Abstract
Previous studies on the identification of ion relations in halophytes have revealed that many members of Chenopodiaceae accumulate high amounts of sodium and chloride even in soils with low salinity, indicating a typical pattern which is genetically fixed. In this study, we followed up with the question of ion relations in different halophyte species with different photosynthetic pathways and different salt tolerance strategies over a complete growing season. Soil and plant samples from five species Climacoptera turcomanica (Litv.) Botsch. (leaf succulent-C4), Salicornia persica Akhani subsp. rudshurensis Akhani (stem succulent-C3), Halimocnemis pilifera Moq. (leaf succulent-C4), Petrosimonia glauca (Pall.) Bunge (leaf succulent-C4) and Atriplex verrucifera M. Bieb. (recreto-halophyte-C3) were collected over a complete growing season from a salt flat 60 km W of Tehran. The contents of main cations (Na+, K+, Ca2+, and Mg2+) and chloride were determined in plant and soil samples. Na+ and Cl− concentration in the shoots of two hygro-halophytes Climacoptera turcomanica and Salicornia persica subsp. rudshurensis were constant over the period of the growing season. In contrast, sodium and chloride in the shoots of Halimocnemis pilifera and Petrosimonia glauca showed respectively an increasing and, in the shoots of Atriplex verrucifera, a decreasing, trend. We did not notice any decreasing trend of K+ together with increasing trend of Na+ in the shoots of the studied species; however K+ in the shoots of all examined species was considerably lower than Na+ and Cl−. It was observed that Climacoptera and Salicornia could absorb and retain calcium even in high salinity conditions, while Halimocnemis and Petrosimonia could not. Na+, K+, Cl−, Ca2+, and Mg2+ contents in the shoots of different types of halophytes (stem-succulent, leaf-succulent and excreting halophyte) or different type of photosynthesis (C3, C4) are independent of those in their rhizosphere. We concluded that it is controlled by the genetic characteristic of the specific taxon rather than by the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salt stress is a major problem in most arid regions of the world limiting biomass production and threatening agricultural productivity and yields in the 21st century (Breckle 2009; Horie and Schroeder 2004). The salinity problem is a common concern in all arid climates, and NaCl must be considered as the most critical compound in those ecosystems which restricts plant growth (Veste et al. 2008). A dry or moist saline soil offers basically similar effects on plants. In both cases, the plants suffer from a physiological drought, because Na+ hydrates more than other ions, which explains why the water retention is higher even if the saline soil is moist (Grigore and Toma 2008). There are about a billion hectares of salt-affected land world-wide: more than 6 % of the world’s total land area (Munns and Tester 2008). In the past or present the most important source of the salt directly or indirectly is the ocean. The oceans occupy about 71 % of the world’s surface area and 97 % of the entire world’s water (Flowers et al. 1986).
Iran, with a surface area of 1,648,000 km2, has many salt deserts, sabkhas and salt marshes. In inner Iran there are over 60 playas (sabkhas) (Krinsley 1970) and numerous salty rivers that support diverse halophytic vegetation with their intermittent or permanent water supply (Akhani 2006). Halophytes are defined as plants which have successful growth and reproductive cycles in saline habitats (Breckle 1995). Some 5 % of the European flora is halophytic, whereas in the Iranian flora the percentage is 7 % (Akhani, unpublished data; Breckle 2002). Studies have shown that halophytes exhibit very different strategies for coping with high NaCl salinity (Koyro et al. 2008). There are no distinctive limits between the various adaptation types (Breckle 1986). It is also true that no single factor is of major importance for the ability to survive at high NaCl salinity. Often combinations of several mechanisms enhance the salt tolerance of individual species (Koyro et al. 2008; Breckle 1995, 2002). Then the process dominant in a distinct species is used to classify the halophytes. The main vascular plant halophyte-types include: stem-succulents; leaf-succulents; recreto-halophytes; pseudo-halophytes; and glycophytes (Breckle 1986, 1990). The result of a study on different types of halophytes along extreme saline gradients showed that various types are arranged according to salinity-zones, as within a salinity variation of 1,000 times, the dominant stem-succulents are gradually substituted by leaf-succulents, those by recreters, those by pseudo-halophytes, until on almost salt-free soil non-halophytes become dominant. The life-form of halophytes is also less variable than the rest of the flora. Annuals and low shrub species dominate in many salt ecosystems (Breckle 1975, 1986). The annual life form comprises 35 % of the halophytic flora of Iran. Many of the annual species belong to the Chenopodiaceae, especially the Salsoleae, Suaedeae and Salicornieae tribes (including many representatives of Caroxylon, Salsola, Climacoptera, Petrosimonia, Halimocnemis, Horaninowia, Girgensohnia, Suaeda, and Salicornia). A total of about 480 species are known to grow in salty habitats in Iran (Akhani 2006, unpublished data). Halophytes are very important in saline ecosystems due to their successful biological ability and reproduction in these regions, grazing forage for livestock, carbon sequestration in saline and arid ecosystems, phyto-remediation, and some agricultural products (Breckle 2009). Therefore, studies of the physiological and eco-physiological parameters of these plants are very important for a better understanding of salt tolerant mechanisms and specification of suitable plants for planting in saline soils (Breckle 2003, 2013; Wucherer et al. 2012).
