Abstract
Aims
Two pot experiments in a “walk-in” growth chamber with controlled day and night temperatures were conducted to investigate the influence of elevated temperatures along with rice straw incorporation on methane (CH4) and nitrous oxide (N2O) emissions as well as rice yield.
Methods
Three temperature regimes–29/25, 32/25, and 35/30 °C (Exp. I) and 29/22, 32/25, and 35/28 °C (Exp. II), representing daily maxima/minima were used in the study. Two amounts of rice straw (0 and 6 t ha−1) were applied with four replications in each temperature regime. CH4 and N2O emissions as well as soil redox potential (Eh) were monitored weekly throughout the rice-growing period.
Results
Elevated temperatures increased CH4 emission rates, with a more pronounced effect from flowering to maturity. The increase in emissions was further enhanced by incorporation of rice straw. A decrease in soil Eh to <−100 mV and CH4 emissions was observed early in rice straw–incorporated pots while the soil without straw did not reach negative Eh levels (Exp. I) or showed a delayed decrease (Exp. II). Moreover, soil with high organic C (Exp. II) had higher CH4 emissions. In contrast to CH4 emissions, N2O emissions were negligible during the rice-growing season. The global warming potential (GWP) was highest at high temperature with rice straw incorporation compared with low temperature without rice straw. On the other hand, the high temperature significantly increased spikelet sterility and reduced grain yield (p < 0.05).
Conclusions
Elevated temperature increased GWP while decreased rice yield. This suggests that global warming may result in a double negative effect: higher emissions and lower yields.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The atmospheric concentration of methane (CH4) and nitrous oxide (N2O), two major greenhouse gases, has increased since pre-industrial times due to anthropogenic activities. The concentration of CH4 increased from a pre-industrial value of 715 ppb to 1,774 ppb and of N2O from 270 ppb to 319 ppb in 2005 (Solomon et al. 2007). Globally, agricultural CH4 and N2O emissions increased by 17 % from 1990 to 2005, with an average annual emission increase of 60 Mt CO2 eq yr−1 (Smith et al. 2007). CH4 and N2O are 25 times and 298 times more potent in terms of global warming potential (GWP) than CO2 on a 100-year time scale, respectively (Solomon et al. 2007). Irrigated lowland rice contributes more than 75 % of global rice production (Fageria et al. 2011), and is considered as one of the major sources of the atmospheric CH4 budget (Minami and Neue 1994; Wassmann et al. 2000; Kruger et al. 2001). Rice fields alone contributed 18 % of the 596 Tg global CH4 flux in 2005 (Denman et al. 2007). In addition to CH4 emissions, rice fields emit N2O (Firestone and Davidson 1989; Khalil et al. 2004), especially when mitigation strategies for CH4 emissions, such as mid-season drainage and/or intermittent irrigation, are adopted (Bronson et al. 1997a; Hua et al. 1997; Minami 1997; Yu et al. 2007; Johnson-Beebout et al. 2009). The dry-wet alternation during mid-season drainage creates a favorable soil environment for both microbial nitrification and denitrification processes; thus, N2O emissions (Wang et al. 2011). Nevertheless, irrigated rice fields are flooded most of the time; the extent of N2O emissions is much lower than that of CH4 emissions (Chen et al. 1997; Ma et al. 2009).
CH4 is the final product of anaerobic degradation of organic matter in a much-reduced environment. Significant CH4 emissions occur when soil redox potential (Eh) is <−100 mV (Hou et al. 2000). Water regime and organic amendment in the rice-growing season are the top two controlling factors for this (Yan et al. 2005). Therefore, when the field is continuously flooded and highly reduced with low sulfate and the absence of other CH4 inhibitors, the availability of methanogenic substrate (organic C) obviously plays a major role (Yao et al. 1999; Fey and Conrad 2000; Yan et al. 2005). Organic C, especially easily decomposable C, acts as both an electron donor and a source of methanogenic substrate (Yao et al. 1999). Hence, soil with high organic C and incorporation of fresh rice straw that contains easily decomposable C increases CH4 emissions (Bossio et al. 1999; Naser et al. 2007). On the other hand, increasing temperature accelerates the decomposition of organic matter (Conant et al. 2011) and stimulates methanogenic activities, thereby increasing CH4 production (Fey and Conrad 2003). Moreover, higher temperature releases more root exudate C from rice plants (Tokida et al. 2010), enhances the reduction of other inorganic electron acceptors such as Fe (Rath et al. 2002), and decreases the duration of flooding to attain steady-state CH4 emissions (i.e., the higher temperature lowers the duration to reach steady-state CH4 emissions) (Devevre and Horwath 2000). All these processes could be compounded and affect CH4 emissions.
Concern is growing about the impact of projected increases in atmospheric temperature with global warming on CH4 emissions. Some previous studies have shown greater CH4 emissions under elevated temperatures (Schulz et al. 1997; Devevre and Horwath 2000; Allen et al. 2003; Fey and Conrad 2003; Tokida et al. 2010). However, conflicting results have also been reported for the effects of elevated temperature. Increased emissions were observed with ambient +2 °C elevated temperature (Schrope et al. 1999) but decreased emissions with ambient +4 °C (Ziska et al. 1998; Schrope et al. 1999). Similarly, Parashar et al. (1993) observed decreased emissions beyond 34 °C. Therefore, the evidence is still inconclusive due to limited data to predict the effect of rising atmospheric temperature on future emissions. On the other hand, rice cultivation has to be intensified to meet increasing global food demand. This intensification would increase not only rice grain yield but also rice straw, which poses a big management challenge. The removal of straw from the field reduces the nutrient content of soil while incorporation increases CH4 emissions (Bossio et al. 1999; Watanabe et al. 1999; Gao et al. 2004; Mandal et al. 2004; Naser et al. 2007). Therefore, the source strength of the CH4 budget may further change with the intensification of rice production along with increasing global temperature (Wassmann et al. 2010). Studies on the effect of elevated temperature in combination with rice straw on CH4 and N2O emissions are still lacking. Furthermore, the increasing temperature directly affects rice growth and yield. High temperature generally increases spikelet sterility and reduces grain yield (Ziska et al. 1996; Peng et al. 2004; Shah et al. 2011). To solve those problems, more studies on elevated temperatures in combination with straw incorporation are critical.
This experiment conducted in a ‘walk-in’ growth chamber forms part of a series of experiments on temperature effects with different approaches. The experiments on elevated canopy temperature in the field and elevated water/soil temperature in the greenhouse will be reported separately. In this paper, we present a study with elevated temperatures in all ecosystem compartments (i.e., air, water, and soil) alongside rice straw incorporation. The specific objectives of this study were:
-
To investigate the effect of elevated temperatures on CH4 and N2O emissions from flooded rice.
-
To assess the interaction effects of temperature elevation and rice straw incorporation on emissions.
-
To record the impacts of temperature and straw on related soil and plant parameters, namely, soil Eh, soil organic carbon, spikelet sterility, straw biomass, rice grain yield, and harvest index.
Materials and methods
Experimental design and treatment
Two studies were conducted consecutively in environment-controlled room type walk-in growth chambers (3.25 × 3.25 × 2.70 m) at IRRI, Los Baños, Philippines, from September, 2010, to August, 2011. The three levels of temperature regimes used in the respective chambers were as follows: 29/25, 32/25, and 35/30 °C (Exp. I) and 29/22, 32/25, and 35/28 °C (Exp. II) representing daily maxima and minima for low, medium, and high temperatures, respectively. These temperatures were controlled with combination of heating and cooling air conditioning system following a sinusoidal-type pattern between daily maxima and minima (Fig. 1). There were two treatments for rice straw: with rice straw added (+Straw: at the rate of 6 t ha−1 or 30 g pot−1) and without rice straw added (−Straw). Each rice straw treatment was arranged in a complete randomized design with four replicates. Thus, each experiment had a total of 24 pots (8 pots each chamber). All the pots were rotated randomly inside the chamber every week after gas sampling.
