families including miR156, miR159, miR166, miR169, miR319, miR395, miR398, miR399, miR447, and miR827 were found to be commonly responsive to Pi deficiency in various plant species, indicating that they are coordinately involved in the conserved Pi signaling pathways in plants (Kuo and Chiou 2011). On the other hand, some Pi-responsive miRNAs seem to be species-specific. Further research is needed to reveal the possible physiological roles of these Pi-responsive miRNAs during plant responses to Pi deficiency.
miRNAs in response to sulfate starvation
S is an essential element for various compounds in plants, such as amino acids and proteins, sulfated polysaccharides, sulfolipids and vitamins, which play critical roles in various physiological processes (Leustek et al. 2000). Low S availability in soils is one of the limiting factors of plant growth and productivity in many countries following significant reduction of anthropogenic sulfur emission (Lewandowska and Sirko 2008). Inorganic sulfate is the major form absorbed and transported into various tissues for assimilation. In Arabidopsis, the uptake of sulfate from soil is mainly achieved by two high-affinity sulfate transporters, SULTR1;1 and SULTR1;2, while low-affinity sulfate transporters, like SULTR2;1, SULTR2;2 and SULTR 3;5, are involved in the translocation of sulfate within the plant (Kataoka et al. 2004; Takahashi et al. 2000). After uptake, sulfate can either be stored in the vacuole within the cell or can be further metabolized in a series of reactions that occur in plastids, where plastidic ATP sulfurylase (APS) acts as the first enzyme in the sulfate assimilation pathway (Saito 2004).
miR395 is involved in regulation of sulfate homeostasis
miR395 was observed to be induced by sulfate starvation, and it targets the transcripts of APS (APS1, APS3 and APS4) and SULTR2;1 genes (Allen et al. 2005; Jones-Rhoades and Bartel 2004; Kawashima et al. 2009; Liang et al. 2010) (Fig. 2). By constructing transgenic plants carrying miR395 promoter-GFP reporter constructs, all of the six Arabidopsis miR395 loci were found to be induced differentially by sulfate starvation (Kawashima et al. 2009). Accompanied by the up-regulation of miR395, the target transcripts of APS1, APS4 and shoot SULTR2;1 were found to be down-regulated by sulfate starvation (Jones-Rhoades and Bartel 2004; Liang et al. 2010). However, APS3 and root SULTR2;1 were up-regulated under sulfate-deficiency stress, which was consistent with the response of miR395 (Kawashima et al. 2009; Liang et al. 2010). Tissue-specific expression analysis demonstrated that miR395 was mainly expressed in phloem companion cells (Kawashima et al. 2009), while SULTR2;1 was predominantly expressed in xylem parenchyma cells (Takahashi et al. 2000). Thus, miR395 suppresses the expression of SULTR2;1 in phloem companion cells under sulfate starvation to strictly limit SULTR2;1 expression in the xylem, which could then increase sulfate translocation from roots to shoots in the xylem and inhibit shoot-to-root transport in the phloem (Kawashima et al. 2009, 2011).
miR395 is regulated by SLIM1 (sulfur limitation 1), a transcription factor of the EIL (ethylene-insensitive-like) family (Kawashima et al. 2009; Maruyama-Nakashita et al. 2006). As one of the key regulatory components of sulfate starvation response, SLIM1 is activated by sulfate limitation and contributes to the induced expression of SULTR1;2, as well as other sulfate-starvation responsive genes (Maruyama-Nakashita et al. 2006). Unlike the induction of miR395 in wild-type plants, no significant variation in the expression of miR395 was found in slim1 mutants under sulfate starvation (Kawashima et al. 2009). The SLIM1-dependent induction of miR395 is critical for increased translocation of sulfate from roots to shoots to ensure efficient sulfate assimilation in the shoots (Kawashima et al. 2011). However, whether SLIM1 directly or indirectly regulates the accumulation of miR395 remains to be elucidated.
Recently, more evidence has been reported for the regulatory role of miR395 in sulfate translocation and assimilation. Sulfate concentration in the shoots of miR395-overexpressing plants was higher than that in wild-type plants even though these plants displayed sulfate deficiency symptoms. The contradictory phenotype of sulfate deficiency exhibited by these sulfate over-accumulator mutant plants might be caused by a repression of sulfate assimilation and sulfate relocation between leaves. Consistent with this hypothesis, these phenotypes of miR395-overexpressing plants are similar to those of the aps1-1 sultr2;1 APS4-RNAi triple mutant. Considered together, these results indicated that miR395 regulates the accumulation of sulfate in the shoot by targeting APS genes and affects the translocation of sulfate between leaves by cleaving SULTR2;1 (Liang et al. 2010) (Fig. 2). In addition, miR395 was found to respond to treatments by metabolites known to regulate sulfate assimilation, implying that miR395 could also function in the regulatory network of sulfate assimilation (Matthewman et al. 2012). However, the molecular mechanism regulating miR395 expression in the sulfate assimilation pathway is still unclear. miR395 has been identified also in Oryza sativa, Medicago truncatula, Solanum lycopersicum, Sorghum bicolor and Brassica napus (Huang et al. 2010; Liang et al. 2010). It is now clear that the miR395/APS-SULTR2;1 regulatory pathway is conserved in plants. Besides miR395, expression of precursors of miR156, miR160, miR164, miR167, miR168 and miR394 are also altered by sulfate starvation in Brassica napus (Huang et al. 2010), suggesting their involvement in adaptation to sulfate limitation.
miRNAs responsive to Cu deficiency
As an essential cofactor for many metalloproteins, such as plastocyanin, copper/zinc (Cu/Zn) superoxide dismutases (CSD), cytochrome c oxidase, plantacyanin and laccases, Cu participates in many important physiological processes in plants (Burkhead et al. 2009). Cu deficiency causes a broad spectrum of problems in plants, including decreased growth and productivity; while Cu excess inhibits plant growth and impairs cellular processes such as photosynthetic electron transport. Various strategies have been evolved to effect Cu homeostasis in plants, which involve a wide range of genes and proteins (Burkhead et al. 2009). In recent years, miR398 and three other miRNAs (miR397, miR408, and miR857) have been implicated in plant response to Cu deficiency by down-regulating genes encoding Cu proteins.