Among salt tolerant plants, the Chenopodiaceae are the largest group (Wucherer et al. 2005; Dimeyeva et al. 2012). A great number of the halophytes and xero-halophytes of the world belong to this family (Akhani et al. 1997; Aronson 1989). It is characterized by a high proportion of halophytic genera (44 %), with some 312 halophytic species. Salt-tolerance is most widespread and best investigated, in such important genera as Salicornia, Atriplex, and Suaeda (Flowers et al. 1986). Previous studies had shown that Chenopodiaceae taxa could accumulate large amounts of sodium and chloride even at low salinity, indicating ion patterns which are genetically fixed (Wucherer et al. 2005; Albert 1982; Breckle 2002). Results from studies in the Great Salt Lake region (U.S.A) of the green leaves of Atriplex confertifolia, A. falcata, Ceratoides lanata and Artemisia tridentata, revealed surprising results in that the sodium content remained constant over a wide range of soil salinities. Similarly, Caroxylon scleranthum (Salsola sclerantha) in various sites in Iran and Afghanistan showed a very similar behaviour in ion pattern under different ecological conditions (Breckle 1986). The results from studies along ecological salinity gradients of the north shore of Mono Lake, California, USA, had shown that, the leaves of Sarcobatus vermiculatus could accumulate large amounts of Na+ independently and over the entire gradient. This plant had the ability to maintain sufficient absorption of N, P, K+, Ca2+, and Mg2+ under high concentrations of Na+ (Donovan et al. 1997). Other investigations on different saline soil types of the Aralkum desert showed no correlation between Na+, K+, Ca2+, Mg2+, and Cl−contents in the shoot of Suaeda acuminata and those contents in different soil types (Wucherer et al. 2005; Breckle and Wucherer 2012). Similar results were obtained from the green stems and leaves of Anabasis articulata and Cornulaca monacantha growing on dunes at the Nizzana experimental site (Veste et al. 2008).
This study aimed to answer the following questions: (1) is the ionic composition of plant shoots dependent on the ionic conditions of their rhizosphere?; (2) are the ion absorption patterns and ionic composition patterns related to taxonomic groups?; and (3) are the ion absorption patterns and ionic composition different in different halophytes having different salt tolerant traits and different photosynthetic pathways?
Materials and methods
Study area and species
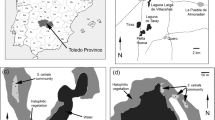
The studies were conducted in salt flats near Mardabad, in saline soils near the Rude-Shur river (salty river), located 60 km west of Tehran, 25 km SE of Karaj (Fig. 1). The saline flats around the Rude-Shur area are partly wasteland from previously, probably unsuccessfully, cultivated ground. The vegetation is dominated by annual C4 halophytic communities with scattered Tamarix androssowii shrubs, and patches of perennial hemi-cryptophytic communities dominated by Atriplex verrucifera. The long roots of most of the annual species may reach the wetter parts of the soil horizon during the dry season. This area is also rich in pseudo-halophytes and ephemerals which benefit from a lower soil salinity caused by leaching from winter and early spring rainfall. Most of the pseudo-halophytes complete their life cycle, or die, as a result of increasing temperatures from May onward (Akhani et al. 2009). Based on the nearest climatic station Karaj located in 25 km NW of area, the annual rainfall is about 240 mm with an annual temperature of 14.9 °C representing an Irano-Turanian arid bioclimate (Fig. 1).
The examined species include three Salsoloid Kranz type C4 species of genera Climacoptera, Halimocnemis, and Petrosimonia, and two C3 species of Atriplex and Salicornia, all belonging to Chenopodiaceae (Table 1). Atriplex verrucifera M. Bieb. belongs to subfamily Chenopodioideae, tribe Atripliceae, widely distributed in temperate deserts and semi-arid areas of SW Asia and has a more northerly range. Climacoptera turcomanica (Litv.) Botsch., Halimocnemis pilifera Moq., and Petrosimonia glauca (Pall.) Bunge are annuals with succulent leaves belonging to subfamily Salsoloideae, tribe Caroxyloneae (Akhani et al. 2007; Hedge et al. 1997). The ranges of these species are centered in the Iranian temperate deserts and salines, and radiate into Central Asia as Irano-Turanian elements. Salicornia persica Akhani subsp. rudshurensis Akhani is an annual with succulent stems and reduced leaves belonging to subfamily Salicornioideae, tribe Salicornieae, described from the locus classicus of the same area (Akhani 2008).