Six high-intensity discharge (HID) lamps (1,000 W each) were fitted into a light box (1.5 × 1.25 m), which was suspended 2.2 m from the floor to maintain the light. These bulbs maintained the photosynthetically active radiation (PAR) during the day as normal sunlight. Hence, the measured light quantity was 200–220 μ mol m−2 s−1 from 0600 to 0900 and 1600 to 1800, while it was 550–650 μ mol m−2 s−1 from 0900 to 1600 (Fig. 1). PAR was measured using a LICOR Quantum/Radio/Photometer. Relative humidity was controlled and maintained at 70–80 %.
Daily water temperature and soil temperature at 5 cm depth were monitored manually using a mercury thermometer between 1330 and 1430. Gas sampling was also conducted at this time. Similarly, air temperature inside the chamber was also recorded at each gas sampling time. All pots were maintained flooded (4–10 cm) throughout the rice-growing period.
Soil and straw preparation
The soil samples (Aquandic epiaquoll) (Dobermann et al. 2000) were collected from an upland site at the IRRI Experimental Farm, Laguna, Philippines (Exp. I: latitude: 14o08′40″, longitude: 121o16′06″ and Exp. II: latitude: 14o08′25″, longitude: 121o16′02″). Soil was collected from the plow layer depth (0–15 cm) during fallow. Removal of non-soil external debris and homogenization by hand mixing were done before transferring soil to individual pots. Two composite samples from each soil were analyzed for their basic characteristics. Soil pH was determined by a glass electrode in a soil-water solution at a ratio of 1:1 (w/v) (Kalra and Maynard 1991). Total C and N were determined simultaneously using an automated dry combustion method (elemental analyzer-TCD method). Soil organic C was measured by oxidizing with K2Cr2O7 and concentrated H2SO4 (Walkley and Black 1934). Total soil Kjeldahl N (Kj N) was determined by the micro-Kjeldahl method (Yoshida et al. 1976). Available P was determined by the Olsen method (Olsen et al. 1954) and Bray method (Bray and Kurtz 1945). Available K and cation exchange capacity (CEC) were determined by the NH4O-Ac extraction method at pH 7 (Helmke and Sparks 1996). Active Fe and Active Mn were determined by the dithionite reduction method (Asami and Kumada 1959). Particle size analysis for sand, silt, and clay was done by the hydrometer method (Kalra and Maynard 1991). The soils used for the two experiments were different from each other in terms of organic C, Kj N, and available P. The soil used in Exp. I had lower organic C, Kj N, and available P. The major physico-chemical properties of the soils are presented in Table 1.
Rice straw (Oryza sativa L. cv Angelica) with 41:1 C-N ratio, 1.98 % total K, and 0.15 % total P was used in the experiment. The micro-Kjeldahl method for N (Yoshida et al. 1976), dry ashing for P (Olsen et al. 1954), and the 1 N HCl extraction method for K (Yoshida et al. 1976) were used for plant tissue analysis. Rice straw was incorporated 23 days (18 August 2010) and 22 days (16 February 2011) before transplanting of rice for Exp. I and Exp. II, respectively. Rice straw was applied at the rate of 6 t ha−1 (30 g pot−1) in straw-treated pots. For water supply, the soil was irrigated two times a week until transplanting of the rice seedlings. This was done to hasten the decomposition of the applied straw in aerobic conditions. The period from straw incorporation to transplanting of rice seedlings represented the fallow period under field conditions.
Handling of plants and soil in pots
Wagner pots (height: 30 cm and diameter: 25 cm) were filled with 10 kg air dry homogenized soil followed by initial flooding. Three rice seedlings (Oryza sativa L. variety Angelica) were transplanted in each pot on 10 September 2010 (Exp. I) and 10 March 2011 (Exp. II). Fertilizers were applied at the rate of 120-30-30 kg N-P2O5-K2O ha−1. Urea was used as a source of N and applied in three equal splits at 14, 35, and 65 days after transplanting (DAT). Basal application for both P and K using single superphosphate (0-20-0) for P and muriate of potash (0-0-60) for K was made. The pots remained flooded throughout the growth period up to harvesting. The rice plants were harvested on 19 January 2011 (Exp. I) and on 25 July 2011 (Exp. II).
Measurements
Soil redox potential (Eh)
Soil Eh was measured using platinum-tipped electrodes; two of these per pot were installed permanently at a depth of 5.0 cm. Soil Eh was measured weekly immediately after gas sampling (1430 to 1530). Eh was monitored in all pots against an Hg/Hg2Cl2 reference electrode using a portable Eh/pH meter. Eh was calculated with the following formula (Sparks 1996):
where Eh (mV) is redox potential, Eh1 and Eh2 are millivolt readings, and the correction factor is 245 for the Hg/Hg2Cl2 reference electrode.
Collection and measurement of gas samples
The closed chamber technique was used to collect gas samples to measure CH4 and N2O emission rates. Each closed chamber was prepared using two plastic pails: one as a base (82 L) and the other as a chamber cover (110 L). These two pails were connected during sampling time by an extension tubing (height: 13 cm, radius: 25.2 cm) made of transparent plastic sheets. The pot with the rice plant was placed in the chamber base. After this, the extension was placed over the chamber base and the chamber cover was fitted over that extension. The pails and extension were fixed in place and made airtight during sampling time using water as a sealing material. Each chamber was equipped with a direct current (DC) fan to maintain thorough mixing of air inside the chamber, a thermometer to measure the inside air temperature during the sampling of gas, and a rubber septum fixed in the chamber wall to collect the gas samples. Gas sampling was conducted throughout the crop growth period. Gas samples from the chamber head space were collected using a 60-mL PVC syringe with stainless steel needle. Four samples were taken at 10-min intervals (0, 10, 20, and 30 min) for 30 min every week from 1300 to 1500. The collected samples were stored (1–3 days) in a previously evacuated vial for laboratory measurement.
The mixing rates of CH4 and N2O were measured simultaneously using a gas chromatograph (GC) (Shimadzu GC-14B, Shimadzu Corporation, Kyoto, Japan) equipped with a flame ionization detector and an electron capture detector. However, N2O measured may be biased due to its interference with CO2 concentration since the ascarite filter pre-column to trap CO2 in air samples was not used (Zheng et al. 2008). The carrier gas was nitrogen (N2) with a flow rate of 14 mL min−1. The column was packed with Porapak Q; column temperature was maintained at 60 °C and detector temperature was set at 340 °C. The retention time for CH4 was 1.0 min, and 3.28 min for N2O. A GC-computer interface was used to quantify the peak area. Temporal increases in CH4 and N2O concentration (ppm min−1) were determined from the slope of the linear regression curve of concentration against time. The emissions of CH4 and N2O gas, expressed as mg m−2 d−1, were calculated using the following equation (IAEA 1992; Gaihre et al. 2011):
where Vc is the volume of the gas chamber in liters (L), MW is the molecular weight of the respective gas, 60 is min h−1, 24 is h d−1, 22.4 is the volume of 1 mol of gas in L at standard temperature and pressure, 273 is the standard temperature in °K, T is the temperature inside the chamber in °C, Ac is the area of the pot with rice in m2, and 1,000 is μg mg−1.
