Abstract
Aim
Phosphate-solubilizing yeasts have been under-exploited in eco-friendly maize cultivation. In this regard, soil yeasts Meyerozyma guilliermondii CC1, Rhodotorula mucilaginosa CC2 and M. caribbica CC3 were investigated for their plant growth-promoting (PGP) activities.
Methods
Soil yeasts were isolated and characterized. Maize (Zea mays L. cv. Tainong No.1) and Chinese cabbage (Brassica rapa L. cv. Pekinensis) were used for seed bioassay. Growth-promoting effects of yeasts under greenhouse conditions were evaluated using maize and lettuce (Lactuca sativa L. cvs. Capitata and Taiwan sword leaf). Ultimately, M. guilliermondii CC1 was tested on field-grown maize; treatments included full-dose chemical fertilizers (CF), yeast (CC1), half-dose chemical fertilizers (½CF), CC1 + ½CF and control. Nutrient uptake, growth, and yield of maize and rhizospheric soil microbes were estimated.
Results
Strain M. guilliermondii CC1 exhibited better seed vigor index in maize and Chinese cabbage. CC1 + ½CF significantly improved the dry-weights, and nutrient uptakes of maize and sword leaf lettuce under greenhouse conditions. In field, CC1 + ½CF application exerted a pronounced effect on growth of maize, cob yield, nutrient-uptake and rhizospheric soil microbial counts.
Conclusion
Our results validated superior biochemical potency and PGP traits of M. guilliermondii CC1 that reduced requisite chemical fertilizer application without affecting the optimal productivity in maize.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Modern agriculture has adversely affected environment and human health. Many studies have been carried out to replace or reduce agrochemical usage, which negatively affects health and environment (Dadhich et al. 2011; Adesemoye and Kloepper 2009; Kumar et al. 2009; Ali et al. 2008; El-Kholy et al. 2005). Increasing human population needs much enhanced crop production, which in turn leads to improper use of chemical fertilizers and chemical pesticides (Hamuda and Patkó 2010; Berg 2009; Leach and Mumford 2008). Both developed as well as developing countries require large amount of chemical fertilizers to meet contemporary and future food demands. About 50 % of the chemical fertilizers are used for the production of cereals (wheat, rice and maize), and 50 % of all chemical fertilizers are consumed in China, USA and India (Roy et al. 2006). China has large cropping area and possesses agriculture industries that are characterized by intensive application of chemical fertilizers. On the contrary, India is a modest user of chemical fertilizer; however, regarded as a great consumer of fertilizers since it has very large cropping areas (Davidson and Gu 2012).
Among cereals, maize is an important crop that requires the application of huge amount of chemical fertilizers. Food and agriculture organization of the United Nations reported that maize receives an average chemical fertilizer application of 136 kg ha−1 with a global planting area of about 115.1 million ha (FAO 2006). World’s largest producers of maize are the United States, China, the European Union, Brazil and Mexico (Meng and Ekboir 2000). Increasing demand for bioethanol to reduce or replace fossil fuel has triggered intense maize cropping in several countries. Enhanced usage of chemical fertilizers consequently escalates environmental pollution (Donner and Kucharik 2008). Alternative ways to solve these environmental issues are organic farming or sustainable agricultural practices (Agamy et al. 2013; Zarabi et al. 2011; El-Kholy et al. 2005). On the other hand, integrated nutrient management systems offer eco-friendly and healthy agricultural output (Dadhich et al. 2011; Adesemoye and Kloepper 2009; El-Kholy et al. 2005; Ali et al. 2008).
Plant growth hormones are commonly used to reduce the usage of chemical fertilizers, pesticides, or as supplements to increase crop growth and yield (Saharan and Nehra 2011; Jeon et al. 2003). Microorganisms produce plant growth hormones such as auxin, gibberellic acid, and ethylene, which promote plant growth and yield (Santi Ferrara et al. 2012; Amprayn et al. 2012; Nassar et al. 2005; Frankenberger and Arshad 1995). Bacteria that dwell in the rhizosphere and exert plant growth-promoting (PGP) traits are termed plant growth-promoting rhizobacteria (PGPR). These PGP traits include N2-fixation, phosphate-solubilization, phytohormone-production, or the ability to confer reduction in plant ethylene levels by ACC deaminase activity, which directly, indirectly, or synergistically supports plant growth and increases nutrient availability in plants (Saharan and Nehra 2011; Fürnkranz et al. 2009). Therefore, PGPR have been regarded as a key factor for the establishment of plants under imbalanced nutrient conditions (Egamberdiyeva and Höflich 2004; Requena et al. 1997).
Yeasts are eukaryotic microfungi, which are widely distributed in natural environments (Lima et al. 2012; Bura et al. 2012; Mestre et al. 2011; Xin et al. 2009). In fact, the role played by yeast in soil agricultural ecosystems is not completely understood. For instance, yeast isolates such as Williopsis californica, W. saturnus, Saccharomyces cerevisiae and Candida tropicalis have been shown to be PGP microorganisms (Amprayn et al. 2012; Nassar et al. 2005; Falih and Wainwright 1995). A soil yeast isolate Cryptococcus podzolicus acts as a decomposer as it can hydrolyze lignocellulosic substances in its natural habitat (Mestre et al. 2011). Rhodotorula mucilaginosa PTD3, an endophytic strain reported to possess exceptional ability of synthesizing xylitol and bioethanol from lignocellulosic hydrolysates (Bura et al. 2012). Strains of Meyerozyma guilliermondii, and M. caribbica have been shown to possess antifungal activities (Bautista-Rosales et al. 2013; Coda et al. 2013; Lima et al. 2012) and thus can be potential biocontrol agents. Nevertheless, to our knowledge, neither any member of the genus Meyerozyma, nor Rhodotorula have been studied in detail for phosphate-solubilization and associated multiple PGP abilities.
Thus, the objective of this study was to evaluate comparatively the PGP activities of phosphate-solubilizing yeasts (PSY) M. guilliermondii CC1, R. muciloginosa CC2, and M. caribbica CC3 and to test a better isolate with superior PGP traits for eco-friendly maize cultivation under field conditions.
Materials and methods
Isolation and cultivation of phosphate-solubilizing yeasts (PSY)
Soil samples were collected at National Chung Hsing University (NCHU) campus, Taiwan (24°07′13.46″ N, 120°40′26.64″ E) during 2005; M. guilliermondii CC1 was isolated from the rhizospheric soil of an uprooted Ficus (Ficus religiosa L.) tree; R. mucilaginosa CC2 and M. caribbica CC3 were isolated from the surface soil. For isolation of PSY, 10 g of rhizospheric (root-adhered) soil from the Ficus tree and 10 g of bulk soil samples collected at NCHU campus were suspended separately in 100 ml sterile distilled water. The soil suspensions were subjected to serial dilutions (10−1 to 10−7); 0.1 ml sample from each dilution was spread on modified Pikovskaya media (NBRIP; Nautiyal 1999) and incubated at 28–30 °C for 5–7 days. The PSY were isolated based on their ability to form clear zone (halo) on NBRIP, purified, identified and stored in potato dextrose broth (PDB, Difco) containing 50 % glycerol at −80 °C.