Plant and soil sampling
The vegetation period begins toward the end of March and ends in November for most halophytes growing in the area. Our sampling started from early spring (mid-April) to the beginning of autumn (mid-October) of 2009: 16 April, 24 May, 25 June, 6 August, 25 September, and 16 October 2009. The plant samples were collected from green stems, young leaves on seedlings, leaves and assimilating organs later in the life cycle (floral leaves, bracts and bracteoles, and photosynthetic shoots). The life cycle of Petrosimonia is shorter than other species and ends in August, so we collected just floral leaves, bracts, and bracteoles at the later time. The soil samples collected from the rhizosphere area of the naturally growing plants. About 2 kg soil volume was gathered from 10 to 30 cm depth. Because of heterogeneity in natural habitats we sampled four random samples from rich colonies of each species in every collection period. Each collection was first homogenized and then three replicates of them were used for laboratory measurements. The average values of these replicates were finally used in statistical analysis.
Soil analysis
The field-collected soil samples were air-dried, crushed, and passed through a 10-mesh (2-mm) sieve for soil analysis. For measurement of the major cation contents, the soil samples were extracted by neutral normal ammonium acetate solution (NH4-Ac-Fraction). Then the sodium and potassium contents were determined by Flame photometery (Jenway, Model PFP7) and the calcium and magnesium contents were determined by Atomic Absorption Spectrometer (Perkin-Elmer AAnalyst100) (Soil and Plant Analysis Council 2000). For measurement of chloride content, the extracted solution is provided by adding 25 mL 0.01 M calcium nitrate solution to 10 g of soil samples. After 15 min shaking at 180 or more epm the solution was immediately filtered using Whatman No. 42 filter paper. Then we used 4 mL 2.02 % ferric nitrate (Fe (NO3)3.9H2O) and 4 mL 0.075 % mercury thiocyanate as a reagent to 10 mL of extracted solution. So after 10 min for colour development the chloride content was determined by spectrophotometer (Shimadzu UV-160) set at 460 nm (Soil and Plant Analysis Council 2000).
EC (Electrical Conductivity) and pH were determined in the saturated paste method in crude saturated extract of the soil samples by EC meter 712 and pH meter (Metrohm 827), respectively (Soil and Plant Analysis Council 2000).
Shoot and root analysis
The collected plant shoots (arial parts consisted of stems and leaves) or roots in the field were transferred immediately to laboratory. The materials were oven-dried at 75 °C to constant weight. After drying and grinding, the powder of plant samples was used for analysis. For measurement of the sodium and potassium contents, the wet digestion of the 0.01 g plant samples was done by 5 mL of 500 mM Nitric acid (HNO3) at 82–86 °C for 2 h in the water bath, and then Na+ and K+ contents were determined by Flame photometer (Jenway, Model PFP7) (Arruda et al. 2003). For measurement of the calcium and magnesium contents, the wet digestion of the 0.05 g plant samples was done by 10 mL of 0.01 N Chloric acid (HCl) at 70–80 °C for 4 h in a water bath, and then Ca2+ and Mg2+ were determined by Atomic Absorption Spectrometer (Perkin-Elmer AAnalyst100) (Sahrawat 1987). For measurement of chloride content, the extracted solution was provided by adding 25 mL de-ionized water to 0.1 g grinded dried plant materials. After 30 min shaking at 180 epm or more the solution was immediately filtered using Whatman No. 42 filter paper. Then we used 4 mL 2.02 % ferric nitrate (Fe(NO3)3.9H2O) and 4 mL 0.075 % Mercury thiocyanate as a regent to 10 mL of extracted solution. So after 10 min for colour development, the chloride content was determined by spectrophotometer (Shimadzu UV-160) set at 460 nm (Lianngxue 1998).
Statistical analysis
In order to evaluate the relationship between time and species on the composition of leaf and extractable soil elements, we analyzed our data using General Linear Model (GLM). Mean separations for univariate (time, species and type of samples e.g. plant and soil), and also for reciprocal effects of two/three subjects (time, species and type of samples e.g. plant and soil) was determined with the Duncan’s multiple-range test using Statistical Analysis System software (SAS). F values for these mean separations were used for testing reciprocal effects of two/three subjects. The correlation of the examined element contents in rhizosphere, shoots and between rhizosphere and shoots of each species in the average of time were determined by Pearson’s correlation coefficient. Similarly, it has been used for determination of correlation between studied elements content in the shoots of every studied halophyte and its rhizosphere during the studied time intervals.