The daily gas emission rate of the period between two consecutive samplings was calculated as the mean of the gas fluxes for those 2 days. This mean was calculated by interpolation of those 2 gas sampling days. Then, total cumulative emissions over the season were estimated from the first sampling of gases up to the last sampling, which was considered as seasonal total emissions. Global warming potential for a 100-year time horizon was calculated based on IPCC (2007). However, the uncertainty associated with diurnal variation is not accounted in seasonal emission and global warming potential.
Observation of rice harvest components
Plant height, tiller number, biomass and grain yield, and percent filled and unfilled grains were recorded during harvesting of the rice crops.
Data analysis
The data were processed and analyzed using the procedure described by Gomez and Gomez (1984). Analysis of variance as a test of fixed effect of temperature (T), rice straw (S), and DAT on different parameters was done using SAS mixed procedure (SAS V9.1) (SAS Institute Inc 2003). Tukey’s test was used for mean comparison and differences were considered significant at P < 0.05. ANOVA of some selected parameters was done using CropStat 7.2 and mean comparison was done by LSD values at the 5 % level.
Results
Temperatures: air, water, and soil
The variations of average air temperature, water temperature, and soil temperature over gas sampling time showed similar patterns throughout the rice-growing season (Fig. 2). Both water and soil temperatures were as uniformly elevated as air temperature. However, the magnitude of both water and soil temperatures was lower than that of air temperature in both experiments. Temperatures remained almost stable with a fluctuation of about 2 °C within a treatment. However, these fluctuations of temperatures were more than 2 °C within a treatment in Exp. I, particularly after 100 DAT.
Seasonal variation of soil Eh in relation to temperature and rice straw
The soil Eh at 5-cm depth showed significant seasonal variations (Table 2). Soil Eh at the beginning of the experiment was relatively higher in all the treatments (Fig. 3). However, because of missing data points, this initial decrease was not recorded in Exp. I. Although the +Straw treatments already had negative Eh values after 20 DAT, it can fairly be assumed that Eh values would have been above 0 mV during the onset of this experiment as in Exp. II. Thereafter, Eh values either remained almost stable (+Straw) or decreased steadily with time (−Straw). The seasonal patterns were similar in all the temperature treatments in a given experiment and the effect of elevated temperature was not significant (p > 0.05). But, the effect of rice straw showed significant differences (p < 0.001) as the Eh values declined rapidly under incorporation of rice straw in contrast to a gradual decrease without straw. Under +straw conditions, soil Eh value reached about −100 mV within the initial 20 DAT. In the latter growth stages, both −Straw and +Straw treatments attained similar Eh values at all temperatures in Exp. II (Fig. 3c and d). On the other hand, the decreasing rate and magnitude of soil Eh values were significantly different (Table 4) between the two experiments, especially under −Straw treatments. Soil Eh remained in a positive range throughout the growing season in Exp. I, with a final Eh value of 40 mV, while it was −200 mV in Exp. II.
Seasonal variation in CH4 and N2O emissions in relation to temperature and rice straw
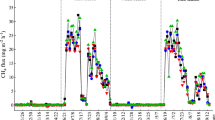
CH4 emission rates showed a seasonal variation with rice growth. Emission rates were increased significantly by both elevated temperatures and rice straw incorporation (Table 2). However, the patterns and magnitude of emissions varied with treatments and experiments (Fig. 4). Temperature and rice straw had an interaction effect; thus, the magnitude of CH4 emission rates was further increased (p < 0.001) with rice straw incorporation under elevated temperatures. Moreover, temperature interacted with time (DAT) (Table 2) and the effect was more prominent at the latter stages of rice growth, particularly in Exp. II (Fig. 4).
In Exp. I under −Straw conditions (Fig. 4a), the considerable CH4 emissions started at only 100 DAT and attained the highest emission rates of 3, 8, and 15 mg m−2 d−1 between 124 and 138 DAT for low, medium, and high temperature, respectively. In contrast, the emissions under +Straw conditions started earlier (20 DAT) in all temperature treatments (Fig. 4b). However, emissions were not measured before 20 DAT as temperature treatments were not employed by that time. The highest emission rates were observed during early tillering stage (21–28 DAT), with 164, 266, and 271 mg m−2 d−1 for low, medium, and high temperature, respectively. There were two seasonal peak emissions, that is, one early-season peak (21–28 DAT) and another mid-season peak (56 DAT), unlike only one late-season peak under −Straw. The emission rates declined gradually (+Straw) with plant growth toward the reproductive and maturity stages in all temperature treatments.
On the other hand, in Exp. II, CH4 emission rates in all temperature treatments under both −Straw and +Straw conditions showed increasing trends with rice growth, and peaks were observed at maturity stage (120 DAT) and a decreasing rate thereafter (Fig. 4c, d). Under −Straw conditions, lower emission rates were recorded from 27 to 83 DAT. Then, emission rates increased, with peak emissions between 118 and 132 DAT. However, under +Straw conditions, significant emissions started earlier (20 DAT) and increased with rice growth. Two seasonal peak emissions were observed: an early-season peak at 34 DAT and a late-season peak at 118–132 DAT. But, there was only a single late-season peak emission observed in the −Straw treatment at all temperatures.
Higher emission rates resulted in higher seasonal average emission rates and cumulative emissions at elevated temperatures (Tables 3 and 4). Cumulative emissions were 2 times and 5 times higher (8 and 35 g m−2) under high temperature than under low temperature (5 and 7 g m−2) in Exp. I and Exp. II, respectively. Similarly, cumulative emissions were almost 49 times and 7 times higher (13 and 42 g m−2) in the +Straw treatment than in the −Straw treatment (0.26 and 6 g m−2) in Exp. I and Exp. II, respectively. Moreover, the high temperature with the +Straw treatment had a far greater magnitude of emissions than the low temperature with −Straw treatment. It increased from 0.04 to 16 and from around 1 to 60 g m−2 for Exp. I and Exp. II, respectively. On the other hand, the average seasonal emission rates and cumulative emissions were significantly higher (4 times) in Exp. II than in Exp. I (Table 3).
N2O emission rates were not detected throughout the rice growth period in Exp. I. However, some emissions were detected in Exp. II, especially in the early period, immediately after flooding the soil until 13 DAT (Fig. 5). Immediately after flooding, very high emissions (100 mg m−2 d−1) were observed in the high-temperature treatment under −Straw conditions. Both emission rates and cumulative emissions were lower at all temperatures in +Straw treatments. Nevertheless, the emission rates declined sharply after 1 week of rice transplanting in −Straw treatments. Then, the emission rates remained low with fluctuating values throughout the growing season in both +Straw and −Straw treatments at all temperatures. The average emission rate under −Straw treatment was observed to be higher (2.76 mg m−2 d−1) in the medium-temperature treatment (Table 3). The seasonal average emission rate from all treatments was observed as 0.91 mg m−2 d−1 and cumulative emissions were 0.25 g m−2.
Seasonal variations of N2O emission rates under different temperatures and rice straw treatments (a: −straw; b: +straw) throughout the rice-growing season in walk-in growth chamber experiment II (error bars are omitted for clarity); negative value on X-axis indicates the days before transplanting of rice
With CH4 emissions, high temperature increased GWP in both experiments. The highest GWP (405 and 1,554 g CO2 eq. m−2) was observed at high temperature under +Straw conditions, respectively, in Exp. I and Exp. II (Table 3). With CH4 emissions, the GWP potential of Exp. II (GWP 677 g CO2 eq. m−2) was more than four times higher than that of Exp. I (GWP 163 g CO2 eq. m−2) (Table 3). This indicates that CH4 is the major contributor to GWP from continuously flooded rice fields.