Molecular identification of yeasts
Yeast genomic DNA (gDNA) was isolated through UltraCleanTM Microbial gDNA Isolation Kit (MO BIO, USA) by following manufacturer’s instructions. For molecular identification of the yeast isolates, variable D2 region near the 5' end of the 26S rRNA (large subunit, LSU) gene was amplified by using gDNA template with primer pair NL-1 (5′-GCA TAT CAA TAA GCG GAG GAA AAG-3′) and NL-4 (5′-GGT CCG TGT TTC AAG ACG G-3′) (Kurtzman and Robnett 1997). PCR conditions were as follows: 95 °C for 4 min followed by 35 cycles at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 1.5 min and finally, 1 cycle at 72 °C for 5 min. NL-1 primer was used for sequencing. Gene sequencing was performed by using the Bigdye terminator kit (Heiner et al. 1998) and an automatic DNA sequencer (ABI PRISM 310, Applied Biosystems, CA, USA) (Watts and MacBeath 2001). Sequences were trimmed using EzTaxon server (Chun et al. 2007) before analysis via BLAST (Altschul et al. 1990) program of National Center for Biotechnology Information (NCBI; http://blast.ncbi.nlm.nih.gov/Blast.cgi). Reference sequences were retrieved from NCBI and analyzed by MEGA 5 (Molecular Evolutionary Genetics Analysis, version 5.0; Tamura et al. 2011), after multiple alignment by Clustal_X (Thompson et al. 1997). Distance matrix method (distance options according to the Kimura two-parameter model, Kimura 1980) including clustering by neighbor-joining (Saitou and Nei 1987), a discrete character-based maximum-parsimony (Fitch 1971) and maximum-likelihood (Felsenstein 1981) methods were used. Tree topologies were evaluated by using bootstrap resampling based on 1,000 replications (Felsenstein 1985).
Morphological, physiological and biochemical characterization of yeasts
Morphology was determined by placing the yeast cells on a carbon-coated copper grid, and staining with 0.2 % uranyl acetate for 5–10 sec, followed by brief air-drying and observation under transmission electron microscope (JEOL JEM-1400). Salt tolerance was studied by using PDB supplemented with 0–12 % NaCl (1 % increment). The pH range for growth was determined using PDB by adjusting the pH to 3–11 before sterilization (pH 0.5 intervals) using appropriate biological buffers. Growth in PDB at 10, 15, 20, 30, 37, 40, 45, 50 and 55 °C was tested after 3 days of incubation at 30 °C. Carbon source utilization, enzyme profiles, and acid production from various carbon sources were assessed by using commercial API 20 C AUX, API ZYM and API 50 CH kits (bioMérieux), respectively. All kit based analyses were performed according to manufacturers’ instructions and the results were recorded after 48 hrs of incubation at 30 °C.
Chitinase and cellulase assay
Activities of chitinase, and cellulase were determined by growing the yeasts on potato dextrose agar (PDA, Difco) plates supplemented with 1 % (v/v) colloidal chitin and 1 % carboxymethyl cellulose, respectively. The respective culture plates were incubated for 7 days at 30 °C and finally flooded with 0.1 % Congo red for 30 min followed by 1 M NaCl treatment for additional 10–15 min. The reaction zone was distinguished from the background and solubilization index (SI) was calculated by subtracting the colony diameter from total diameter of the reaction zone.
Quantitative phosphate-solubilization assay
Phosphate-solubilizing activity of yeasts was determined quantitatively according to Chen et al. (2006). Initially, solid agar media containing aluminum-, ferrous- and tricalcium-phosphates (5 g l−1 each) were used. Ten microliter culture suspension of yeasts were spotted separately on above given agar media in triplicates and incubated at 30 °C for 7 days. SI was calculated by subtracting the colony diameter from total diameter of halo. In addition, the tricalcium-phosphate-solubilizing activities of yeasts were also determined by Mo blue spectrophotometry (880 nm) using liquid tricalcium-phosphate medium.
Quantitative indole-3-acetic acid (IAA) production assay
Yeasts were cultured in yeast extract peptone dextrose (YPD, Difco) broth, with or without the presence of 0.1 % (w/v) L-tryptophan (L-trp, Sigma) for 5 days. Cells were collected by centrifugation (10,000 g, 5 min, 25 °C), 1 ml of culture supernatant was mixed with 4 ml of Salkowski reagent (in perchloric acid) in a glass test tube and incubated at room temperature for 30 min. The optical density (530 nm) of the reaction mixture was recorded by using a UV–visible spectrophotometer (U-3010, Hitachi).
Preparation of yeast inocula
Yeast cells were grown separately using PDB at 30 °C on a shaker at 150 rpm. After 72 h, cells were harvested by centrifugation (6,000 × g for 10 min) and cell pellets were washed twice with sterile water. Washed cells were re-suspended, and diluted by using sterile water and applied as respective inocula for seed germination bioassays in vitro and greenhouse pot experiments. Similarly, for field application, inoculum of M. guilliermondii CC1 was exclusively prepared. Yeast density was determined by dilution-plating on PDA and expressed as colony forming units (CFU).
Seed germination bioassay in vitro
Seed germination bioassays were performed by using M. guilliermondii CC1, R. mucilaginosa CC2, and M. caribbica CC3 inoculations on maize (Zea mays L. cv. Tainong No.1) and Chinese cabbage (Brassica rapa L. cv. Pekinensis). Seeds were surface sterilized with 1 % sodium hypochlorite solution for 20 min and washed several times with sterile water. Eight seeds were arranged randomly on two sheets of 70-mm sterile filter paper (Advantec, Toyo, Roshi Kaisha Ltd., Japan), which was kept in a Petri dish and inoculated with 2 ml of yeast culture suspension (100-fold diluted, final cell density ~3.52–7.12 × 106CFU ml−1). This assay was conducted in triplicates for each treatment. After 4 days, percentage seed germination, and root and shoot lengths were measured. The seedling vigor index (SVI) was calculated using the following formula: SVI = % Germination × (shoot length + root length) (Amprayn et al. 2012).