Results
Mean sodium (Na+) contents in the shoots and rhizosphere of species during the growing season are given in Table 1S. This shows that sodium content in the shoots of Salicornia persica subsp. rudshurensis and Climacoptera turcomanica do not differ significantly (Fig. 2a, c), even though in their rhizosphere increase significantly during the growing season (Fig. 2b, d). Similarly the increasing trend of soil electrical conductivity (EC) was observed from April to October in their rhizosphere (Fig. 3a, b). Sodium content in their rhizosphere is significantly more than that in the rhizosphere of the other studied species (Table 1S). Na+-concentration in the shoots of Halimocnemis pilifera and Petrosimonia glauca significantly shows an increasing trend, while in the shoots of Halimocnemis it indicates that Na+- concentration is going down after August (Fig. 2e, g). However it does not vary significantly in their rhizosphere over the growing season (Fig. 2f, h). Similarly the soil EC of Halimocnemis and Petrosimonia showed a relatively constant trend (Fig. 3e, d). Na+ content in the shoots of Atriplex verrucifera is decreasing (Fig. 2i), while in their rhizosphere it does not differ significantly and it is constant during growth period (Fig. 2j), the EC data also showed that (Fig. 3c).
Median of the sodium content in the shoots (left panel) and rhizospheres (right panel) of examined plants during a growth period. a shoots and b rhizospheres of Salicornia persica subsp. rudshurensis (Salicornia), c shoots and d rhizospheres of Climacoptera turcomanica (Climacoptera), e shoots and f rhizospheres of Halimocnemis pilifera (Halimocnemis), g shoots and h rhizospheres of Petrosimonia glauca (Petrosimonia), and i shoots and j rhizospheres of Atriplex verrucifera (Atriplex)
There is a positive correlation between Na+ and Cl− contents in the shoots of Salicornia, Halimocnemis, and Atriplex (Fig. 4a, b, c).
Mean potassium (K+) content in the shoots and rhizosphere of species during the growing season are shown in Table 1S. It indicates that the potassium content in the shoots of Salicornia and Climacoptera significantly shows an increasing trend (Fig. 5a, c). In the rhizosphere of these two species, it shows a relatively constant trend over the growing season; however it is significantly increased in October (Fig. 5b, d). K+ content in the shoot of Halimocnemis and Petrosimonia significantly shows an increasing trend until August and then, in Halimocnemis it is significantly decreased in October (Fig. 5e, g), even though K+ in the rhizosphere of these two species do not differ significantly (exception of K+ in the rhizosphere of Halimocnemis in April which is significantly high) over the time intervals (Fig. 5f, h). Potassium content in the shoots and rhizospheres of Atriplex is relatively constant during a growth period; however it is significantly decreased in October (Fig. 5i, j).
Median of the potassium content in the shoots and rhizosphere of studied plants during a growth period. a shoots and b rhizospheres of Salicornia persica subsp. rudshurensis (Salicornia), c shoots and d rhizospheres of Climacoptera turcomanica (Climacoptera), e shoots and f rhizospheres of Halimocnemis pilifera (Halimocnemis), g shoots and h rhizospheres of Petrosimonia glauca (Petrosimonia) and i shoots and j rhizospheres of Atriplex verrucifera (Atriplex)
Potassium content in the shoots of Salicornia and Climacoptera is correlated positively with sodium content in their rhizosphere (Fig. 6a, b). Similarly, there are positive correlation between K+ and Na+ content in the shoots of Halimocnemis and Petrosimonia (Fig. 7a, b).
Mean calcium (Ca2+) content in the shoots and rhizosphere of species during the growing season is shown in Table 1S. It indicates that the calcium content in the shoots of Salicornia, Climacoptera, and Atriplex significantly shows an increasing trend (Fig. 8a, c, i), while it does not differ significantly in their rhizosphere throughout the growing season (Fig. 8b, d, j). On the other hand Ca2+- concentration in the shoots of Halimocnemis and Petrosimonia significantly shows a decreasing trend (Fig. 8e, g); similarly a decreasing trend occurs in the rhizosphere of Halimocnemis (Fig. 8f), but not in the rhizosphere of Petrosimonia, which is constant during time intervals (Fig. 8h).
Median of the calcium content in the shoots and rhizospheres of examined plants during a growth period. a shoots and b rhizospheres of Salicornia persica subsp. rudshurensis (Salicornia), c shoots and d rhizospheres of Climacoptera turcomanica (Climacoptera), e shoots and f rhizospheres of Halimocnemis pilifera (Halimocnemis), g shoots and h rhizospheres of Petrosimonia glauca (Petrosimonia) and i shoots and j rhizospheres of Atriplex verrucifera (Atriplex)
There exists a positive correlation between the calcium content in the shoots of Salicornia and Climacoptera and the sodium content of their rhizosphere (Fig. 9a, b). In contrast, a negative correlation is observed between Na+ and Ca2+ content in the shoots of Halimocnemis, Petrosimonia and Atriplex (Fig. 10a, b, c).