Relationship between soil Eh and CH4 and N2O emissions
Segmented non-linear regression analysis showed a significant exponential relationship between CH4 emissions and soil Eh, particularly below soil Eh value of −40 mV (y = abx). The good match between actual data points and computed correlation is shown in Fig. 6 (R2 = 0.61, p = <0.001, n = 126). Since emissions were negligible above −40 mV, they were segmented on that point. Below −40 mV, emissions increased exponentially with a decrease in soil Eh (y = 2.4084 * 0.9745x). CH4 emission rates were higher from −100 to −200 mV (Fig. 6). On the other hand, N2O emissions were observed when soil Eh values were in positive ranges (>0 mV) (soil Eh data before transplanting not shown). However, this was observed for only a short time immediately after flooding of the soil. The emission trend was limited to only a few points with positive Eh values. When soil Eh became negative, no N2O emissions occurred throughout the rice-growing season. Furthermore, rice straw incorporation rapidly decreased soil Eh and increased the magnitude of CH4 emissions as well as emitted CH4 earlier than without rice straw.
Rice growth and yield
The analysis of variance between temperatures and some agronomic parameters such as plant height at maturity, the number of panicles hill−1, filled spikelets panicle−1, and spikelet sterility was significantly affected by temperature in both experiments (Table 5). Plant height increased with temperature. The highest plant height was observed at medium (Exp. II) to high temperature (Exp. I), whereas the lowest height was at low temperature. A variable effect was observed on panicles hill−1 in two experiments. Significantly more panicles (21 hill−1) were observed at high temperature in Exp. I whereas more were observed (33 hill−1) at low temperature in Exp. II. However, there was no significant difference between high and medium temperature in both experiments. Similarly, a higher number of both total and filled spikelets panicle−1 was observed at medium temperature in both experiments. In contrast, spikelet sterility was significantly higher (73 % and 40 %) at high temperature and lower (17 % each) at medium temperature in Exp. I and Exp. II, respectively. This indicates that medium temperature was optimum for that rice variety. In both experiments, straw weight was significantly higher, while grain weight was significantly lower in the high-temperature treatments (Table 6). The average grain yield was higher (23 and 60 g pot−1) at medium temperature and lower (10 and 38 g pot−1) at high temperature in Exp. I and Exp. II, respectively. Similarly, 1,000-grain weight was also significantly higher at medium temperature than at high temperature. The drastic decrease in grain yield under high temperature was due to high spikelet sterility (Table 5). As with grain yield, harvest index was also higher at medium temperature. Lower grain yield, harvest index, and 1,000-grain weight were observed at high temperature.
Moreover, rice straw had a significant effect on plant height, number of panicles hill−1, total spikelets panicle−1, and both straw and grain weight in Exp. I. All those values were higher under −Straw conditions. This is probably because of the immobilization of soil N with straw incorporation, since the soil used in Exp. I had very low organic C and N. There was no straw × temperature interaction effect on agronomic parameters in both experiments (Tables 5 and 6).
Discussion
Effect of temperature and rice straw on soil Eh
The soil Eh showed a clear seasonal trend and it decreased starting from high positive values (Table 2; Fig. 3). According to literature results, soil redox reactions are mainly controlled by temperature in addition to moisture regime, C availability, and microbial activities. Longer flooding time is required to induce soil reduction at low temperature due to lower microbial activities. Vaughan et al. (2009) investigated the intensity of reduction processes in the soil by using an Eh-pH stability line (equilibrium boundary) for ferrihydrite. When the soil temperature was between 1 and 3.9 °C, longer periods of saturation (for 20 day) were required for the redox potential to drop below the ferrihydrite stability line, while it took only 2 days at temperature above 9 °C. A similar experiment on soil reduction with respect to ferrihydrite conducted by Rabenhorst and Castenson (2005) showed no measurable reduction below 2 °C, while a 35 % to 45 % reduction was observed within 7 days when the temperature was in between 8 and 20 °C. Those findings clearly suggest that the higher the soil temperature, the faster the soil reduction in flooded soils in given soil temperature ranges. In our study, however, soil Eh was not significantly affected by temperature, which can most likely be attributed to the temperature range investigated. We elevated temperatures from 29 to 35 °C, that is, temperature changes were small and basically remained within the optimum temperature range of microbial activities.
The decreasing trend and magnitude of soil Eh varied with rice straw incorporation. Generally, soil Eh decreased rapidly with rice straw incorporation. Rice straw supplies electrons during its decomposition and thus its incorporation in flooded soils decreased soil Eh (Bossio et al. 1999; Gao et al. 2004; Minamikawa and Sakai 2006). The faster decrease in soil Eh, especially in the early growth stage of rice, was associated with early emissions of CH4. However, a gradual decrease in soil Eh with continuous flooding can be observed even without rice straw incorporation (Fig. 3c). Therefore, the soil Eh in both +Straw and −Straw treatments became similar, especially in the latter growth stages of rice (Exp. II). During flowering to maturity stages, rice plants supply organic matter as root exudates, which serve as an electron donor and decrease soil Eh (Fey and Conrad 2000). On the other hand, soil Eh can also be influenced by soil properties. Generally, greater C abundance leads to increased microbial activity and therefore more rapid reduction (Rabenhorst and Castenson 2005). Hou et al. (2000) reported that soil with higher organic matter was reduced faster and attained lower Eh values than soil with lower organic matter. In this study, the soil Eh in Exp. I (−Straw) remained positive throughout the growing period, probably due to very low soil organic C compared with the soil of Exp. II (Table 1). This result was in close agreement with Gao et al. (2004), in which no soil reduction was observed until the end of the experiment when rice straw was not applied. Nevertheless, the magnitude of the final soil Eh values was also lower in Exp. I even when rice straw was incorporated.
Seasonal variations in CH4 and N2O emissions
CH4 emission rates showed significant seasonal variation, with peak emissions at tillering (Exp. I, +Straw) to maturity stage (Exp. II). In continuously flooded rice fields, 2–3 peak emissions can be observed. The early-season peak emissions appear in soil with high organic matter and that with organic amendment (Wassmann et al. 2000). In this study, the higher CH4 emission rates in earlier stage and decreasing with rice growth in Exp. I (+Straw) might be due to the effect of rice straw, since the soil had low organic carbon (Table 1) and the emission was negligible under −Straw conditions. On the other hand, emission rates were increasing with plant growth in Exp. II under all the treatments which had high organic carbon. The late-season peak emissions from flowering to maturity stages are due to the supply of plant-borne C through root exudates and decaying tissues (Neue et al. 1997). These substances from rhizodeposition are readily available for methanogenesis, which increases and becomes higher at flowering to maturity (Kimura et al. 2004; Gaihre et al. 2011). Moreover, the higher emissions at flowering are associated with the well-developed aerenchyma tissues, which serve as a medium for CH4 transport (Adhya et al. 1994). Though all soils remained continuously flooded, CH4 emission rates decreased after maturity stage in all treatments. This decreasing rate from maturity to harvesting stage and thereafter may be due to the decreasing dissolved organic C (DOC), reduced root exudates, and decreasing transport capacity of the aerenchyma tissues (Zhan et al. 2010).