Greenhouse experiments
Pot experiments were performed on maize (Zea mays L. cv. Tainong No.1), sword leaf lettuce (Lactuca sativa L. cv. Taiwan sword leaf) and head lettuce (Lactuca sativa L. cv. Capitata) and under greenhouse conditions at NCHU, Taiwan (24°07′09.93″ N, 120°40′31.04″ E). Soil for greenhouse experiments was collected from the top soil (0–30 cm) of an agriculture farmland located at Guoxing (24°03′37″ N, 120°53′59″ E), Nantou county, Taiwan. Soil physicochemical properties were determined as follows: soil texture was assessed by hydrometer method (Gee and Bauder 1986); soil pH and electrical conductivity were measured by using soil suspension prepared in deionized water (1:5, w/v) (Rayment and Higginson 1992); total N in soil was analyzed according to Kjeldahl’s digestion procedure (Keeney and Nelson 1982); other soil nutrients (P, K, Ca, Mg, Fe, Mn and Zn) were extracted using Mehlich-3 solution (Mehlich 1984) and analyzed by inductively coupled plasma–atomic emission spectrometry (ICP–AES) with a sequential Jobin Yvon JY 138 Ultrace spectrometer (Faithfull 2002); soil organic matter was determined by loss-on-ignition method (Ben-Dor and Banin 1989). Physicochemical properties of soil were as follows: texture, loamy (45.3 % silt, 36.1 % sand and 18.6 % clay); pH, 5.61; electrical conductivity, 345 μS cm−1; organic matter, 74.80 g kg−1; N, 1.88 g kg−1; P, 0.16 mg kg−1; K, 0.06 mg kg−1; Ca, 1.22 mg kg−1; Mg, 0.15 mg kg−1; Fe, 0.39 mg kg−1; Mn, 0.02 mg kg−1.
Treatments included (1) control (without any yeast or chemical fertilizer applications); (2) CF (full-dose chemical fertilizers: 100 kg N, 50 kg P2O5 and 100 kg K2O ha−1 for maize; 100 kg N, 75 kg P2O5 and 110 kg K2O ha−1 for sword leaf lettuce and head lettuce; (3) ½CF (half-dose chemical fertilizers: 50 kg N, 25 kg P2O5 and 50 kg K2O ha−1 for maize; 50 kg N, 37.5 kg P2O5 and 55 kg K2O ha−1 for sword leaf lettuce and head lettuce; (4) inoculation of M. guilliermondii CC1; (5) inoculation of R. mucilaginosa CC2; (6) inoculation of M. caribbica CC3; (7) ½CF+M. guilliermondii CC1; (8) ½CF+R. mucilaginosa CC2; (9) ½CF+M. caribbica CC3. Maize consisted of 2.5 kg non-sterile soil pot−1, whereas sword leaf lettuce and head lettuce each contained 1 kg non-sterile soil pot−1.
During chemical fertilization, full-doses of P2O5 and K2O, and half-dose of N were applied at the time of sowing and only half-dose of N was applied after 20 days of sowing at root-zone. Four seeds each of maize, sword leaf lettuce and head lettuce were introduced in to respective pots at a depth of 1–3 cm. After emergence of three leaves, two plants pot−1 were retained and their roots were exposed by thinning. Respective yeast inocula (~10 ml pot−1 of 100-fold diluted inocula; final cell density, ~5.3 × 106CFU ml−1) were applied directly on the exposed roots, which were eventually covered-up by soil. Each treatment was repeated four times. Soil moisture was maintained ~25 % throughout the experiment.
Greenhouse experimental conditions were as follows: for maize, experimental timing was 27th November 2009 to 14th February 2010 (period of harvesting was 80 days after sowing); average humidity was 68 %; average air temperature was 26.4 °C; total sunshine duration was 407.7 h. For sword leaf lettuce, experimental timing was 26th June 2009 to 9th August 2009 (period of harvesting was 45 days after sowing); average humidity was 71.5 %; average air temperature was 27.3 °C; total sunshine duration was 303.2 h. For head lettuce, experimental timing was 27th November 2009 to 15th January 2010 (period of harvesting was 50 days after sowing); average humidity was 68 %; average air temperature was 26.2 °C; total sunshine duration was 258.1 h.
Plant heights were recorded at the time of harvesting. Plant shoots were dried at 72 °C (~74 h) immediately after harvesting for the determination of dry-weight. The dried samples were ground to powder and sieved (0.5 mm). Plant nutrients such as P, K, Ca, Mg, Mn, Fe, and Zn were extracted from powdered sample as given in Singh et al. (2011) and analyzed by ICP-AES. Plant N was determined by Kjeldahl’s digestion method (Keeney and Nelson 1982).
Field experiment
Field experiment was performed exclusively on maize using inoculum of M. guilliermondii CC1 and chemical fertilizer combination at an agriculture farm located at Guoxing, Nantou County, Taiwan (24°03′37″ N, 120°53′59″ E). Physicochemical properties of the field soil were as given earlier. Maize was harvested after 90 days of sowing.
Climatic conditions during the period of experimentation based on Central Weather Bureau, Taiwan were as follows: during the month of October, November, December and January, the total precipitation were 9.5, 39.0, 35.5 and 58.0; average air temperature were 21.9, 18.1, 25.2 and 16.6 °C; average relative humidity were 72.6, 72.7, 72.3 and 72.6 %; total sunshine duration were 176.6, 203.7, 179.4 and 182.3 h, respectively.
The randomized block design was used with 5 treatments having 3 replicates (block), and each block had five plots. Net plot size for each treatment was 4.2 m × 3.0 m (12.6 m2) with a row-to-row spacing of 70 cm and plant-to-plant spacing of 30 cm. Treatments included (1) control (without any yeast or chemical fertilizer applications); (2) CF (full-dose chemical fertilizers: 200 kg N, 100 kg P2O5 and 50 kg K2O ha−1, local recommendation); (3) ½CF (half-dose chemical fertilizers: 100 kg N, 50 kg P2O5 and 25 kg K2O ha−1); (4) CC1 (inoculation of M. guilliermondii CC1 alone); (5) CC1 + ½CF (combination of M. guilliermondii CC1 and ½CF).
During chemical fertilization, full-doses of P2O5 and K2O, and half-dose of N were applied 6 days before sowing and remaining half-dose of N was applied after 6 weeks of sowing by broad-cast practice. For sowing, shallow furrows were opened and two maize seeds were placed at a depth of 4–5 cm. After two weeks, one plant hill−1 was retained, its roots were exposed by thinning and first-dose of M. guilliermondii CC1 inoculum (~30 ml plant−1 of 100-fold diluted inocula; final cell density, ~5.3 × 106CFU ml−1) was introduced directly over exposed roots and covered-up with soil. Subsequent doses were applied at the root zone after 3rd, 4th and 5th weeks of sowing.
Plant heights were recorded at the time of harvesting. Plant shoots and cobs were dried at 72 °C (~74 h) immediately after harvesting for the determination of respective dry-weights. The dried plant shoots were ground to powder for the nutrient analyses (N, P, K, Ca, Mg, Mn, Fe and Zn) as given above.