The mean contents of sodium, potassium, chloride, calcium, and magnesium of roots and shoots at time intervals are shown in Table 2S. The Na+, Cl−, K+, and Mg2+ contents in the shoots of the examined halophytes are considerably higher than that in their roots, while the calcium content in their roots is more than that in their shoots (Fig. 11).
The Mg2+ content in the shoots of all examined halophytes was high and significantly more than K+ (Table 1S).
It was observed that the contents of the elements (Na+, Cl−, K+, and Mg2+) in the shoots of examined species are significantly more than those in their rhizosphere (Table 1S). The correlations between Na+, K+, Cl−, Ca2+, and Mg2+ in the shoots of studied halophytes and their rhizosphere during the growing season show that there is no significantly correlation between them (Fig. 12, and Figs. 1-4S).
Missing correlation between sodium in the shoots and rhizospheres of a Salicornia persica subsp. rudshurensis (Salicornia), (R = 0.11), b Climacoptera turcomanica (Climacoptera) (R = −0.16), c Halimocnemis pilifera (Halimocnemis) (R = 0.38), d Petrosimonia glauca (Petrosimonia) (R = −0.089), and e Atriplex verrucifera (Atriplex) (R = −0.25) in the entire time intervals (for all diagram p < 0.05)
Discussion
The ionic patterns in the shoots and rhizosphere fall into three groups:
-
Group1
Ion patterns in Salicornia and Climacoptera
In this group, sodium content in the shoots does not vary significantly during the growth period (Fig. 2a, c), whereas the Na+ in their rhizosphere exhibits an increasing trend toward the end of the growing season (Fig. 2b, d). It was noted that the ability to uptake and maintain Na+-concentration in these species was more than the other species studied in this research. These two species are halo-succulent, hygro-halophytic and sodiophilic species occurring on solonchak soils along margin of saline rivers, moist sabkhas and shores of salty lakes and sea (Breckle 1986). Previous studies have shown that some species like Salicornia europaea have the ability to accumulate up to 50 % NaCl (dry basis), and 93.2 % water (fresh basis) in the vacuole of the cells of their succulent stems (Ushakova et al. 2005). Additionally, it has been reported that Salicornia bigelovii can grow optimally in 200 mM NaCl by increasing their succulent tissues. High uptake of Na+ could be achieved by some Na+ transporters (like low-affinity carriers e. g. LCT1 and HKT1), and channels (e. g. VIC) (Maathuis and Amtmann 1999) through activation of H+-ATPase influenced by Na+-concentration as an activator and following Na+ influx. Water is also accumulated in the large vacuoles of water storage tissues (Ayala et al. 1996). Furthermore it is possible that Salicornia bigelovii like Suaeda maritima were able to retain Na+, but not K+in vacuoles, for osmotic adjustment and some other roles substitution of K+ (Flowers and Colmer 2008).
We can conclude that Salicornia and Climacoptera are obligate halophytes because they maintain high Na+-concentration even at such sensitive stages of their life-cycle as seedling, flowering and fruiting time. Similarly, studies on Salicornia pacifica indicated that this species could germinate under high salinity conditions (Khan and Weber 1986).
The Na+ content in the shoots of the studied species is much higher than in their rhizosphere (Table 1S). This is advantageous in obligatory eu-halophytes by using Na+ as an osmoticum in the saline substrate (Wang et al. 2004; Albert 1982; Steiner 1939; Keller 1925). It seems that Na+ plays a role as one of the activators of V-H+-ATPase for osmotic adjustment (Blumwald et al. 2000), and in many halophytes has a major role in osmotic equilibrium as substitute for K+ (Blumwald 2000), while glycophytes do not (Ghnaya et al. 2007).
We observed a positive correlation between Na+ and Cl− content in the shoots of examined species of Salicornia, Halimocnemis, and Atriplex (Fig. 4a, b, c). It could be not only for a similar role of Na+ and Cl− in osmo-regulation and salt-tolerance, but also for maintaining a charge balance in the cell vacuoles (Flowers et al. 1986; Maathuis 2006). Similarly the toxic accumulation of ions such as Cl− and Na+ in the cytosol of Arabidopsis thaliana ecotype Columbia has been reported in the water stress and high saline condition (Maathuis 2006). In addition it is documented by observation of chloride channel, which is activated by the V-H+-ATPase (Blumwald et al. 2000) by high concentration of Cl− as one of the activators of V-H+-ATPase (Maathuis 2006).