N2O emissions were not detected throughout the rice-growing period in Exp. I. Generally, N2O emissions are not occurring when the field is continuously flooded. This result is in good agreement with earlier studies stating that N2O emissions from continuously flooded fields are usually negligible (Bronson et al. 1997b; Chen et al. 1997; Ma et al. 2009). But, the high emission rates immediately after flooding the soil to 10 DAT (Fig. 5a) under −Straw treatments in Exp. II may be due to the denitrification of NO3-N present in the soil. On the other hand, emissions were lower under +Straw treatments (Fig. 5b), probably due to the immobilization of soil N by rice straw incorporation (Yao et al. 2010; Muhammad et al. 2011). Nevertheless, emissions decreased at 7 DAT because the large amount of N2O was further reduced to N2 before being emitted from the soil. Hence, N2O emissions are negligible throughout the growing season. However, the possible emission peaks after application of urea (14, 35 and 65 DAT) were not captured since gas sampling was done weekly.
Effect of temperature and rice straw on CH4 emissions
CH4 emissions increased significantly in elevated temperature treatments. Temperature plays a key role in CH4 emissions in anoxic soils (Fey and Conrad 2003). Generally, higher temperature increases the decomposition of organic matter (Fey and Conrad 2000; Plante et al. 2010; Das and Adhya 2012) and increases root exudates and root sloughing (Allen et al. 2003; Tokida et al. 2010), which in turn provides more substrates for methanogenic bacteria. Moreover, higher temperature increases biomass production due to higher photosynthesis leading to greater rhizodeposition. This higher rhizodeposition shows a positive effect on CH4 emissions (Wang et al. 1999; Tokida et al. 2010). In the present study, increases by a factor of 2 (Exp. I) and 5 (Exp. II) in CH4 emissions were observed in high-temperature treatment compared with low temperature. These results are in line with the results of Allen et al. (2003), who obtained fourfold higher total seasonal emission in high temperature (38 vs. 32 °C). However, Tokida et al. (2010) and Yun et al. (2012) showed a smaller increase in emissions (8–44 %) by elevating soil/water temperature (1.7–2 °C above ambient) in field conditions. The higher increase reported by Allen et al. (2003) and in this study may be due to a higher temperature increment. However, the effect of temperature on CH4 emissions was not linear (Fig. 4). The higher response was observed from low temperature to medium compared with the increment from medium to high. High temperature had relatively higher emissions and GWP than medium temperature, but these differences were below statistical significance. This suggests that the temperature response between 32 and 35 °C is lower than between 29 and 32 °C, and probably the temperature range of 32–35 °C is the near optimum range for methane emissions. The majority of methanogens are mesophilic and these microorganisms thrive at temperatures between 30 and 35 °C (Minami and Neue 1994). Any temperature beyond that limit may decrease CH4 emissions. Parashar et al. (1993) and Schulz et al. (1997) demonstrated increasing CH4 emissions with increasing soil temperature up to 34–35 °C and a decrease beyond this limit. Similarly, Schrope et al. (1999) reported decreasing CH4 emission rates with increasing temperature (ambient +4 °C) when ambient temperature was around 34 °C during gas sampling time. This suggests that the change in emissions with elevated temperature depends on the actual temperature range, that is, starting from a low ambient temperature will increase emissions while starting from a high temperature may in fact decrease emissions. The inhibitory effect of higher temperature is probably due to a combination of stress to the methanogens as well as the rice plants (Schrope et al. 1999) that act as conduits for methane.
On the other hand, the effects of elevated night temperatures alone on emissions are not well studied. Cheng et al. (2008) reported higher CH4 emissions when night temperature increased from 22 to 32 °C, keeping day temperature constant at 32 °C. In this study, night temperatures (daily minimum) were maintained higher by 2–3 °C in Exp. I, particularly in low- and medium-temperature treatments compared with Exp. II. (Figure 1). The increment here (2–3 °C) was much lower than the 10 °C of their study and those high temperatures were maintained for only a few hours at night. Thus, this increment in night temperature was assumed to have a negligible effect on emissions. However, its effect on grain yield was expected, which is discussed later under “Rice growth and yield in relation to temperature”.
Rice straw incorporation increased CH4 emissions in all temperature treatments. Rice straw acts as an electron donor and provides methanogenic substrate during its decomposition, thus lowering soil Eh and increasing CH4 emissions (Bossio et al. 1999). The magnitude of CH4 emissions is correlated to the amount of rice straw (Naser et al. 2007). A higher amount of rice straw incorporation accelerated soil reduction (Gao et al. 2004). The earlier accumulation of acetate generated through decomposition of rice straw triggers CH4 emissions at an early stage (Glissmann and Conrad 2000). In this study, the impacts of rice straw were very evident in both experiments. In Exp. I, negligible emissions were observed without straw incorporation. Exp. II showed a late peak without straw, but nevertheless much lower cumulative emissions than with straw incorporation. Watanabe et al. (1999) assessed the contribution of different organic sources to CH4 emissions under a straw application rate of 6 t/ha. Straw had the highest percentage (42 %), followed by plant-borne material (37–40 %) and soil organic matter (18–21 %). The percentage of straw was especially high at the beginning of the growing period.
Elevated temperature with rice straw incorporation revealed a significant additive effect on CH4 emissions. Methane emission rates were far greater at elevated temperature with rice straw incorporation (Table 3). Decomposition rates increase with high temperature, but substrate availability determines the upper limit of decomposition (von Lutzow and Kogel-Knabner 2009). Thus, the temperature effect will be enhanced with incorporation of rice straw. Moreover, the more pronounced effect of temperature during flowering to maturity stages is probably due to higher rhizodeposition and accelerated root senescence in addition to decomposition of rice straw (Allen et al. 2003; Cheng et al. 2008; Tokida et al. 2010).
Methane emission potential is influenced by soil properties. Accordingly, the response of rice straw may differ with soil type and the fastest response is observed in soil with high inherent CH4 production potential (Lu et al. 2000). In this study, the response of rice straw to total CH4 emissions was higher in the soil with low organic C. There was a higher increase in CH4 emissions in low-carbon soil (OC = 0.55 %) (Exp. I) than in soil with high carbon (OC = 0.99 %) (Exp. II). However, the magnitude of emissions was higher in soil with high organic C. Moreover, the incorporation of rice straw significantly increased soil organic carbon and available K in soil (data not shown).
The effect of temperature on N2O emissions was not significant. Emissions of N2O were below the detection limit throughout the growing period and considerable emissions were observed only immediately after flooding the soil. However, incorporation of rice straw reduced N2O emissions, probably because of immobilization as discussed earlier (“Seasonal variations in CH4 and N2O emissions”).
Relationship between soil Eh and CH4 and N2O emissions
Soil Eh and CH4 emissions showed a significant exponential relationship. Emissions increased with decreasing soil Eh, particularly below −40 mV, and became higher from −100 to −200 mV. Soil Eh could be roughly divided into two ranges to illustrate CH4 emissions, considering −100 mV as a threshold. CH4 emissions were significant below that, but negligible above that. Soil Eh decreased with flooding and this has a direct effect on CH4 emissions (Gaunt et al. 1997; Minamikawa and Sakai 2006). Flooding of the soil reduced the molecular O2 in the soil. When molecular O2 is absent, soil microorganisms use the alternative electron acceptors as an O2 source. The reduction of these oxidized soil components follows the thermodynamic sequence. Microorganisms sequentially use NO3 −, Mn4+, Fe3+, SO4 2−, and lastly CO2. At the terminal step, CO2 is reduced to CH4 (Kogel-Knabner et al. 2010). Hence, CH4 production takes place under much reduced conditions and higher CH4 emissions could be observed with the lowest soil Eh (Wang et al. 1993; Supparattanapan et al. 2009). This result suggests that CH4 emissions are a function of soil Eh, and a soil Eh value of ≤100 mV is critical for CH4 emissions. On the other hand, emissions of N2O were observed only immediately after flooding of the soil until soil Eh was >0 mV and CH4 emissions had not yet started. Methane emissions were the highest when soil Eh was the lowest and N2O emissions were negligible.