Rhizospheric soil microbial counts and detection of M. guilliermondii CC1
Rhizospheric soil sample was randomly collected from each treatment immediately after harvesting the maize. One gram each of rhizospheric soil was mixed with 10 ml sterile water (1:10, w/v). Samples were then serially diluted from 10−1 to 10−4, and 0.1 ml each was separately spread on PDA and YPD agars in duplicates. The plates were incubated at 25 °C under darkness and the colonies were counted after 96 h of incubation. Cell counts were presented as log CFU g−1 rhizosphere soil (wet-weight). M. guilliermondii CC1 was detected on PDA and YPD primarily based on its colony morphology followed by colony PCR targeted towards 26S rRNA gene.
Statistical analyses
The randomized complete design (RCD), and randomized complete block design (RCBD) were used for the statistical analyses of greenhouse and field experimental data, respectively. One-way analysis of variance (ANOVA) was used for analyzing each set of data. All statistical analyses were performed using software package SAS (Statistical Analysis System; version 9.2, SAS Institute Inc., Cary, North Carolina, USA). A p value of <0.05 was considered as significant throughout.
Results
Strains CC1 and CC3 were identified to represent two different species of the genus Meyerozyma, whereas CC2 was a Rhodotorula species as determined by NCBI BLAST search based on D1/D2 region of 26S rRNA gene sequence. Corresponding gene sequence of M. guilliermondii CC1, M. caribbica CC3 (532 bp each), and R. mucilaginosa CC2 (526 bp) were submitted to the GenBank under the accession numbers JX970461, JX970462 and JX970463, respectively. M. guilliermondii CC1 shared pairwise sequence similarities of 99.8 %, 99.4 %, and 91.7 % to M. guilliermondii PGU45709T, M. caribbica NRRL Y-27274T and R. mucilaginosa ATCC 32763T, respectively. R. mucilaginosa CC2 shared 99.8 % similarity to R. mucilaginosa ATCC 32763T, and 91.7 % sequence similarity to both M. guilliermondii PGU45709T and M. caribbica NRRL Y-27274T. M. caribbica CC3 shared 100 %, 99.8 %, and 91.7 % sequence similarities to M. caribbica NRRL Y-27274T, M. guilliermondii PGU45709T and R. mucilaginosa ATCC 32763T, respectively. In the neighbor-joining phylogenetic tree, these yeasts clustered tightly associated with respective type strains, with which they shared a maximum pairwise sequence similarity (Fig. 1). Furthermore, bootstrap confidence of the nodes were very high (> 95 %) that indicated a stable phylogenetic position of these yeasts. Similarly, their phylogenetic positions were conserved in the maximum-parsimony and maximum-likelihood trees (not shown).
Unrooted neighbor-joining tree depicting the phylogenetic position of the yeast strains used in this study (highlighted as bold) and related type strains based on the 26S rRNA gene D1/D2 domain sequences. Bootstrap values of > 70 % after 1,000 bootstrap replicates are shown at the branch points. Bar, 0.05 substitutions per site. Reference sequences were retrieved from GenBank under the accession numbers given in parenthesis. Type strains are marked with superscript ‘T’
Cells of yeast strains possessed distinct morphology as shown in Fig. S1. They exhibited good growth at temperature 20–40 °C, pH 3–12 and tolerated 1–12 % NaCl. M. guilliermondii CC1 assimilated variety of carbon sources particularly D-glucose, glycerol, calcium 2-keto-gluconate, D-galactose, D-sorbitol, methyl-αD-glucopyranoside, N-acetyl-glucosamine, D-cellobiose, D-maltose and D-trehalose (Table S1). R. muciloginosa CC2 strongly assimilated D-glucose, L-arabinose, adonitol, D-sorbitol, D-maltose and D-saccharose. M. caribbica CC3 assimilated strongly D-glucose, calcium 2-keto-gluconate, D-galactose, N-acetyl-glucosamine and D-cellobiose.
M. guilliermondii CC1 showed strong positive reactions for lipase, leucine arylamidase, acid phosphatase, napthtol phosphohydrolase and α-glucosidase (Table S2). R. muciloginosa CC2 exhibited strong activities for esterase C4, leucine arylamidase, acid phosphatase and β-glucosidase. M. caribbica CC3 displayed strong esterase C4, esterase lipase C8, lipase, leucine arylamidase, acid phosphatase, alkaline phosphatase and α-glucosidase activities.
M. guilliermondii CC1 produced acid from variety of carbon sources including glycerol, D-adonitol, D-galactose, D-glucose, D-fructose, D-mannose, D-mannitol, D-sorbitol, esculin ferric citrate, D-cellobiose, D-maltose, D-saccharose, D-trehalose, D-melezitose and D-raffinose (Table S3). M. caribbica CC3 exhibited similar acid production profile excluding D-raffinose, but including excessive L-arabinose and D-xylose for strong acid production. Acid production ability of R. muciloginosa CC2 was remarkably poor.
M. guilliermondii CC1 displayed pronounced chitinase activity followed by strain M. caribbica CC3, whereas no detectable chitinase activity was recorded for R. muciloginosa CC2 (Fig. 2). None of the yeasts exhibited cellulase activity.
Chitinolytic ability of yeasts as determined on potato dextrose agar plates supplemented with 1 % (v/v) colloidal chitin. CC1, Meyerozyma guilliermondii CC1; CC2, Rhodotorula mucilaginosa CC2; CC3, M. caribbica CC3. Different letters denote significant differences (P < 0.05) according to Duncan’s multiple range test. ND, not detected
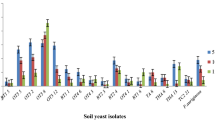
In liquid tricalcium-phosphate media, M. guilliermondii CC1 showed highest phosphate-solubilizing capability (190.8 mg l−1) followed by M. caribbica CC3 (170.4 mg l−1) and R. mucilaginosa CC2 (97.7 mg l−1). The solid tricalcium-phosphate media also showed a similar trend (M. guilliermondii CC1 > M. caribbica CC3 > R. mucilaginosa CC2) with a SI of 1.8, 1.5 and 1.2, respectively (Fig. 3). Interestingly, none of these yeasts exhibited ferrous-phosphate- and aluminum-phosphate-solubilizing capabilities.
Without-L-trp, M. guilliermondii CC1, R. mucilaginosa CC2, and M. caribbica CC3 produced low amounts of IAA, which were 2.3, 1.9 and 2.6 μg ml−1, respectively (Fig. 4). After introducing L-trp into the media, both M. guilliermondii CC1 (10.6 μg ml−1) and R. mucilaginosa CC2 (8.9 μg ml−1) produced significantly higher amounts of IAA than M. caribbica CC3 (5.5 μg ml−1).