miR398, encoded by three miR398 genes in Arabidopsis, regulates at least four genes including CSD1 and CSD2 (encoding cytosolic and chloroplastic CSD, respectively), COX5b-1 (encoding a subunit of the mitochondrial cytochrome c oxidase), and CCS1 (encoding copper chaperone for superoxide dismutase (SOD)), by transcript cleavage and translational inhibition (Beauclair et al. 2010; Brodersen et al. 2008; Dugas and Bartel 2008; Jones-Rhoades and Bartel 2004; Zhu et al. 2011). Expression of miR398 is substantially induced by Cu starvation, while its target genes CSD1 and CSD2 are down-regulated (Yamasaki et al. 2007). CSD can be functionally replaced by Fe superoxide dismutase, which is also induced by Cu starvation. Thus, the reduction of Cu-containing protein CSD might be required to increase the Cu availability for other essential Cu proteins such as plastocyanin (Yamasaki et al. 2007) (Fig. 2). However, the expression of miR398 is down-regulated when under high Cu stress, leading to the post-transcriptional induction of CSD1 and CSD2 (Sunkar et al. 2006). The induction of CSD under high Cu stress can effectively detoxify superoxide radicals and accumulate more Cu in the form of Cu proteins. Transgenic plants overexpressing a miR398-resistant form of CSD2 are thus much more tolerant to oxidative stresses including high Cu stress (Sunkar et al. 2006). It seems that miR398 acts as a key modulator in the regulation of Cu homeostasis by down-regulating nonessential Cu protein CSD when Cu is deficient or excessive.
miR397, miR408, and miR857 were also observed to target nonessential Cu proteins such as laccases and plantacyanin (Abdel-Ghany and Pilon 2008) (Fig. 2). Similar to miR398, these three miRNAs were induced by Cu starvation, and the expression pattern of each of these miRNAs was found to be negatively correlated with that of their respective target genes. Similar to the function of miR398 in Cu homeostasis, these three additional Cu-responsive miRNAs can also help optimizing Cu provision for the most essential Cu-containing proteins.
Recently, the transcription factor SPL7 (SQUAMOSA promoter binding protein-like 7) was identified to be responsible for regulating Cu homeostasis in Arabidopsis (Yamasaki et al. 2009). Interestingly, the expression of miR397, miR398, miR408, and miR857 were all found to be regulated by SPL7 (Yamasaki et al. 2009). SPL7 binds directly to GTAC motifs in the promoter of the miR398 gene in vitro, and these motifs have been found to be essential and sufficient for the response to Cu deficiency in vivo. In contrast to miR398b and miR398c, the promoter sequence of miR398a does not contain the GTAC motifs, which could account for the lower expression of miR398a and unresponsiveness to Cu deficiency (Sunkar et al. 2006). Besides miR398b and miR398c, the levels of miR397a, miR408, and miR857 were also much lower in the spl7 mutant indicating that SPL7 activates the transcription of these Cu-responsive miRNAs. Thus, SPL7 appears to be a critical factor regulating the levels of many Cu proteins, including Cu/Zn SOD, laccases and plantacyanin via the Cu-responsive miRNAs (Yamasaki et al. 2009).
miRNAs responsive to Fe deficiency
Fe is an essential nutrient required for many cellular reactions. In most neutral or basic soils, Fe is often unavailable for plants due to its limited solubility. On the other hand, Fe toxicity to plants may be triggered by anaerobic conditions in acidic soils (Hell and Stephan 2003). In plants, the molecular components involved in responses to Fe deficiency are regulated at both transcriptional and post-transcriptional levels (Kobayashi and Nishizawa 2012). Recently, miRNAs have been suggested to be involved in plant adaptation to Fe deficiency. Eight conserved miRNAs from five families (miR159, miR169, miR172, miR173, miR394) were first cloned from a small RNA library treated by Fe starvation in Arabidopsis, and were shown to be differentially expressed in response to Fe deficiency (Kong and Yang 2010). The Fe-deficiency-responsive cis-acting elements, IDE1 and IDE2, which were identified from the barley IDS2 promoter, are located in the promoters of many Fe-deficiency-inducible genes (Kobayashi et al. 2003). Interestingly, many Arabidopsis miRNAs harboring the IDE1/IDE2 motifs in their promoters were found to be responsive to Fe deficiency (Kong and Yang 2010). In addition, eight miRNAs belonging to seven conserved families (miR172, miR158, miR163, miR165, miR166, miR397, and miR398) were found to be significantly and differentially expressed in Fe-deficiency-treated Arabidopsis (Waters et al. 2012). Also, through microarray and northern blot analysis, the expression of some miRNAs (e.g., miR167, miR397, miR398, and miR408) have been found to be responsive to Fe deficiency in common bean (Valdes-Lopez et al. 2010). Notably, limitation in Fe supply represses the expression of miR397 and miR398, which target the mRNAs of multiple Cu proteins, implying an association of Fe deficiency with Cu homeostasis (Waters et al. 2012).