Rice growth and yield in relation to temperature
In this study, high temperature significantly increased spikelet sterility and decreased grain yield (Tables 5 and 6). The lower grain yield in high temperature is associated with high spikelet sterility. Generally, high temperature leads to greater sink demand due to increased growth and respiration, and therefore more rapid use of assimilates. This leads to less use of assimilates for grain production. The effect of high temperature on the growth and yield of rice depends on the sensitivity of the growth stage and duration of the exposure. High temperature can affect all growth stages, but the reproductive phase, especially booting and flowering stage, is considered to be the most susceptible, which leads to high spikelet sterility and reduced grain yield (Shah et al. 2011). Horie et al. (2004) also stated that extremely high temperature during flowering can drastically decrease rice yield because of spikelet sterility. On the other hand, high temperature significantly increased the straw biomass in both experiments (Table 6). This shows that vegetative growth is generally much more tolerant of high temperatures than reproductive-stage growth. Hence, high temperature up to a certain limit may not have a negative effect on vegetative growth and photosynthesis. Therefore, higher straw dry matter weight was observed in high-temperature treatments. Medium temperature (32/25 °C) in this study resulted in higher grain yield with low spikelet sterility, and probably this is the optimum temperature for that rice cultivar. Ziska et al. (1996) also observed 50 % higher grain yield at temperature 29/21 °C while yield at higher growth temperature (37/29 °C) was almost zero because of temperature-induced spikelet sterility. Variations exist in the effect of high temperature. Higher minimum temperature (night temperature) affects rice yields more than higher maximum temperature (day temperature). In this study, an increase in nighttime temperature by 2 °C (28 to 30 °C in Exp. I) as compared to the 28 °C of Exp. II (with constant day temperature of 35 °C) increased spikelet sterility by more than 30 % (Table 5). This finding is supported by Welch et al. (2010), who reported that higher minimum temperature reduced yield, whereas higher maximum temperature raised it. Hence, an increase in nighttime temperature is more critical than an increase in day temperature. It is reported that a 1 °C increase in growing-season minimum temperature in the dry season in the Philippines decreased rice yield by 10 % (Peng et al. 2004). This suggests a net negative impact on rice yield with increased temperature associated with global warming (Welch et al. 2010). On the other hand, the higher straw and grain yield in Exp. II compared with Exp. I was mainly due to the higher soil fertility (high organic C and N).
Conclusions
This study has investigated different temperature regimes, that is, combinations of maximum and minimum temperatures over one 24-h cycle. We are referring to these three regimes as (i) low (29/25, 29/22 °C), (ii) medium (32/25 °C), and (iii) high (35/30, 35/28 °C) temperature regimes, respectively. It is understood, however, that these descriptive terms have to be seen in the context of a tropical/subtropical climate during the rice-growing season. Temperature increases from low to medium caused higher GWP by factors of 3.0–11.0 without straw and factors of 1.4–3.7 with straw incorporation. This means that we can expect a relatively strong impact of atmospheric warming during periods of the rice crop when background temperatures are low. These low-temperature regimes are typically observed in the subtropics during spring and autumn. In tropical regions, this low-temperature regime will essentially be at higher altitudes or in particular meteorological situations. In all these cases, temperature increases will largely increase GHG emission rates from rice, namely, rice grown in China and northern India during spring and autumn as well as higher altitude rice in Southeast Asia. The increase from medium to high temperature, however, causes much lower changes in GWP than from low to medium temperature (see Table 3). In fact, the temperature increment ratios were around one in all these inter-comparisons, that is, there has been no significant increase in GWP with higher temperatures in this range. This will apply to the subtropics during summer as well as to the low-altitude tropics throughout the year. However, this experiment encompassed temperature changes in all three ecosystem components (air, water, soil). This suggests that increasing global temperature will increase emissions of greenhouse gases, as long as the increase in air temperature translates into a warming of the soil/water components as well. Temperature increments of air/plant canopy and soil/water alone do not produce the same effect as increments of the entire system (Gaihre 2012).
More importantly, elevated temperature had an additive effect with straw incorporation and far greater CH4 emissions and GWP were observed under high temperature with straw incorporation than under low temperature without straw. This suggests that the effect of temperature depend on general carbon such as soil carbon, organic amendment and inherent supply from plants. Moreover, the increased supply of straw with intensive cultivation of rice could further increase emissions unless alternative management strategies are adopted instead of incorporation. On the other hand, high temperature increased plant biomass but reduced grain yield with low harvest index. This indicates that increasing atmospheric temperature may have a direct effect on grain yield, especially due to increasing spikelet sterility. Thus, we conclude that global warming may result in a double negative effect: higher emissions and lower yields. However, these results were from environment-controlled growth chambers, and more field studies with experimental warming are needed to come up with comprehensive conclusions.
References
Adhya TK, Rath AK, Gupta PK, Rao VR, Das SN, Parida KM, Parashar DC, Sethunathan N (1994) Methane emission from flooded rice fields under irrigated conditions. Biol Fertil Soils 18:245–248
Allen LH Jr, Albrecht SL, Colon-Guasp W, Covell SA, Baker JT, Pan D, Boote KJ (2003) Methane emissions of rice increased by elevated carbon dioxide and temperature. J Environ Qual 32:1978–1991
Asami T, Kumada K (1959) A new method for determining free iron in paddy soils. Soil Sci Plant Nutr 5:141–146
Bossio DA, Horwath WR, Mutters RG, Kessel CV (1999) Methane pool and flux dynamics in a rice field following straw incorporation. Soil Biol Biochem 31:1313–1322
Bray RH, Kurtz LT (1945) Determination of total organic and available form of phosphorus in soils. Soil Sci 59:39–46
Bronson KF, Neue HU, Abao EB, Singh U (1997a) Automated chamber measurements of methane and nitrous oxide flux in a flooded rice soil: I. Residue, nitrogen, and water management. Soil Sci Soc Am J 61:981–987
Bronson KF, Neue HU, Abao EB, Singh U (1997b) Automated chamber measurements of methane and nitrous oxide flux in a flooded rice soil: II. Fallow period emissions. Soil Sci Soc Am J 61:988–993