IAA-producing ability of Meyerozyma guilliermondii CC1, Rhodotorula mucilaginosa CC2 and M. caribbica CC3 as determined in potato dextrose broth (PDB) supplemented with- (black-column) or without-L-tryptophan (L-trp) (white-column). Error bars represent standard deviation (SD, n = 3). Different letters denote significant differences (P < 0.05) according to Duncan’s multiple range test
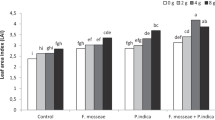
Maize seed germination was not altered significantly by yeast inoculations (Table 1). However, root length of maize was significantly increased after inoculating by yeasts as compared to control. Shoot length of maize was highest after inoculating M. guilliermondii CC1; inoculation of M. caribbica CC3 and control exhibited similar shoot lengths, whereas R. mucilaginosa CC2 inoculated seeds possessed lowest shoot length. Maximum maize SVI was recorded for M. guilliermondii CC1, whereas R. mucilaginosa CC2 and M. caribbica CC3 offered statistically similar SVI. The Chinese cabbage seeds treated with M. guilliermondii CC1 significantly improved SVI as compared to other treatments.
Plant growth results obtained from the greenhouse experiments are summarized in Table 2. Highest height and dry-weight were recorded for maize plants after CF application. Plant heights documented for ½CF and CC1 + ½CF were mutually-similar, which were also significantly higher than that of other treatments excluding CF. Application of CC2 + ½CF and CC3 + ½CF yielded statistically similar plant heights. Inoculation of yeasts alone enhanced dry-weight of maize plant as compared to control. Significantly higher plant dry-weights of maize were recorded during CC1 + ½CF and CC3 + ½CF application as compared to other treatments excluding CF. Application of CC1 + ½CF also enhanced the height, and dry-weight of lettuce plants followed by CC3 + ½CF and CC2 + ½CF.
The macronutrient data obtained from the greenhouse experiments are summarized in Table 3. As expected, plants supplied with chemical fertilizers exhibited better nutrient uptake when compared to other treatments including the control. The results showed that CC1 + ½CF, CC2 + ½CF, and CC3 + ½CF can enhance N, P and K uptake in maize than with ½CF alone. Application of yeast alone was not so effective in enhancing N, P and K uptake in maize. The uptakes of N, P and K in lettuce plants were significantly higher during CC1 + ½CF application as compared to other treatments excluding CF.
The secondary and micronutrient data obtained from the greenhouse experiments are summarized in Table 4. In maize, when compared to ½CF application, CC1 + ½CF promoted Ca-, Mg-, Mn- and Zn-uptakes; CC2 + ½CF enhanced Ca-, Mn- and Zn-uptakes; CC3 + ½CF promoted Ca- and Mg-uptakes. Further, the application of CC1 + ½CF significantly enhanced the nutrient uptake in both the verities of lettuce than that of ½CF. On the contrary, CC2 + ½CF and CC3 + ½CF yielded heterogeneous nutrient uptake in lettuce.
Due to relatively better response that was obtained for CC1 + ½CF as compared to CC2 + ½CF and CC3 + ½CF during greenhouse experiments, M. guilliermondii CC1 was selected for further field trial. The combinatory effects of M. guilliermondii CC1, and chemical fertilizers on plant growth, yield and nutrient uptake were studied after 90 days of cultivation of maize under field conditions. Inoculation of M. guilliermondii CC1 alone showed significant increase in plant height, and shoot dry-weight than that of control, but it was not as effective as ½CF and CF treatments (Table 5). Inoculation of M. guilliermondii CC1 moderately increased the cob yield (18 %) as compared to control. When CC1 + ½CF was applied, plant height, and cob yield was significantly enhanced than that of ½CF treatment and was as effective as chemical fertilizer treatment. Shoot dry-weight did not differ significantly between CC1 + ½CF and ½CF treatments.
Plant nutrient uptakes are summarized in Table 6. Application of M. guilliermondii CC1 alone showed significant increase in N-, P-, K-, Fe-, Mn- and Zn-uptakes in the shoot of maize as compared to that of control. The Ca-uptake did not differ significantly among control, ½CF and CC1 treatments. The Mg-uptake after M. guilliermondii CC1 treatment was comparable to that of control, and both the treatments showed significantly higher Mg-uptake than that of ½CF and chemical fertilizer treatments. Interestingly, CC1 and CC1 + ½CF application showed increased uptake of Mg as compared to that of CF.
Colonies of M. guilliermondii CC1 were detected on PDA and YPD agar during post-harvest analysis of cultivable rhizospheric soil microorganisms. We preferred PDA and YPD instead of universal bacteriological media in order to facilitate the recovery of M. guilliermondii CC1 from the rhizospheric soil. Further, the rhizospheric soil microbial CFU as determined on PDA, and YPD agar were in the log range of 4.3–6.7 and 5.4–6.7, respectively (Fig. 5) for various treatments. On PDA and YPD agar, the rhizospheric soil microbial CFU for the treatment CC1 and CC1 + ½CF were mutually similar but relatively higher (log 6.6–6.7) as compared to rest of the treatments. The rhizospheric soil microbial CFU did not differ considerably on PDA for control, chemical fertilizer and ½CF treatments. The rhizospheric soil microbial cell density was relatively higher on YPD agar than PDA for control, chemical fertilizer and ½CF treatments. In conclusion, inoculation of M. guilliermondii CC1 alone and its combination with ½CF (CC1 + ½CF) stimulated the cell density of cultivable microbes in root-adhered soil.
Effect of Meyerozyma guilliermondii CC1, and chemical fertilizer treatments on the total microbial colony-forming unit (CFU) of maize rhizospheric soils as determined by using potato dextrose (PDA, black-column) and yeast extract peptone dextrose (YPD, white-column) agars. Error bars represent standard deviation (SD, n = 2)
Discussion
Among the wealth of PGP bacteria described so far, most isolates also exhibit clinical origins and cause devastating health hazards in human (Young et al. 2013; Tyler and Triplett 2008). On the contrary, exploitation of yeast in agriculture has gained considerable attention due to its analogous beneficial bioactivity and enhanced safety (Agamy et al. 2013). Kurtzman and Suzuki (2010) dissected the genus Pichia and proposed a novel genus Meyerozyma and reclassified Pichia guilliermondii and P. caribbica as M. guilliermondii and M. caribbica, respectively. In this study, strains of the genus Meyerozyma and Rhodotorula were screened for their PGP traits. Although, our primary focus was to assess PGP effects of PSY on maize plants, Chinese cabbage and two varieties of lettuce were evaluated simultaneously at different stages of this study in order to gain broader perspective of corresponding PSY in agricultural applications. During seed bioassay, yeast inoculations enhanced root development as compared to control. Present enhancement is partly attributed to the IAA producing ability of yeasts that was shown earlier in W. saturnus and C. tropicalis (Amprayn et al. 2012; Nassar et al. 2005). Indeed, all the yeasts used in our study produced almost-similar amount of IAA in the absence of L-trp. However, they exhibited significantly varied IAA production when supplemented with L-trp, which might be due to the metabolic disparity existing among those strains. The microbial ability of producing IAA influences plant-microbe interactions (Santi Ferrara et al. 2012; Hsu 2010; Ludwig-Müller 2004). IAA performs many regulatory functions, including stimulating plant cell enlargement, cambium cell division, differentiation of phloem and xylem, root initiation and lateral root formation (Hsu 2010).