miRNAs responsive to metal stress
Elevated concentration of both essential (such as Fe, Cu and Zn) and non-essential (such as Al, Cd and Hg) metals in the soil can inhibit plant growth and cause toxicity to plants. In order to maintain the concentration of essential metals within physiological limits, and to minimize the toxicity of non-essential metals, plants have evolved a range of physiological and cellular mechanisms to cope with metal stresses by detoxification and/or development of metal tolerance (Clemens 2001). Recent studies have revealed that plant miRNAs are involved in adaptive responses to metal stresses caused by Cu, Fe, Mn, Al, Cd, Hg and As (Chen et al. 2012; Ding et al. 2011; Huang et al. 2009, 2010; Lima et al. 2011; Liu and Zhang 2012; Sunkar et al. 2006; Valdes-Lopez et al. 2010; Zeng et al. 2012; Zhang et al. 2013; Zhou et al. 2012a, b). miRNAs responsive to metal stresses and their potential roles in metal stress responses have recently been reviewed (Gielen et al. 2012; Mendoza-Soto et al. 2012; Yang and Chen 2013). Most of the target genes regulated by metal-responsive miRNAs function in diverse metabolic pathways including sulphate metabolism, phytohormone signaling, antioxidation, and miRNA biogenesis (Yang and Chen 2013). It is worth noting that some miRNAs (such as miR156, miR319, miR390, miR393, miR396 and miR398) that function in controlling plant growth and development, are commonly regulated by various metal stresses (Gielen et al. 2012; Mendoza-Soto et al. 2012; Yang and Chen 2013). However, further studies are needed to understand the exact roles of the metal-responsive miRNAs and their targets in the regulatory network involved in plant tolerance to metal stress.
miRNA is involved in regulation of oxidative stress induced by nutrient stress
Various stresses including nutrient deficiency and metal stresses increase the production of reactive oxygen species (ROS) in plants (Gill and Tuteja 2010; Shin et al. 2005). Excessive ROS are toxic and cause damage to proteins, lipids, carbohydrates, and nucleic acids, which ultimately results in oxidative stress. To protect against oxidative stress damage, plants have evolved a series of antioxidant defense mechanisms that could be grouped into enzymatic (e.g., SOD, catalase, ascorbate peroxidase, and glutathione reductase) and non-enzymatic (e.g., ascorbic acid, glutathione, and phenolic compounds) systems (Gill and Tuteja 2010). The potential role of miRNAs in antioxidant defense has recently been demonstrated. CSD1 and CSD2, which are important ROS scavengers, are targeted by miR398 (Sunkar et al. 2006). In coincidence with the increased ROS under nutrient stress, miR398 was found to be repressed by N, P, K, or Fe deficiency, as well as by Cd or Hg toxicity in plants (Hsieh et al. 2009; Kuo and Chiou 2011; Pant et al. 2009; Yang and Chen 2013), indicating the important role of miR398 in regulating ROS homeostasis under nutrient stress. Another nutrient stress responsive miRNA, miR528, was also predicted to target a CSD gene in maize (Trevisan et al. 2012). Plantacyanin, which may affect ROS production (Dong et al. 2005), is targeted by a Cu-responsive miR408 (Abdel-Ghany and Pilon 2008). miR408 as well as miR528 were also repressed by N or Pi starvation in maize (Pei et al. 2013; Trevisan et al. 2012; Xu et al. 2011; Zhao et al. 2012); and notably, expression localizations of the two miRNAs were similar (Trevisan et al. 2012). These data suggest important roles of these miRNAs in controlling ROS accumulation under nutrient stress. Very recently, eight miRNAs including miR398 and miR528 were identified to be responsive to zinc (Zn) deficiency in Sorghum bicolor, and two CSD genes SbCSD1 and SbCSD2 were identified to be targeted by miR398 and miR528 respectively (Li et al. 2013). Interestingly, the expression patterns of the two miRNAs were opposite in roots and leaves under Zn-deficiency (Li et al. 2013), which suggests that the activity of CSD is fine-tuned by miRNAs under Zn deficiency stress.
miRNA is involved in systemic regulation of nutrient stress response
Systemic signaling through phloem-mediated long-distance transport of macromolecules including RNAs and proteins is involved in many developmental processes and stress responses in plants (Lough and Lucas 2006). In the past few years, small RNAs have been found to move via the phloem and play a potential role in systemic regulation of nutrient homeostasis (Marín-González and Suárez-López 2012; Kehr 2013; Liu et al. 2009). miR399 was observed to accumulate specifically in phloem sap of Pi-deficient, but not in Pi-sufficient rapeseed (Brassica napus) plants (Buhtz et al. 2008); and grafting experiments using Arabidopsis verified the translocation of miR399 via the phloem from shoots to roots (Lin et al. 2008; Pant et al. 2008). Thus, the phloem-mobile miR399 might be a key component mediating plant Pi homeostasis as a systemic messenger of Pi deficiency response (Liu et al. 2009). Besides miR399, many other aforementioned nutrient-responsive miRNAs have also been detected in phloem sap of various nutrient-stressed plants (Buhtz et al. 2008, 2010; Pant et al. 2009). For instance, expression levels of miR399, miR399*, miR827, miR2111 and miR2111* were increased greatly in phloem sap from plants under Pi-deficiency; miR395 was increased dramatically in phloem sap from sulfate-starved plants; miR397, miR398 and miR408 were increased in phloem sap from plants stressed by Cu-deficiency; miR169 was decreased significantly in phloem sap from N- or Pi-starved plants. It is conceivable that these miRNAs including miRNAs* may also function as candidate for long-distance signalling to systemically regulate plant response to these nutrient stresses. However, more studies are needed to validate this hypothesis and to explore their possible roles in systemic regulation of nutrient response.
Conclusions and futher perspectives
Since the finding of sulfate-responsive miR395 in 2004, many nutrient-responsive miRNAs have been identified by various approaches, and some of these miRNAs have been found to play critical roles in nutrient stress sensing, signaling and adaptation. For instance, miR167 and miR393 function in plant response to N availability by regulating root development; miR399 and miR827 are essential in regulating Pi homeostasis under Pi deficiency, miR395 is important for controlling sulfate allocation and assimilation during sulfate starvation; and the Cu-limitation-induced miR398 plays a critical role in maintaining Cu homeostasis (Figs. 1 and 2). However, mechanistic details regarding the roles of nutrient-responsive miRNAs are still limited. Further characterization of these miRNAs and their target genes will improve our understanding of the regulatory network involved in plant adaption to nutrient stress.