Chen GX, Huang GH, Huang B, Yu KW, Wu J, Xu H (1997) Nitrous oxide and methane emissions from soil-plant systems. Nutr Cycl Agroecosyst 49:41–45
Cheng W, Sakai H, Hartley A, Yagi K, Hasegawa T (2008) Increased night temperature reduces the stimulatory effect of elevated carbon dioxide concentration on methane from rice paddy soil. Global Change Biol 14:644–656
Conant R, Ryan MG, Agren GI, Birge HE, Davidson EA, Eliasson PE, Evans SE, Frey SD, Fiardina CP, Hopkins FM, Hyvonen R, Kirschubaum MF, Lavallee JM, Leifeld J, Parton W, Steinweg JM, Wallenstein MD, Wetterstedt JAM, Bradford MA (2011) Temperature and soil organic matter decomposition rates: synthesis of current knowledge and a way forward. Global Change Biol 17:3392–3404
Das S, Adhya TK (2012) Dynamics of methanogenesis and methanotrophy in tropical paddy soils as influenced by elevated CO2 and temperature interaction. Soil Biol Biochem 47:36–45
Denman KL, Brasseur G, Chidthaisong A et al (2007) Couplings between changes in the climate system and biogeochemistry. In: Solomon S et al (eds) Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge (UK) and New York (USA), pp 501–587
Devevre OC, Horwath WR (2000) Decomposition of rice straw and microbial carbon use efficiency under different soil temperatures and moistures. Soil Biol Biochem 32:1773–1785
Dobermann A, Dawe D, Roetter RP, Cassman KG (2000) Reversal of rice yield decline in a long-term continuous cropping experiment. Agron J 92:633–643
Fageria NK, Carvalho GD, Santos AB, Ferreira EPB, Knupp AM (2011) Chemistry of lowland rice soils and nutrient availability. Commun Soil Sci Plant Anal 42:1913–1933. doi:10.1080/00103624.2011.591467
Fey A, Conrad R (2000) Effect of temperature on carbon and electron flow and on the archaeal community in methanogenic rice field soil. Appl Environ Microbiol 66:4790–4797
Fey A, Conrad R (2003) Effect of temperature on the rate-limiting step in the methanogenic degradation pathway in rice field soil. Soil Biol Biochem 35:1–8
Firestone MK, Davidson EA (1989) Microbiological basis of NO and N2O production and consumption in soil. In: Andreae MO, Schimel DS (eds) Exchange of trace gases between terrestrial ecosystems and the atmosphere. Wiley, New York, pp 7–21
Gaihre YK (2012) Greenhouse gas emissions and soil redox potential in irrigated lowland rice as affected by elevated temperatures. PhD thesis, University of the Philippines Los Baños
Gaihre YK, Tirol-Padre A, Wassmann R, Aquino E, Pangga GV, Sta-Cruz PC (2011) Spatial and temporal variations in methane fluxes from irrigated lowland rice fields. Philipp Agric Sci 94:335–342
Gao S, Tanji KK, Scardaci SC (2004) Impact of rice straw incorporation on soil redox status and sulfide toxicity. Agron J 96:70–76
Gaunt JL, Neue HU, Bragais J, Grant IF, Giller KE (1997) Soil characteristics that regulate soil reduction and methane production in wetland rice soils. Soil Sci Soc Am J 61:1526–1531
Glissmann K, Conrad R (2000) Fermentation pattern of methanogenic degradation of rice straw in anoxic paddy soil. FEMS Microbiol Ecol 31:117–126
Gomez KA, Gomez AA (1984) Statistical procedures in agricultural research, 2nd edn. Wiley, New York, 680 p
Helmke PA, Sparks DL (1996) Lithium, sodium, potassium, rubidium, and sesium. In: Sparks DL (ed) Methods of soil analysis. Part 3. Chemical methods. Soil Science Society of America, Inc., and American Society of Agronomy, Inc, Madison, pp 551–574
Horie T, Yoshida H, Kawastu S, Katsura K, Homma K, Shiraiwa T (2004) Effect of elevated atmospheric CO2 concentration and increased temperature on rice: implications for Asian rice production. In: Toriyama K, Heong KL, Hardy B (eds) Rice is life: scientific perspectives for the 21st century. Proceedings of the World Rice Research Conference, Tsukuba, pp 536–539
Hou AX, Chen GX, Wang ZP, Cleemput OV, Patrick WH (2000) Methane and nitrous oxide emission from a rice field in relation to soil redox and microbial processes. Soil Sci Soc Am J 64:2180–2186
Hua X, Guangxi X, Cai Z, Tsuruta H (1997) Nitrous oxide emissions from three rice paddy fields in China. Nutr Cycl Agroecosyst 49:23–28
IAEA (International Atomic Energy Agency) (1992) Manual on measurement of methane and nitrous oxide emissions from agriculture. IAEATECHDOC-674. IAEA, Vienna
IPCC (Intergovernmental Panel on Climate Change) (2007) Summary for policymakers. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp 1–18
Johnson-Beebout SE, Angeles OR, Alberto MCR, Buresh RJ (2009) Simultaneous minimization of nitrous oxide and methane emission from rice paddy soils is improbable due to redox potential changes with depth in a greenhouse experiment without plants. Geoderma 149:45–53
Kalra YP, Maynard DG (1991) Methods manual for forest soil and plant analysis, For. Can., Northwest Reg., North For. Cent., Edmonton, Alberta, Canada. Inf. NOR-X-315
Khalil K, Mary B, Renault P (2004) Nitrous oxide production by nitrification and denitrification in soil aggregates as affected by O2 concentration. Soil Biol Biochem 36:687–699
Kimura M, Murase J, Lu Y (2004) Carbon cycling in rice field ecosystems in the context of input, decomposition and translocation of organic materials and fates of their end products (CO2 and CH4). Soil Biol Biochem 36:1399–1416
Kogel-Knabner I, Amelung W, Cao Z, Fiedler S, Frenzel P, Jahn R, Kalbitz K, Kolbl A, Schloter M (2010) Biogeochemistry of paddy soils. Geoderma 157:1–14
Kruger M, Frenzel P, Conrad R (2001) Microbial processes influencing methane emission from rice fields. Global Change Biol 7:49–63
Lu Y, Wassmann R, Neue HU, Huang C, Bueno CS (2000) Methanogenic responses to exogenous substances in anaerobic rice soils. Soil Biol Biochem 32:1683–1690
Ma J, Ma E, Xu H, Yagi K, Cai Z (2009) Wheat straw managment affects CH4 and N2O emissions from rice fields. Soil Biol Biochem 41:1022–1028
Mandal KG, Misra AK, Hati KM, Bandhopadhyay KK, Ghosh PK, Mohanty M (2004) Rice residue-management options and effects on soil properties and crop productivity. Food Agric Environ 2:224–231
Minami K (1997) Atmospheric methane and nitrous oxide: sources, sinks and strategies for reducing agricultural emissions. Nutr Cycl Agroecosyst 49:203–211
Minami K, Neue HU (1994) Rice paddies as a methane source. Clim Chang 27:13–26
Minamikawa K, Sakai N (2006) The practical use of water management based on soil redox potential for decreasing methane emission from a paddy field in Japan. Agric Ecosyst Environ 116:181–188
Muhammad W, Vaugham SM, Dalal RC, Menzies NW (2011) Crop residues and fertilizer nitrogen influence residue decomposition and nitrous oxide emission from a vertisol. Biol Fertil Soils 47:15–23
Naser HM, Nagata O, Tamura S, Hatano R (2007) Methane emissions from five paddy fields with different amounts of rice application in central Hokkaido, Japan. Soil Sci Plant Nutr 53:95–101
Neue HU, Wassmann R, Kludze HK, Wang B, Lantin RS (1997) Factors and processes controlling methane emissions from rice fields. Nutr Cycl Agroecosyst 49:111–117
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circular 939. Washington, DC
Parashar DC, Gupta PK, Rai J, Sharma RC, Singh N (1993) Effect of soil temperature on methane emission from paddy fields. Chemosphere 26:247–250