The increasing trend of K+ content in the shoots of studied halophytes is more remarkable in Salicornia and Climacoptera than the other species (Fig. 5a, c). It could be for no reduction in xylem K+ loading (Pandolfi et al. 2012), and retention of K+ by maintain negative membrane potential in root cells of halophytes (Teakle et al. 2013). Additionally this is explained by their ability to maintain uptake of essential elements even under high saline conditions (Donovan et al. 1997). In Salsola kali, a very distinct K+-uptake can be observed, leading to exceptionally high K+-concentrations (Reimann and Breckle 1995).
In this study, we observed that K+/Na+ in the shoots of all studied species is low and the mean potassium content is significantly lower than that of Na+ and Cl− (Table 1S). The ability to maintain a high cytosolic K+/Na+ ratio is evidently one of the key determinants of plant salt tolerance (Maathuis and Amtmann 1999). Na+ is extruded from the cytosol to the external medium or vacuoles and K+ is maintained in cytosol. The fact that cytosol contains only 10 % of cell volume comparing with valcuoles which constitute 90 % of the cell volume, a K+/Na+ ratio less than one is predictable in most halophytes especially in succulent species having large vacuoles and water storage tissues (Chen et al. 2007). Additionally K+ (as KCl) cannot substitute for the role of Na+ in all halophytes (Flowers and Colmer 2008). This is in accordance with previous findings in Sarcobatus, Salicornia europaea, and Suaeda maritima by absorbing large amounts of Na+ and a K+/Na+ leaf value less than one (Donovan et al. 1997; Breckle 1976, 1986). Interestingly, there is a strong positive correlation between K+ content in the shoots and Na+ content in the rhizosphere of Salicornia and Climacoptera (Fig. 6a, b). It could be explained that increasing Na+ in the rhizosphere would be a signal for increasing K+ uptake from the soil (Reimann and Breckle 1993).
Our study showed that the positive correlation of Na+ in rhizosphere and Ca2+ in the shoots of Climacoptera and Salicornia indicates that these species could preserve and retain calcium as an essential element in their tissues even under high salinity condition (Fig. 9a, b). This might play a role in amelioration toxic effects of Na+, characteristic of halophytes in saline conditions (Albert 1982). Ca2+ could play this role by reduction Na+ influx, through blocking of nonselective cation channels (Maathuis and Amtmann 1999), inhibition Na+-induced K+ efflux, through K+-permeable channels, and prevention K+ efflux through regulating outward-rectifying KOR channels (Shabala et al. 2006). Furthermore nanomolar concentration of calcium as a signalling molecule play a role in activation of SOS-regulatory pathway for Na+ compartmentalization into the vacuoles (Han et al. 2005). The same correlation has been reported in maize and the North American Sarcobatus, as they could preserve N, P, K+, Ca2+, and Mg2+ uptake together in a highly external Na+-concentration (Donovan et al. 1997; Breckle 1995; Blumwald 2000). Observation of Mg2+ concentrations higher than K+ in the shoots of all examined halophytes in this paper (Table 1S) is a characteristic feature already documented in other species of extreme saline habitats (Albert and Popp 1977). The maintaining of high amount of Mg2+ in leaves of halophytes plays a role as a co-factor for tonoplast ATPases and helps to vacuolar compartmentalization of Na+ for osmo-regulation. Additionally, leaf Mg2+ is required for protein translation in the presence of high Na+ (Donovan et al. 1997).
-
Group2
Ion patterns in Halimocnemis and Petrosimonia
In the second group, the Na+-concentration in shoots generally shows an increasing trend up till August (Fig. 2e, g), and later in September and October a slight decline can be observed in Halimocnemis. This species has much larger leaves during the vegetative period, but these are shed and replaced by smaller floral leaves during flowering. During that time, organs such as green and young stems, bracts, bracteoles and even perianths act as substitutes for the assimilating role of normal leaves (Akhani 2006). Lower Na+-concentration is therefore related to loss of vegetative leaves which usually have higher concentration of Na+; it could also be a self-regulatory mechanism to get rid of some sodium and chloride and to reduce uptake of soluble elements like Na+ with the transpiration stream. It seems that reduction of Na+ in the reproductive development of halophytes is important because of the high sensitivity at this stage to salt stress (Munns and Rawson 1999; Walker et al. 1979; Breckle 1976). It was also reported in non-halophytes such Vitis vinifera (Hawker and Walker 2010) and other cultivated plants (Breckle 1996).
Observation of the positive correlation between K+ and Na+ content in the shoots of Halimocnemis and Petrosimonia (Fig. 7a, b) could, for similar reasons, be described in Salicornia and Climacoptera. However in these two genera, there is a higher degree of compatibility than the other examined species.