With the development of high-throughput small RNA and degradome sequencing, many nutrient-responsive miRNAs and their target genes have been identified. However it is still unclear whether these miRNAs also regulate targets via translational inhibition, as in the case of miR172 and miR398 (Brodersen et al. 2008; Chen 2004). In addition, more studies, based on molecular and genetic approaches, are required to identify components upstream (e.g. transcription factors and relative cis-elements) and downstream (e.g. miRNA target genes or related proteins) to these nutrient-responsive miRNAs. Phenotypic and physiological analysis of plants with modified expression of a specific miRNA and/or its target genes is very helpful for elucidating the roles of miRNAs in nutrient stress responses. Besides miRNAs, miRNA* and other classes of small RNAs, such as small RNAs derived from transposons, and tRNA-derived small RNAs, are also responsive to nutrient stress (Hsieh et al. 2009), but their physiological function is still unclear and deserves the attention of scientists in this field.
MicroRNA-based genetic modification technology is a useful strategy to develop crop cultivars with increased biomass productivities and enhanced tolerance to biotic and abiotic stress (Macovei et al. 2012; Zhou and Luo 2013). By modulating the levels of nutrient-responsive miRNAs and/or of their target genes, plants can be engineered to be more tolerant to nutrient stresses and/or efficient in utilizing nutrients (Fischer et al. 2013). For example, transgenic Arabidopsis plants overexpressing miR399 accumulate more Pi in shoots than wild type plants because of increased Pi uptake, Pi translocation from roots to shoots, and retention of Pi in the shoots (Fujii et al. 2005; Chiou et al. 2006). Moreover, overexpressing a miR398-resistant form of CSD2 confers tolerance to oxidative stress induced by high intensity light and heavy metals in transgenic Arabidopsis plants (Sunkar et al. 2006). In addition to overexpression, other strategies, such as moderate constitutive expression, stress-induced expression, time- or tissue-specific expression, downregulation of miRNAs by target mimicry, gene silencing by artificial miRNAs, RNA interference, and expression of miRNA-resistant target genes could all be utilized to modulate the levels of nutrient-responsive miRNAs and/or their target genes, providing multiple strategies to create crop cultivars with improved nutrient use efficiency and/or nutrient stress tolerance.
References
Abdel-Ghany SE, Pilon M (2008) MicroRNA-mediated systemic down-regulation of copper protein expression in response to low copper availability in Arabidopsis. J Biol Chem 283:15932–15945
Alam SM (1999) Nutrient uptake by plants under stress conditions. In: Pessarakli M (ed) Handbook of plant and crop stress. CRC Press, Marcel Dekker Inc, New York, pp 285–313
Allen E, Xie Z, Gustafson AM, Carrington JC (2005) microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121:207–221
Aung K, Lin SI, Wu CC, Huang YT, Su CL, Chiou TJ (2006) pho2, a phosphate overaccumulator, is caused by a nonsense mutation in a microRNA399 target gene. Plant Physiol 141:1000–1011
Baek D, Kim MC, Chun HJ, Kang S, Park HC, Shin G, Park J, Shen M, Hong H, Kim WY, Kim DH, Lee SY, Bressan RA, Bohnert HJ, Yun DJ (2013) Regulation of miR399f transcription by AtMYB2 affects phosphate starvation responses in Arabidopsis. Plant Physiol 161:362–373
Bari R, Pant BD, Stitt M, Scheible WR (2006) PHO2, microRNA399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol 141:988–999
Beauclair L, Yu A, Bouche N (2010) microRNA-directed cleavage and translational repression of the copper chaperone for superoxide dismutase mRNA in Arabidopsis. Plant J 62:454–462
Branscheid A, Sieh D, Pant BD, May P, Devers EA, Elkrog A, Schauser L, Scheible WR, Krajinski F (2010) Expression pattern suggests a role of MiR399 in the regulation of the cellular response to local Pi increase during arbuscular mycorrhizal symbiosis. Mol Plant Microbe Interact 23:915–926
Brodersen P, Sakvarelidze-Achard L, Bruun-Rasmussen M, Dunoyer P, Yamamoto YY, Sieburth L, Voinnet O (2008) Widespread translational inhibition by plant miRNAs and siRNAs. Science 320:1185–1190
Buhtz A, Springer F, Chappell L, Baulcombe DC, Kehr J (2008) Identification and characterization of small RNAs from the phloem of Brassica napus. Plant J 53:739–749
Buhtz A, Pieritz J, Springer F, Kehr J (2010) Phloem small RNAs, nutrient stress responses, and systemic mobility. BMC Plant Biol 10:64
Burkhead JL, Reynolds KA, Abdel-Ghany SE, Cohu CM, Pilon M (2009) Copper homeostasis. New Phytol 182:799–816
Bustos R, Castrillo G, Linhares F, Puga MI, Rubio V, Perez-Perez J, Solano R, Leyva A, Paz-Ares J (2010) A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in Arabidopsis. PLoS Genet 6:e1001102
Chen X (2004) A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303:2022–2025