Several fungi and bacteria have been shown to be phosphate-solubilizers (Young et al. 2013; Sharma 2011; Nenwani et al. 2010; Kumar et al. 2009). Similarly, there are some reports on PSY as well (Xiao et al. 2013; Hesham and Mohamed 2011; Narsian et al. 2010; Al-Falih 2005). However, very few yeast isolates have been studied in detail for their PGP effects so far (Agamy et al. 2013; Amprayn et al. 2012; Nassar et al. 2005; Falih and Wainwright 1995). Earlier we have shown that the strain CC1 can solubilize tricalcium-phosphate and promote uptake of elemental phosphorus in garden lettuce using pot experiments (Nakayan et al. 2009). In this study, we have collectively evaluated PGP abilities including phosphate-solubilization of two more soil yeasts (R. mucilaginosa CC2 and M. caribbica CC3) with reference to M. guilliermondii CC1. The results suggest that the phosphate-solubilizing potential is widespread in soil yeasts and it may vary considerably at genus- and species-level.
Integrated application approaches that include the combination of microbial inoculants, and reduced level of chemical fertilizers to obtain better growth and yield has gained significant interest (Dadhich et al. 2011; Kumar et al. 2009; Ali et al. 2008; El-Kholy et al. 2005). Here, we tested the inoculum of individual soil yeast species combined with ½CF under greenhouse conditions. Application of CC1 + ½CF resulted in efficient uptake of macro- and micro-nutrients in both maize and lettuce. Plant dry-weight also significantly increased due to CC1 + ½CF application. Superior PGP traits including acid production from various carbon sources, chitinase activity, phosphate-solubilization and IAA-production are predicted to be collectively responsible for observed enhancements. Earlier reports suggest that the increase in nutrient uptake by plant was due to the release of some plant growth-regulating hormones such as IAA by soil microorganisms that improve root hair (Shahab et al. 2009) and its efficiency to uptake nutrients including phosphorus (Medina et al. 2004). Our greenhouse experimental results correlated well with the findings of Kumar et al. (2009), who showed that the application of bacterial strain Pseudomonas aeruginosa LES4, a tomato rhizosphere isolate, with ½CF resulted in plant growth equivalent to CF treatment, without compromising with the growth and yield of sesame. We further performed field trial to understand better the possible beneficial impact exerted by soil yeast M. guilliermondii CC1 on maize.
During field experiment, as compared to control, although the M. guilliermondii CC1 treatment alone produced 7 %, and 41 % higher plant height and shoot dry-weight, respectively, the cob yield did not change significantly. When inoculation of M. guilliermondii CC1 was combined with ½CF, the plant height was significantly enhanced than ½CF treatment, which was similar to that of chemical fertilizer treatment. CC1 + ½CF application resulted in similar cob yield as that of chemical fertilizer treatment, which was also far superior to that of ½CF treatment. The fact that CC1 + ½CF treatment resulted in lower shoot dry-weight but identical cob yield as that of chemical fertilizer treatment indicated that the inoculation of M. guilliermondii CC1 could assist in allocation of nutrients to the cob instead of the shoot. Moreover, this result suggests that M. guilliermondii CC1 inoculation will increase the nutrient-uptake efficacy of maize and can reduce half the amount of recommended chemical fertilizers. Nutrient data revealed that M. guilliermondii CC1 treatment alone increased the overall uptake of N, P, K, Fe, Mn and Zn as compared to that of control. More significantly, CC1 + ½CF treatment promoted uptake of most of the nutrients tested.
Inoculation of M. guilliermondii CC1 remarkably increased the rhizospheric soil microbial counts, which probably indicates a healthy plant and soil microbial interactions (Berg 2009). The root exudates secreted by maize plants contain diverse array of chemical compounds (Carvalhais et al. 2011; Badri and Vivanco 2009; Rasmann et al. 2005). Carbohydrate derivatives such as glucose, galactose, fructose, arabinose, xylose and mannitol are found to be in root exudates (Badri and Vivanco 2009), which can serve as potential carbon source for rhizospheric soil microbial flora. In fact, glucose and galactose act as sole energy source for the growth of M. guilliermondii CC1 as stated earlier. Additionally, M. guilliermondii CC1 produced acid from glucose, and galactose besides from arabinose, xylose, fructose and mannitol. The chemical composition of root exudates varies in maize plants according to the mineral nutrient availability and the plants secrete high amounts of carbohydrates specifically during phosphate-limited conditions (Carvalhais et al. 2011). The carbohydrates secreted by maize plant serve as a rich energy source for soil microorganisms, particularly for the rhizospheric phosphate-solubilizing M. guilliermondii CC1. Since organic acids play a definite role in phosphate-solubilization (Lin et al. 2006; Chen et al. 2006), potential acid-producing ability from carbohydrates of M. guilliermondii CC1 may in turn facilitate mobilization of insoluble phosphate in the rhizospheric soil. Eventually, the chemical components of root exudates and excreted metabolic byproducts of actively flourishing yeasts may collectively promote the growth of other rhizospheric soil microbial flora, which could partly explain the increased soil microbial counts at maize rhizosphere after M. guilliermondii CC1 inoculation.
Fungal population under field conditions varies significantly according to various growth phases of maize (Gomes et al. 2003) and infestation by phytopathogenic fungi could negatively affect maize yield (Channon and Farina 1991; Sumner and Minton 1989). A major structural component of fungal cell wall is chitin and interestingly, M. guilliermondii CC1 showed superior ability of chitin hydrolysis besides having several other PGP traits. M. guilliermondii CC1 can even metabolize N-acetyl-glucosamine, monomeric chitin derivative, as a sole carbon/nitrogen source and hence exhibits active growth. Although we have not investigated the fungal dynamics during this study, we hypothesize that the chitinolytic biocontrol activity of M. guilliermondii CC1 could have played a definite role in the enhancement of maize yield. It was interesting to note that CC1, like other yeasts, lacked cellulase activity that might be detrimental to plant tissues. Furthermore, the soil origin, multiple PGP traits and resistance to extreme environmental conditions offer competitive advantage for M. guilliermondii CC1 over other microbial flora that colonizes maize rhizosphere. As supporting evidence, M. guilliermondii CC1 was detected consistently in the maize rhizospheric soil during this study. Taken together, the present work demonstrated that M. guilliermondii CC1 is beneficial soil yeast that merits further development in agricultural applications.