In this group, Ca2+ suddenly decreased during the time intervals and accompanied with decreasing Na+ in the flowering and fruiting stages (Figs. 2e and 8e, g). It seems that decreasing Na+ in low Ca2+ condition in those stages is the compatibility of these species for preventing harmful effects of Na+ such as decreasing antioxidant enzyme activities (Gautier et al. 2009).
On the other hand, the strong negative correlation between Na+ and Ca2+ was observed in the shoots of Halimocnemis and Petrosimonia (Fig. 10a, b). These species are xero-halophytic, C4 plants with high water-use efficiency. Although the content of Ca2+ is high in dry soils (Albert and Popp 1977), it seems that the decrease of Ca2+ is because of the low availability of this element. In addition, the members of Chenopodiaceae are very tolerant of high sodium chloride, while they are sensitive to soluble calcium under high saline condition because of many reasons, such as decreasing oxygen uptake (Wiebe and Walter 1972). Therefore, the halophytic Chenopodiaceae use oxalate as a chelator (Osmond 1963; Wiebe and Walter 1972) which is apparent in many species of the families Caryophyllaceae, Chenopodiaceae and Polygonaceae (more than 5 % oxalate by dry weight) (Albert and Popp 1977). Calcium oxalate could be soluble or insoluble depending on the type of species and increasing level of hydration (Libert and Franceschi 1987). We have observed a large number of salt crystals in water storage tissues (not shown). By using dilute (500mM) HNO3 (which has not enough acidity to take insoluble crystals of Ca2+-Ox or other scarcely soluble bound Ca2+ and Mg2+); we have extracted only a small portion of Ca2+. The observed low Ca2+ could be the result of precipitation of calcium oxalate in their tissues (Wiebe and Walter 1972; Albert 1982).
-
Group3
Ion patterns in Atriplex
The Na+ content in shoot of Atriplex shows a decreasing trend (Fig. 2i), in spite of a slight increase of this element in their rhizosphere (Fig. 2j) during growth. It is well known that species of Atriplex reduce toxicity of sodium and chloride by accumulating these ions in bladder-like trichomes, and then shedding those salt trichomes (Breckle 1976; Schirmer and Breckle 1982; Freitas and Breckle 1992; Breckle et al. 1990). The quantity of sodium removed would depend on the age of leaves, rate of formation and bursting of hairs, which is different in various species (Reimann and Breckle 1988) and the salinity of the growing medium (Mozafar and Goodin 1970; Uchiyama and Sugimura 1985). In this study, we observed the reduction of Na+ and Cl− in the shoots during flowering and fruit development - like the case of Halimocnemis - is maximal. Therefore, it seems that the gradual loss of larger leaves with more sodium content than the flowering bracteoles is an important reason for such a pattern.
It is likely that the retention of potassium in shoots of Atriplex is a similar case.
Observation of a negative correlation between Ca2+ and Na+ in shoots of Atriplex (Fig. 10c) may represent the importance of Ca2+ in floral and fruit development. During the vegetative period, large amounts of Na+ and insoluble Ca2+ are stored in salt crystals of leaves. By losing leaves during reproductive development, the amount of soluble Ca2+ is increased in non-leafy organs (Libert and Franceschi 1987), whereas the Na+ content decreased. Moreover, decrease of Na+ is necessary to prevent harmful antagonistic effects of these two elements on oxygen uptake (Wiebe and Walter 1972).
Ion patterns in roots compared with shoots
In all the species studied, the concentration of higher amount of Na+, Cl−, K+, and Mg2+ in shoots in comparison with roots (Table 2S, Fig. 11) is due to the exclusive presence of the adaptation mechanism in their shoots. This is their ability to compartmentalize these elements by dilution mechanism or forming salt crystals in vacuoles, or recreating by salt glands or shedding of salt trichomes (Liangpeng et al. 2007; Balnokin et al. 2005; Breckle 1990, 1995). In contrast, it was observed that the calcium content in the roots of examined halophytes is more than that in their shoots (Table 2S, Fig. 11). It is known that the higher amount of Ca2+ content in the root cells is to induce specific expression of aquaporin with an increase of NaCl in the substrate of Arabidopsis (Munns and Tester 2008).
Are ion patterns correlated with types of photosynthetic pathways?
It is well known that C4 plants dominant under high temperature and osmotic stress conditions because of their higher water-use efficiency (Dajic 2006; Welkie and Caldwell 1970; Osmond 1974). Additionally, Na+ is essential for translocation of pyruvate across the chloroplast envelope, and then this element is needed as an essential micro-nutrient in C4 species (Brownell and Crosslan 1972; Maathuis and Amtmann 1999). However, under natural conditions there is never any sodium-deficiency in plants (Albert 1982). So it seems advantageous that a significant number of halophytes perform a C4 type of photosynthesis (Brownell and Crosslan 1972; Ohnishi and Kanai 1990) with better water-use efficiency.