Chen ZH, Bao ML, Sun YZ, Yang YJ, Xu XH, Wang JH, Han N, Bian HW, Zhu MY (2011) Regulation of auxin response by miR393-targeted transport inhibitor response protein 1 is involved in normal development in Arabidopsis. Plant Mol Biol 77:619–629
Chen L, Wang T, Zhao M, Tian Q, Zhang WH (2012) Identification of aluminum-responsive microRNAs in Medicago truncatula by genome-wide high-throughput sequencing. Planta 235:375–386
Chiou TJ (2007) The role of microRNAs in sensing nutrient stress. Plant Cell Environ 30:323–332
Chiou TJ, Lin SI (2011) Signaling network in sensing phosphate availability in plants. Annu Rev Plant Biol 62:185–206
Chiou TJ, Aung K, Lin SI, Wu CC, Chiang SF, Su CL (2006) Regulation of phosphate homeostasis by microRNA in Arabidopsis. Plant Cell 18:412–421
Clarkson DT, Hanson JB (1980) The mineral nutrition of higher plants. Annu Rev Plant Physiol 31:239–298
Clemens S (2001) Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212:475–486
Combier JP, Frugier F, de Billy F, Boualem A, El-Yahyaoui F, Moreau S, Vernie T, Ott T, Gamas P, Crespi M, Niebel A (2006) MtHAP2-1 is a key transcriptional regulator of symbiotic nodule development regulated by microRNA169 in Medicago truncatula. Genes Dev 20:3084–3088
Delhaize E, Randall PJ (1995) Characterization of a phosphate-accumulator mutant of Arabidopsis thaliana. Plant Physiol 107:207–213
Ding Y, Chen Z, Zhu C (2011) Microarray-based analysis of cadmium-responsive microRNAs in rice (Oryza sativa). J Exp Bot 62:3563–3573
Dong J, Kim ST, Lord EM (2005) Plantacyanin plays a role in reproduction in Arabidopsis. Plant Physiol 138:778–789
Dugas DV, Bartel B (2008) Sucrose induction of Arabidopsis miR398 represses two Cu/Zn superoxide dismutases. Plant Mol Biol 67:403–417
Fischer JJ, Beatty PH, Good AG, Muench DG (2013) Manipulation of microRNA expression to improve nitrogen use efficiency. Plant Sci 210:70–81
Franco-Zorrilla JM, Valli A, Todesco M, Mateos I, Puga MI, Rubio-Somoza I, Leyva A, Weigel D, Garcia JA, Paz-Ares J (2007) Target mimicry provides a new mechanism for regulation of microRNA activity. Nat Genet 39:1033–1037
Fujii H, Chiou TJ, Lin SI, Aung K, Zhu JK (2005) A miRNA involved in phosphate-starvation response in Arabidopsis. Curr Biol 15:2038–2043
Gao N, Su Y, Min J, Shen W, Shi W (2010) Transgenic tomato overexpressing ath-miR399d has enhanced phosphorus accumulation through increased acid phosphatase and proton secretion as well as phosphate transporters. Plant Soil 334:123–136
Gielen H, Remans T, Vangronsveld J, Cuypers A (2012) MicroRNAs in metal stress: specific roles or secondary responses? Int J Mol Sci 13:15826–15847
Gifford ML, Dean A, Gutierrez RA, Coruzzi GM, Birnbaum KD (2008) Cell-specific nitrogen responses mediate developmental plasticity. Proc Natl Acad Sci U S A 105:803–808
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gu M, Xu K, Chen A, Zhu Y, Tang G, Xu G (2010) Expression analysis suggests potential roles of microRNAs for phosphate and arbuscular mycorrhizal signaling in Solanum lycopersicum. Physiol Plant 138:226–237
Hackenberg M, Huang PJ, Huang CY, Shi BJ, Gustafson P, Langridge P (2013) A comprehensive expression profile of microRNAs and other classes of non-coding small RNAs in barley under phosphorous-deficient and -sufficient conditions. DNA Res 20:109–125
Hell R, Stephan U (2003) Iron uptake, trafficking and homeostasis in plants. Planta 216:541–551
Hsieh LC, Lin SI, Shih AC, Chen JW, Lin WY, Tseng CY, Li WH, Chiou TJ (2009) Uncovering small RNA-mediated responses to phosphate deficiency in Arabidopsis by deep sequencing. Plant Physiol 151:2120–2132
Hu B, Zhu C, Li F, Tang J, Wang Y, Lin A, Liu L, Che R, Chu C (2011) LEAF TIP NECROSIS1 plays a pivotal role in the regulation of multiple phosphate starvation responses in rice. Plant Physiol 156:1101–1115
Huang SQ, Peng J, Qiu CX, Yang ZM (2009) Heavy metal-regulated new microRNAs from rice. J Inorg Biochem 103:282–287
Huang SQ, Xiang AL, Che LL, Chen S, Li H, Song JB, Yang ZM (2010) A set of miRNAs from Brassica napus in response to sulphate deficiency and cadmium stress. Plant Biotechnol J 8:887–899
Huang CY, Shirley N, Genc Y, Shi B, Langridge P (2011) Phosphate utilization efficiency correlates with expression of low-affinity phosphate transporters and noncoding RNA, IPS1, in barley. Plant Physiol 156:1217–1229
Jones-Rhoades MW, Bartel DP (2004) Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol Cell 14:787–799
Jones-Rhoades MW, Bartel DP, Bartel B (2006) MicroRNAs and their regulatory roles in plants. Annu Rev Plant Biol 57:19–53
Kant S, Bi YM, Rothstein SJ (2011a) Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. J Exp Bot 62:1499–1509
Kant S, Peng M, Rothstein SJ (2011b) Genetic regulation by NLA and microRNA827 for maintaining nitrate-dependent phosphate homeostasis in Arabidopsis. PLoS Genet 7:e1002021
Kataoka T, Hayashi N, Yamaya T, Takahashi H (2004) Root-to-shoot transport of sulfate in Arabidopsis. Evidence for the role of SULTR3;5 as a component of low-affinity sulfate transport system in the root vasculature. Plant Physiol 136:4198–4204