References
Adesemoye AO, Kloepper JW (2009) Plant–microbes interactions in enhanced fertilizer-use efficiency. Appl Microbiol Biotechnol 85:1–12
Agamy R, Hashem M, Alamri S (2013) Effect of soil amendment with yeasts as bio-fertilizers on the growth and productivity of sugar beet. Afr J Agric Res 8:46–56
Al-Falih AM (2005) Phosphate solubilization in vitro by some soil yeasts. Qatar Univ Sci J 25:119–125
Ali S, Khan AR, Mairaj G, Arif M, Fida M, Bibi S (2008) Assessment of different crop nutrient management practices for yield improvement. Aust J Crop Sci 2:150–157
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Amprayn K, Rose MT, Kecskés M, Pereg L, Nguyen HT, Kennedy IR (2012) Plant growth promoting characteristics of soil yeast (Candida tropicalis HY) and its effectiveness for promoting rice growth. Appl Soil Ecol 61:295–299
Badri DV, Vivanco JM (2009) Regulation and function of root exudates. Plant Cell Environ 32:666–681
Bautista-Rosales PU, Calderon-Santoyo M, Servín-Villegas R, Ochoa-Álvarez NA, Ragazzo-Sánchez JA (2013) Action mechanisms of the yeast Meyerozyma caribbica for the control of the phytopathogen Colletotrichum gloeosporioides in mangoes. Biol Control 65:293–301
Ben-Dor E, Banin A (1989) Determination of organic matter content in arid-zone soils using a simple “loss-on-ignition method”. Commun Soil Sci Plant Anal 20:1675–1695
Berg G (2009) Plant–microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl Microbiol Biotechnol 84:11–18
Bura R, Vajzovic A, Doty SL (2012) Novel endophytic yeast Rhodotorula mucilaginosa strain PTD3 I: production of xylitol and ethanol. J Ind Microbiol Biotechnol 39:1003–1011
Carvalhais LC, Dennis PG, Fedoseyenko D, Hajirezaei MR, Borriss R, von Wirén N (2011) Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, phosphorus, potassium, and iron deficiency. J Plant Nutr Soil Sci 174:3–11
Channon P, Farina MPW (1991) Are soil-borne diseases depressing yields of continuously-grown maize in Natal? S Afr J Plant Soil 8:141–145
Chen YP, Rekha PD, Arun AB, Shen FT, Lai WA, Young CC (2006) Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl Soil Ecol 34:33–41
Chun J, Lee JH, Jung Y, Kim M, Kim S, Kim BK, Lim YW (2007) EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int J Syst Evol Microbiol 57:2259–2261
Coda R, Rizzello CG, Cagno RD, Trani A, Cardinali G, Gobbetti M (2013) Antifungal activity of Meyerozyma guilliermondii: identification of active compounds synthesized during dough fermentation and their effect on long-term storage of wheat bread. Food Microbiol 33:243–251
Dadhich SK, Somani LL, Shilpkar D (2011) Effect of integrated use of fertilizer P, FYM and biofertilizers on soil properties and productivity of soybean-wheat crop sequence. J Adv Dev Res 2:42–46
Davidson D, Gu FX (2012) Materials for sustained and controlled release of nutrients and molecules to support plant growth. J Agr Food Chem 60:870–876
Donner SD, Kucharik CJ (2008) Corn-based ethanol production compromises goal of reducing nitrogen export by the Mississippi River. Proc Natl Acad Sci USA 105:4513–4518
Egamberdiyeva D, Höflich G (2004) Effect of plant growth-promoting bacteria on growth and nutrient uptake of cotton and pea in a semi-arid region of Uzbekistan. J Arid Environ 56:293–301
El-Kholy MA, El-Ashry S, Gomaa AM (2005) Biofertilization of maize crop and its impact on yield and grains nutrient content under low rates of mineral fertilizers. J App Sci Res 1:117–121
Faithfull NT (2002) Methods in agricultural chemical analysis: A practical handbook. CABI Publishing, Wallingford
Falih AM, Wainwright M (1995) Nitrification, S-oxidation and P-solubilization by the soil yeast Williopsis californica and by Saccharomyces cerevisiae. Mycol Res 99:200–204
FAO (2006) Fertilizer use by crop: FAO fertilizer and plant nutrition bulletin 17. Food and Agriculture Organization of the United Nation, Rome, pp 60–61
Felsenstein J (1981) Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol 17:368–376
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Fitch WM (1971) Toward defining the course of evolution: minimum change for a specific tree topology. Syst Zool 20:406–416
Frankenberger WT, Arshad M (1995) Phytohormones in soils: Microbial production and function. Marcel Dekker Inc, New York
Fürnkranz M, Müller H, Berg G (2009) Characterization of plant growth promoting bacteria from crops in Bolivia. J Plant Dis Prot 116:149–155
Gee GW, Bauder JW (1986) Particle-size analysis. In: Klute A (ed) Methods of soil analysis, part 1, physical and mineralogical methods, 2nd edn. American Society if Agronomy, Inc. and and Soil Science Society of America, Inc, Madison, pp 383–411
Gomes NCM, Fagbola O, Costa R, Rumjanek NG, Buchner A, Mendona-Hagler L, Smalla K (2003) Dynamics of fungal communities in bulk and maize rhizosphere soil in the tropics. Appl Environ Microbiol 69:3758–3766
Hamuda HEAFB, Patkó I (2010) Relationship between environmental impacts and modern agriculture. Óbuda University e-Bulletin 1:87–98
Heiner CR, Hunkapiller KL, Chen SM, Glass JI, Chen EY (1998) Sequencing multimegabase-template DNA with BigDye terminator chemistry. Genome Res 8:557–561
Hesham AE, Mohamed HM (2011) Molecular genetic identification of yeast strains isolated from Egyptian soils for solubilization of inorganic phosphates and growth promotion of corn plants. J Microbiol Biotechnol 21:55–61
Hsu SY (2010) IAA production by Streptomyces scabies and its role in plant microbe interaction. M.Sc. A thesis, Cornell University
Jeon JS, Lee SS, Kim HY, Ahn TS, Song HG (2003) Plant growth promotion in soil by some inoculated microorganisms. J Microbiol 41:271–276
Keeney DR, Nelson DW (1982) Nitrogen-inorganic forms. In: Page AL, Miller DR, Keeney DR (eds) Methods of soil analysis, part 2, chemical and microbiological methods, 2nd edn. American Society of Agronomy, Inc. and Soil Science Society America, Inc, Madison, pp 643–698
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kumar S, Pandey P, Maheshwari DK (2009) Reduction in dose of chemical fertilizers and growth enhancement of sesame (Sesamum indicum L.) with application of rhizospheric competent Pseudomonas aeruginosa LES4. Eur J Soil Biol 45:334–340
Kurtzman CP, Robnett CJ (1997) Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5' end of the large-subunit (26S) ribosomal DNA gene. J Clin Microbiol 35:1216–1223