Peng S, Huang J, Sheehy JE, Laza RC, Visperas RM, Zhong X, Centeno G, Khush GS, Cassmann KG (2004) Rice yields decline with higher night temperature from global warming. Proc Natl Acad Sci USA. www.pnas.org/cgi/doi/10.1073/pnas.0403720101
Plante AF, Conant RT, Carlson J, Greenwood R, Shulman JM, Haddix ML, Paul EA (2010) Decomposition temperature sensitivity of isolated soil organic matter fractions. Soil Biol Biochem 42:1991–1996
Rabenhorst MC, Castenson KL (2005) Temperature effects on iron reduction in hydric soil. Soil Sci 170:734–742
Rath AK, Ramakrishnan B, Sethunathan N (2002) Temperature dependence of methane production in tropical rice soils. Geomicrobiol J 19:581–592
SAS Institute Inc (2003) Proceedings of the 28th Annual SAS® Users Group International Conference. SAS Institute Inc., Cary
Schrope MK, Chanton JP, Allen LH, Baker JT (1999) Effect of CO2 enrichment and elevated temperature on methane emissions from rice, Oryza sativa. Global Change Biol 5:587–599
Schulz S, Matsuyama H, Conrad R (1997) Temperature dependence of methane production from different precursors in a profundal sediment (Lake Constance). FEMS Microbiol Ecol 22:207–213
Shah F, Huang J, Cui K, Nie L, Sah T, Chen C, Wang K (2011) Impact of high-temperature stress on rice plant and its traits related to tolerance. J Agric Sci 149:545–556
Smith P, Martino D, Cai Z, Gwary D, Janzen H, Kumar P, McCarl B, Ogle S, Omara F, Rice C, Scholes B, Sirotenko O (2007) Agriculture. In: Metz B, Davidson OR, Bosch PR, Dave R, Meyer LA (eds) Climate change 2007: mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp 498–540
Solomon S, Qin D, Manning M et al. (2007) Technical summary. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp 21–91
Sparks DL (ed) (1996) Methods of soil analysis. Part 3. Chemical methods, 3rd edn. American Society of Agronomy, Inc., Soil Science Society of America, Inc, Madison, pp 1255–1273
Supparattanapan S, Saenjan P, Quantin C, Maeght JL, Grunberger O (2009) Salinity and organic amendment effects on methane emission from a rain-fed saline paddy field. Soil Sci Plant Nutr 55:142–149
Tokida T, Fumoto T, Cheng W, Matsunami T, Adachi M, Katayangi N, Matsushima M, Okawara Y, Nakamura H, Okada M, Sameshima R, Hasegawa T (2010) Effect of free-air CO2 enrichment (FACE) and soil warming on CH4 emission from a rice paddy field: impact assessment and stoichiometric evaluation. Biogeoscience 7:2639–2653
Vaughan KL, Rabenhorst MC, Needelman BA (2009) Saturation and temperature effects on the development of reducing conditions in soils. Soil Sci Soc Am J 73:663–667
von Lutzow M, Kogel-Knabner I (2009) Temperature sensitivity of soil organic matter decomposition: what do we know? Biol Fertil Soils 46:1–15
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter and proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Wang ZP, Delaune RD, Masscheleyn PH, Patrick WH Jr (1993) Soil redox and pH effects on methane production in a flooded rice soil. Soil Sci Soc Am J 57:382–385
Wang B, Xu Y, Wang Z, Li Z, Guo Y, Shao K, Chen Z (1999) Methane emissions from rice fields as affected by organic amendment, water regime, crop establishment, and rice cultivar. Environ Monit Assess 57:213–228
Wang JY, Xia JX, Xiong ZQ, Khalil MAK, Xing GX (2011) Water regime-nitrogen fertilizer-straw incorporation interaction: field study on nitrous oxide emissions from a rice agroecosystem in Nanjing, China. Agric Ecosyst Environ 141:437–446
Wassmann R, Neue HU, Lantin RS, Makarim K, Chareonslip N, Buendia LV, Rennenberg H (2000) Characterization of methane emissions from rice fields in Asia. II. Differences among irrigated, rainfed, and deepwater rice. Nutr Cycl Agroecosyst 58:13–22
Wassmann R, Nelson GC, Peng SB, Sumfleth K, Jagadish SVK, Hosen Y, Rosegrant MW (2010) Rice and global climate change. In: Pandey S et al (eds) Rice in the global economy: strategic research and policy issue for food security. International Rice Research Institute, Manila (Philippines), pp 411–432
Watanabe A, Takeda T, Kimura M (1999) Evaluation of origins of CH4 carbon emitted from rice paddies. J Geophys Res 104:23623–23629
Welch JR, Vincent JR, Auffhammer M, Moya PM, Dobermann A, Dawe D (2010). Rice yields in tropical/subtropical Asia exhibit large but opposing sensitivities to minimum and maximum temperatures. Proc Natl Acad Sci U S A. www.pnas.org/cgi/doi/10.1073/pnas.1001222107
Yan XY, Yagi K, Akiyama H, Akimoto H (2005) Statistical analysis of the major variables controlling methane emission from rice fields. Global Change Biol 11:1131–1141
Yao H, Conrad R, Wassmann R, Neue HU (1999) Effect of soil characteristics on sequential reduction and methane production in 16 rice paddy soils from China, the Philippines, and Italy. Biogeochemistry 47:269–295
Yao Z, Zhou Z, Zheng X, Xie B, Mei B, Wang R, Butterbach-Bahl K, Zhu J (2010) Effect of organic matter incorporation on nitrous oxide emissions from rice-wheat rotation ecosystems in China. Plant Soil 327:315–330
Yoshida S, Forno DA, Cock JH, Gomez KA (1976) Laboratory manual for physiological studies of rice. International Rice Research Institute, Los Baños, Philippines
Yu K, Bohme F, Rinklebe J, Neue HU, Delaune RD (2007) Major biogeochemical processes in soils: a microcosm incubation from reducing to oxidizing conditions. Soil Sci Soc Am J 71:1406–1417
Yun SI, Kang BM, Lim SS, Choi WJ, Ko J, Yoon S, Ro HM, Kim HY (2012) Further understanding CH4 emissions from a flooded rice field exposed to experimental warming with elevated [CO2]. Agric For Meteorol 154–155:75–83
Zhan M, Cougui C, Wang J, Iangy J, Cai M, Yue L, Shahrear A (2010) Dynamics of methane emission, active soil organic carbon and their relationships in wetland integrated rice-duck systems in Southern China. Nutr Cycl Agroecosyst. Published online: 15 May 2010. doi:10.1007/s10705-010-9371-7
Zheng X, Mei B, Wang Y, Xie B, Wang Y, Dong H, Xu H, Chen G, Cai Z, Yue J, Gu J, Su F, Zou J, Zhu J (2008) Quantification of N2O fluxes from soil-plat systems may be biased by the applied gas chromatograph methodology. Plant Soil 311:211–234
Ziska LH, Manalo PA, Ordonez RA (1996) Intraspecific variation in the response of rice (Oryza sativa L.) to incrased CO2 and temperature: growth and yield response of 17 cultivars. J Exp Bot 47:1353–1359
Ziska LH, Moya TB, Wassmann R, Namuco OS, Lantin RS, Aduna JB, Abao E Jr, Bronson KF, Neue HU, Olszyk K (1998) Long-term growth at elevated carbon dioxide stimulates methane emission in tropical paddy rice. Global Change Biol 4:657–665
Acknowledgment
The authors would like to express their sincere gratitude to the International Rice Research Institute for its support of the experiment and the Asian Development Bank-Japan Scholarship Program (ADB-JSP) for the scholarship given to the senior author. We thank to Mr. Eugenio Aquino and Mr. Rodolfo Delos Reys for their assistance in the experiment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Klaus Butterbach-Bahl.
Rights and permissions
About this article
Cite this article
Gaihre, Y.K., Wassmann, R. & Villegas-Pangga, G. Impact of elevated temperatures on greenhouse gas emissions in rice systems: interaction with straw incorporation studied in a growth chamber experiment. Plant Soil 373, 857–875 (2013). https://doi.org/10.1007/s11104-013-1852-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1852-4