Kawashima CG, Yoshimoto N, Maruyama-Nakashita A, Tsuchiya YN, Saito K, Takahashi H, Dalmay T (2009) Sulphur starvation induces the expression of microRNA-395 and one of its target genes but in different cell types. Plant J 57:313–321
Kawashima CG, Matthewman CA, Huang S, Lee BR, Yoshimoto N, Koprivova A, Rubio-Somoza I, Todesco M, Rathjen T, Saito K, Takahashi H, Dalmay T, Kopriva S (2011) Interplay of SLIM1 and miR395 in the regulation of sulfate assimilation in Arabidopsis. Plant J 66:863–876
Kehr J (2012) Roles of miRNAs in nutrient signaling and homeostasis. In: Sunkar R (ed) MicroRNAs in plant development and stress responses. Springer, Berlin, pp 197–217
Kehr J (2013) Systemic regulation of mineral homeostasis by microRNAs. Front Plant Sci 4:145
Khraiwesh B, Zhu JK, Zhu J (2012) Role of miRNAs and siRNAs in biotic and abiotic stress responses of plants. Biochim Biophys Acta 1819:137–148
Kobayashi T, Nishizawa NK (2012) Iron uptake, translocation, and regulation in higher plants. Annu Rev Plant Biol 63:131–152
Kobayashi T, Nakayama Y, Itai RN, Nakanishi H, Yoshihara T, Mori S, Nishizawa NK (2003) Identification of novel cis-acting elements, IDE1 and IDE2, of the barley IDS2 gene promoter conferring iron-deficiency-inducible, root-specific expression in heterogeneous tobacco plants. Plant J 36:780–793
Kong WW, Yang ZM (2010) Identification of iron-deficiency responsive microRNA genes and cis-elements in Arabidopsis. Plant Physiol Biochem 48:153–159
Kraiser T, Gras DE, Gutiérrez AG, González B, Gutiérrez RA (2011) A holistic view of nitrogen acquisition in plants. J Exp Bot 62:1455–1466
Kruszka K, Pieczynski M, Windels D, Bielewicz D, Jarmolowski A, Szweykowska-Kulinska Z, Vazquez F (2012) Role of microRNAs and other sRNAs of plants in their changing environments. J Plant Physiol 169:1664–1672
Kuo HF, Chiou TJ (2011) The role of microRNAs in phosphorus deficiency signaling. Plant Physiol 156:1016–1024
Leustek T, Martin MN, Bick JA, Davies JP (2000) Pathways and regulation of sulfur metabolism revealed through molecular and genetic studies. Annu Rev Plant Physiol Plant Mol Biol 51:141–165
Lewandowska M, Sirko A (2008) Recent advances in understanding plant response to sulfur-deficiency stress. Acta Biochim Pol 55:457–471
Li WX, Oono Y, Zhu J, He XJ, Wu JM, Iida K, Lu XY, Cui X, Jin H, Zhu JK (2008) The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and posttranscriptionally to promote drought resistance. Plant Cell 20:2238–2251
Li Y, Zhang Y, Shi D, Liu X, Qin J, Ge Q, Xu L, Pan X, Li W, Zhu Y, Xu J (2013) Spatial-temporal analysis of zinc homeostasis reveals the response mechanisms to acute zinc deficiency in Sorghum bicolor. New Phytol. doi:10.1111/nph.12434
Liang G, Yang F, Yu D (2010) MicroRNA395 mediates regulation of sulfate accumulation and allocation in Arabidopsis thaliana. Plant J 62:1046–1057
Liang G, He H, Yu D (2012) Identification of nitrogen starvation-responsive microRNAs in Arabidopsis thaliana. PLoS ONE 7:e48951
Lima JC, Arenhart RA, Margis-Pinheiro M, Margis R (2011) Aluminum triggers broad changes in microRNA expression in rice roots. Genet Mol Res 10:2817–2832
Lin SI, Chiang SF, Lin WY, Chen JW, Tseng CY, Wu PC, Chiou TJ (2008) Regulatory network of microRNA399 and PHO2 by systemic signaling. Plant Physiol 147:732–746
Lin SI, Santi C, Jobet E, Lacut E, Kholti NE, Karlowski WM, Verdeil JL, Breitler JC, Perin C, Ko SS, Guiderdoni E, Chiou TJ, Echeverria M (2010) Complex regulation of two target genes encoding SPX-MFS proteins by rice miR827 in response to phosphate starvation. Plant Cell Physiol 51:2119–2131
Liu Q, Zhang H (2012) Molecular identification and analysis of arsenite stress-responsive miRNAs in rice. J Agric Food Chem 60:6524–6536
Liu TY, Chang CY, Chiou TJ (2009) The long-distance signaling of mineral macronutrients. Curr Opin Plant Biol 12:312–319
Liu F, Wang Z, Ren H, Shen C, Li Y, Ling HQ, Wu C, Lian X, Wu P (2010) OsSPX1 suppresses the function of OsPHR2 in the regulation of expression of OsPT2 and phosphate homeostasis in shoots of rice. Plant J 62:508–517
Liu TY, Huang TK, Tseng CY, Lai YS, Lin SI, Lin WY, Chen JW, Chiou TJ (2012) PHO2-dependent degradation of PHO1 modulates phosphate homeostasis in Arabidopsis. Plant Cell 24:2168–2183
Lough TJ, Lucas WJ (2006) Integrative plant biology: role of phloem long-distance macromolecular trafficking. Annu Rev Plant Biol 57:203–232
Lundmark M, Korner CJ, Nielsen TH (2010) Global analysis of microRNA in Arabidopsis in response to phosphate starvation as studied by locked nucleic acid-based microarrays. Physiol Plant 140:57–68
Macovei A, Gill SS, Tuteja N (2012) microRNAs as promising tools for improving stress tolerance in rice. Plant Signal Behav 7:1296–1301
Marín-González E, Suárez-López P (2012) “And yet it moves”: cell-to-cell and long-distance signaling by plant microRNAs. Plant Sci 196:18–30
Maruyama-Nakashita A, Nakamura Y, Tohge T, Saito K, Takahashi H (2006) Arabidopsis SLIM1 is a central transcriptional regulator of plant sulfur response and metabolism. Plant Cell 18:3235–3251