Kurtzman CP, Suzuki M (2010) Phylogenetic analysis of ascomycete yeasts that form coenzyme Q-9 and the proposal of the new genera Babjeviella, Meyerozyma, Millerozyma, Priceomyces, and Scheffersomyces. Mycoscience 51:2–14
Leach AW, Mumford JD (2008) Pesticide environmental accounting: a method for assessing the external costs of individual pesticide applications. Environ Pollut 151:139–147
Lima JR, Gonçalves LRB, Brandão LR, Rosa CA, Viana FMP (2012) Isolation, identification and activity in vitro of killer yeasts against Colletotrichum gloeosporioides isolated from tropical fruits. J Basic Microbiol 52:1–10
Lin TF, Huang HI, Shen FT, Young CC (2006) The protons of gluconic acid are the major factor responsible for the dissolution of tricalcium phosphate by Burkholderia cepacia CC-Al74. Bioresour Technol 97:957–960
Ludwig-Müller J (2004) From auxin homeostasis to understanding plant pathogen and plant symbiont interaction: editor’s research interests. J Plant Growth Regul 23:1–8
Medina A, Vassileva M, Caravaca F, Roldán A, Azcón R (2004) Improvement of soil characteristics and growth of Dorycnium pentaphyllum by amendment with agrowastes and inoculation with AM fungi and/or the yeast Yarowia lipolytica. Chemosphere 56:449–456
Mehlich A (1984) Mehlich 3 soil test extractant: a modification of Mehlich 2 extractant. Commun Soil Sci Plant Anal 15:1409–1416
Meng E, Ekboir J (2000) Current and future trends in maize production and trade. CIMMYT World Maize Facts and Trends 35–44
Mestre MC, Rosa CA, Safar SVB, Libkind D, Fontenla SB (2011) Yeast communities associated with the bulk-soil, rhizosphere and ectomycorrhizosphere of a Nothofagus pumilio forest in northwestern Patagonia, Argentina. FEMS Microbiol Ecol 78:531–541
Nakayan P, Shen FT, Hung MH, Young CC (2009) Effectiveness of Pichia sp. CC1 in decreasing chemical fertilization requirements of garden lettuce in pot experiments. As J Food Ag-Ind Special S66–S68
Narsian V, Samaha SM, Patel HH (2010) Rock phosphate dissolution by specific yeast. Indian J Microbiol 50:57–62
Nassar AH, El-Tarabily KA, Sivasithamparam K (2005) Promotion of plant growth by an auxin-producing isolate of the yeast Williopsis saturnus endophytic in maize (Zea mays L.) roots. Biol Fertil Soils 42:97–108
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170:265–270
Nenwani V, Doshi P, Saha T, Rajkumar S (2010) Isolation and characterization of a fungal isolate for phosphate solubilization and plant growth promoting activity. J Yeast Fungal Res 1:9–14
Rasmann S, Köllner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, Gershenzon J, Turlings TCJ (2005) Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434:732–737
Rayment GE, Higginson FR (1992) Australian laboratory handbook of soil and water chemical methods, vol 3. Inkata Press, Melbourne
Requena N, Jimenez I, Toro M, Barea JM (1997) Interactions between plant-growth-promoting rhizobacteria (PGPR), arbuscular mycorrhizal fungi and Rhizobium spp. in the rhizosphere of Anthyllis cytisoides, a model legume for revegetation in mediterranean semi-arid ecosystems. New Phytol 136:667–677
Roy RN, Finck A, Blair GJ, Tandon HLS (2006) Plant nutrition for food security ‒ a guide for integrated nutrient management. FAO Fertilizer and plant nutrition bulletin 16. Food and Agriculture Organization of the United Nation, Rome
Saharan BS, Nehra V (2011) Plant growth promoting rhizobacteria: a critical review. Life Sci Med Res 2011:1–30
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Santi Ferrara FI, Oliveira ZM, Gonzales HHS, Floh EIS, Barbosa HR (2012) Endophytic and rhizospheric enterobacteria isolated from sugar cane have different potentials for producing plant growth-promoting substances. Plant Soil 353:409–417
Shahab S, Ahmed N, Khan NS (2009) Indole acetic acid production and enhanced plant growth promotion by indigenous PSBs. Afr J Agric Res 4:1312–1316
Sharma K (2011) Inorganic phosphate solubilization by fungi isolated from agriculture soil. J Phytol 3:11–12
Singh S, Rekha PD, Arun AB, Hameed A, Singh S, Shen FT, Young CC (2011) Glutamate wastewater as a culture medium for Azospirillum rugosum production and its impact on plant growth. Biol Fert Soils 47:419–426
Sumner DR, Minton NA (1989) Crop losses in corn induced by Rhizoctonia solani AG-2-2 and nematodes. Phytopathology 79:934–941
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Tyler HL, Triplett EW (2008) Plants as a habitat for beneficial and/or human pathogenic bacteria. Annu Rev Phytopathol 46:53–73
Watts D, MacBeath JRE (2001) Automated fluorescent DNA sequencing on the ABI PRISM 310 Genetic Analyzer. Methods Mol Biol 167:153–170
Xiao C, Chi R, Pan X, Liu F, He J (2013) Rock phosphate solubilization by four yeast strains. Ann Microbiol 63:173–178
Xin G, Glawe D, Doty SL (2009) Characterization of three endophytic, indole-3-acetic acid-producing yeasts occurring in Populus trees. Mycol Res 113:973–980
Young LS, Hameed A, Peng SY, Shan YH, Wu SP (2013) Endophytic establishment of the soil isolate Burkholderia sp. CC-Al74 enhances growth and P-utilization rate in maize (Zea mays L.). Appl Soil Ecol 66:40–47
Zarabi M, Alahdadi I, Akbari GA, Akbari GA (2011) A study on the effects of different biofertilizer combinations on yield, its components and growth indices of corn (Zea mays L.) under drought stress condition. Afr J Agric Res 6:681–685
Acknowledgments
Authors would like to thank the editor and anonymous reviewers for their constructive criticism and suggestions that contributed to the improvement of this paper. This research work was supported by grants from the National Science Council of Taiwan, R.O.C. and Council of Agriculture, Executive Yuan, Taiwan, R.O.C.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Peter A.H. Bakker.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 417 kb)
Rights and permissions
About this article
Cite this article
Nakayan, P., Hameed, A., Singh, S. et al. Phosphate-solubilizing soil yeast Meyerozyma guilliermondii CC1 improves maize (Zea mays L.) productivity and minimizes requisite chemical fertilization. Plant Soil 373, 301–315 (2013). https://doi.org/10.1007/s11104-013-1792-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1792-z