Matthewman CA, Kawashima CG, Huska D, Csorba T, Dalmay T, Kopriva S (2012) miR395 is a general component of the sulfate assimilation regulatory network in Arabidopsis. FEBS Lett 586:3242–3248
McAllister CH, Beatty PH, Good AG (2012) Engineering nitrogen use efficient crop plants: the current status. Plant Biotechnol J 10:1011–1125
Mendoza-Soto AB, Sanchez F, Hernandez G (2012) MicroRNAs as regulators in plant metal toxicity response. Front Plant Sci 3:105
Meng YJ, Ma XX, Chen DJ, Wu P, Chen M (2010) MicroRNA-mediated signaling involved in plant root development. Biochem Biophys Res Commun 393:345–349
Nischal L, Mohsin M, Khan I, Kardam H, Wadhwa A, Abrol YP, Iqbal M, Ahmad A (2012) Identification and comparative analysis of microRNAs associated with low-N tolerance in rice genotypes. PLoS ONE 7:e50261
Ohkama-Ohtsu N, Wasaki J (2010) Recent progress in plant nutrition research: cross-talk between nutrients, plant physiology and soil microorganisms. Plant Cell Physiol 51:1255–1264
Pant BD, Buhtz A, Kehr J, Scheible WR (2008) MicroRNA399 is a long-distance signal for the regulation of plant phosphate homeostasis. Plant J 53:731–738
Pant BD, Musialak-Lange M, Nuc P, May P, Buhtz A, Kehr J, Walther D, Scheible WR (2009) Identification of nutrient-responsive Arabidopsis and rapeseed microRNAs by comprehensive real-time polymerase chain reaction profiling and small RNA sequencing. Plant Physiol 150:1541–1555
Pei L, Jin Z, Li K, Yin H, Wang J, Yang A (2013) Identification and comparative analysis of low phosphate tolerance-associated microRNAs in two maize genotypes. Plant Physiol Biochem 70:221–234
Peng M, Hannam C, Gu H, Bi YM, Rothstein SJ (2007) A mutation in NLA, which encodes a RING-type ubiquitin ligase, disrupts the adaptability of Arabidopsis to nitrogen limitation. Plant J 50:320–337
Raghothama KG, Karthikeyan AS (2005) Phosphate acquisition. Plant Soil 274:37–49
Richardson AE, Barea JM, McNeill AM, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321:305–339
Richardson AE, Lynch JP, Ryan PR, Delhaize E, Smith FA, Smith SE, Harvey PR, Ryan MH, Venek EJ, Lambers H, Oberson A, Culvenor RA, Simpson RJ (2011) Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 349:121–156
Rubio V, Linhares F, Solano R, Martin AC, Iglesias J, Leyva A, Paz-Ares J (2001) A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev 15:2122–2133
Rubio V, Bustos R, Irigoyen ML, Cardona-Lopez X, Rojas-Triana M, Paz-Ares J (2009) Plant hormones and nutrient signaling. Plant Mol Biol 69:361–373
Saito K (2004) Sulfur assimilatory metabolism. The long and smelling road. Plant Physiol 136:2443–2450
Schachtman DP, Shin R (2007) Nutrient sensing and signaling: NPKS. Annu Rev Plant Biol 58:47–69
Scheible WR, Pant B, Musialak-Lange M, Nuc P (2011) Nutrient-responsive plant microRNAs. In: Erdmann VA, Barciszewski J (eds) Non coding RNAs in plants. Springer, Berlin, pp 313–337
Schroeder JI, Delhaize E, Frommer WB, Guerinot ML, Harrison MJ, Herrera-Estrella L, Horie T, Kochian LV, Munns R, Nishizawa NK, Tsay YF, Sanders D (2013) Using membrane transporters to improve crops for sustainable food production. Nature 497:60–66
Sha A, Chen Y, Ba H, Shan Z, Zhang X, Wu X, Qiu D, Chen S, Zhou X (2012) Identification of Glycine max microRNAs in response to phosphorus deficiency. J Plant Biol 55:268–280
Shin R, Berg RH, Schachtman DP (2005) Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiol 46:1350–1357
Sunkar R, Zhu JK (2004) Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 16:2001–2019
Sunkar R, Kapoor A, Zhu JK (2006) Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell 18:2051–2065
Sunkar R, Li YF, Jagadeeswaran G (2012) Functions of microRNAs in plant stress responses. Trends Plant Sci 17:196–203
Takahashi H, Watanabe-Takahashi A, Smith FW, Blake-Kalff M, Hawkesford MJ, Saito K (2000) The roles of three functional sulphate transporters involved in uptake and translocation of sulphate in Arabidopsis thaliana. Plant J 23:171–182
Todesco M, Rubio-Somoza I, Paz-Ares J, Weigel D (2010) A collection of target mimics for comprehensive analysis of microRNA function in Arabidopsis thaliana. PLoS Genet 6:e1001031
Trevisan S, Nonis A, Begheldo M, Manoli A, Palme K, Caporale G, Ruperti B, Quaggiotti S (2012) Expression and tissue-specific localization of nitrate-responsive miRNAs in roots of maize seedlings. Plant Cell Environ 35:1137–1155
Tsay YF, Ho CH, Chen HY, Lin SH (2011) Integration of nitrogen and potassium signaling. Annu Rev Plant Biol 62:207–226
Valdes-Lopez O, Arenas-Huertero C, Ramirez M, Girard L, Sanchez F, Vance CP, Luis Reyes J, Hernandez G (2008) Essential role of MYB transcription factor: PvPHR1 and microRNA: PvmiR399 in phosphorus-deficiency signalling in common bean roots. Plant Cell Environ 31:1834–1843
Valdes-Lopez O, Yang SS, Aparicio-Fabre R, Graham PH, Reyes JL, Vance CP, Hernandez G (2010) MicroRNA expression profile in common bean (Phaseolus vulgaris) under nutrient deficiency stresses and manganese toxicity. New Phytol 187:805–818
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157:423–447
Vidal EA, Araus V, Lu C, Parry G, Green PJ, Coruzzi GM, Gutierrez RA (2010) Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proc Natl Acad Sci U S A 107:4477–4482
Voinnet O (2009) Origin, biogenesis, and activity of plant microRNAs. Cell 136:669–687
Wang Y, Wu WH (2013) Potassium transport and signaling in higher plants. Annu Rev Plant Biol 64:451–476
Wang C, Huang W, Ying Y, Li S, Secco D, Tyerman S, Whelan J, Shou H (2012) Functional characterization of the rice SPX-MFS family reveals a key role of OsSPX-MFS1 in controlling phosphate homeostasis in leaves. New Phytol 196:139–148
Wang Y, Zhang C, Hao Q, Sha A, Zhou R, Zhou X, Yuan L (2013) Elucidation of miRNAs-mediated responses to low nitrogen stress by deep sequencing of two soybean genotypes. PLoS One 8:e67423
Waters BM, McInturf SA, Stein RJ (2012) Rosette iron deficiency transcript and microRNA profiling reveals links between copper and iron homeostasis in Arabidopsis thaliana. J Exp Bot 63:5903–5918
Wu G (2013) Plant microRNAs and development. J Genet Genomics 40:217–230
Xie Z, Allen E, Fahlgren N, Calamar A, Givan SA, Carrington JC (2005) Expression of Arabidopsis MIRNA genes. Plant Physiol 138:2145–2154
Xu Z, Zhong S, Li X, Li W, Rothstein SJ, Zhang S, Bi Y, Xie C (2011) Genome-wide identification of microRNAs in response to low nitrate availability in maize leaves and roots. PLoS ONE 6:e28009
Xu G, Fan X, Miller AJ (2012) Plant nitrogen assimilation and use efficiency. Annu Rev Plant Biol 63:153–182
Xu F, Liu Q, Chen L, Kuang J, Walk T, Wang J, Liao H (2013) Genome-wide identification of soybean microRNAs and their targets reveals their organ-specificity and responses to phosphate starvation. BMC Genomics 14:66
Yamasaki H, Abdel-Ghany SE, Cohu CM, Kobayashi Y, Shikanai T, Pilon M (2007) Regulation of copper homeostasis by micro-RNA in Arabidopsis. J Biol Chem 282:16369–16378
Yamasaki H, Hayashi M, Fukazawa M, Kobayashi Y, Shikanai T (2009) SQUAMOSA promoter binding protein-like7 is a central regulator for copper homeostasis in Arabidopsis. Plant Cell 21:347–361
Yang ZM, Chen J (2013) A potential role of microRNAs in plant response to metal toxicity. Metallomics. doi:10.1039/c3mt00022b
Zeng HQ, Zhu YY, Huang SQ, Yang ZM (2010) Analysis of phosphorus-deficient responsive miRNAs and cis-elements from soybean (Glycine max L.). J Plant Physiol 167:1289–1297
Zeng QY, Yang CY, Ma QB, Li XP, Dong WW, Nian H (2012) Identification of wild soybean miRNAs and their target genes responsive to aluminum stress. BMC Plant Biol 12:182
Zhang LW, Song JB, Shu XX, Zhang Y, Yang ZM (2013) miR395 is involved in detoxification of cadmium in Brassica napus. J Hazard Mater 250–251:204–211
Zhao M, Ding H, Zhu JK, Zhang F, Li WX (2011) Involvement of miR169 in the nitrogen-starvation responses in Arabidopsis. New Phytol 190:906–915
Zhao M, Tai H, Sun S, Zhang F, Xu Y, Li WX (2012) Cloning and characterization of maize miRNAs involved in responses to nitrogen deficiency. PLoS One 7:e29669
Zhao X, Liu X, Guo C, Gu J, Xiao K (2013a) Identification and characterization of microRNAs from wheat (Triticum aestivum L.) under phosphorus deprivation. J Plant Biochem Biotechnol 22:113–123
Zhao Y, Xu Z, Mo Q, Zou C, Li W, Xu Y, Xie C (2013b) Combined small RNA and degradome sequencing reveals novel miRNAs and their targets in response to low nitrate availability in maize. Ann Bot 112:633–642
Zhou M, Luo H (2013) MicroRNA-mediated gene regulation: potential applications for plant genetic engineering. Plant Mol Biol. doi:10.1007/s11103-013-0089-1
Zhou ZS, Song JB, Yang ZM (2012a) Genome-wide identification of Brassica napus microRNAs and their targets in response to cadmium. J Exp Bot 63:4597–4613
Zhou ZS, Zeng HQ, Liu ZP, Yang ZM (2012b) Genome-wide identification of Medicago truncatula microRNAs and their targets reveals their differential regulation by heavy metal. Plant Cell Environ 35:86–99
Zhu YY, Zeng HQ, Dong CX, Yin XM, Shen QR, Yang ZM (2010) microRNA expression profiles associated with phosphorus deficiency in white lupin (Lupinus albus L.). Plant Sci 178:23–29
Zhu C, Ding Y, Liu H (2011) MiR398 and plant stress responses. Physiol Plant 143:1–9
Acknowledgments
We apologize to the authors whose work is not cited here due to space limitation. We thank Professor Zhi Min Yang for his critical reading of the manuscript and valuable comments. Our current research is supported by grants from the National Science Foundation of China (31201679 and U1130304) and the Program of New Century Excellent Talent in Universities (NCET-11-0672).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Philip John White.
Rights and permissions
About this article
Cite this article
Zeng, H., Wang, G., Hu, X. et al. Role of microRNAs in plant responses to nutrient stress. Plant Soil 374, 1005–1021 (2014). https://doi.org/10.1007/s11104-013-1907-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1907-6