In this study, we observed that Salicornia, by a C3 type of photosynthesis, and Climacoptera, by a C4 type, have occupied different micro-habitats, while they have a similar ionic pattern and can be classified in one group. So we can conclude that there is no relation between type of photosynthesis and the ion patterns of these halophytes. However, along a moisture gradient there is always a tendency of higher colonization of C4 species in transition between hygrohalophytes and xerohalophytes (Akhani et al. 2003; Frey et al. 1985).
Conclusion
Our studies have shown that there is no noteworthy correlation between the ion contents in the shoots and rhizosphere of the five studied halophytes, with different strategies for tolerating saline conditions (stem-succulent, leaf-succulent and broad-leaf with hair bladders) belonging to different types of photosynthesis and different taxa (Fig. 12, and Figs. 1-4s). Therefore we can suggest that sodium-concentration or ionic composition patterns in the shoots of these halophytes are controlled by fixed genetic processes rather than by the environment, albeit for determinate conclusion we need more studies on these examined species in different localities and also in the controlled condition.
This study has also shown that ionic composition pattern does not relate to the taxonomic group of examined halophytes; like the case of Climacoptera and Salicornia that have a similar ion relation but are in different taxonomic groups. Similarly, the same ion relation has been reported for Caroxylon scleranthum, Atriplex confertifolia, and some other halophytes (Breckle 1986). Consequently, it is suggested that the ion relations exhibit a specific ionic composition pattern which is genetically fixed and for larger groups was termed physiotype (Kinzel 1982; Albert 1982). Practically we can conclude that the halophytic species of Chenopodiaceae are not only very successful in carbon sequestration under high salinity but also they have high potential to be used for reclamation of saline soils by their genetical ability to take off large amount of salt from deeper soil layers.
References
Akhani H (2006) Biodiversity of halophytic and sabkha ecosystems in Iran. In: Ajmal Khan M et al (eds.) (ed) Sabkha Ecosystems Volume II: West and Central Asia. Springer, pp 71–88
Akhani H (2008) Taxonomic revision of the genus Salicornia L. (Chenopodiaceae) in Central and Southern Iran. Pak J Bot 40(4):1635–1655
Akhani H, Trimborn P, Ziegler H (1997) Photosynthetic pathways in Chenopodiaceae from Africa, Asia and Europe with their ecological, phytogeographical and taxonomical importance. Plant Syst Evol 206(1–4):187–221
Akhani H, Ghobadnejhad M, Hashemi SMH (2003) Ecology, biogeography and pollen morphology of Bienertia cycloptera Bunge ex Boiss. (Chenopodiaceae), an Enigmatic C4 plant without Kranz anatomy. Plant Biol 5(2):167–178
Akhani H, Edwards G, Roalson EH (2007) Diversification of the Old World Salsoleae s.l. (Chenopodiaceae): molecular phylogenetic analysis of nuclear and chloroplast data sets and a revised classification. Int J Plant Sci 168(6):931–956
Akhani H, Lara MV, Ghasemkhani M, Ziegler H, Edwards GE (2009) Does Bienertia cycloptera with the single-cell system of C4 photosynthesis exhibit a seasonal pattern of delta C-13 values in nature similar to co-existing C4 Chenopodiaceae having the dual-cell (Kranz) system? Photosynth Res 99(1):23–36
Albert R (1982) Halophyten. In: Kinzel H (ed) Pflanzenökologie und Mineralstoffwechsel. Ulmer, Stuttgart, pp 33–204
Albert R, Popp M (1977) Chemical composition of Halophytes from Neusiedler Lake Region in Austria. Oecologia 27(2):157–170
Aronson JA (1989) HALOPH, a database of salt tolerant plants of the world. Office of Arid Lands Studies. University of Arizona, Tucson
Arruda SCC, Rodriguez APM, Arruda MAZ (2003) Ultrasound-assisted extraction of Ca, K and Mg from in vitro citrus culture. J Braz Chem Soc 14(3):470–474
Ayala F, Oleary JW, Schumaker KS (1996) Increased vacuolar and plasma membrane H+ −ATPase activities in Salicornia bigelovii Torr in response to NaCl. J Exp Bot 47(294):25–32
Balnokin YV, Myasoedov NA, Shamsutdinov ZS, Shamsutdinov NZ (2005) Significance of Na + and K + for sustained hydration of organ tissues in ecologically distinct halophytes of the family Chenopodiaceae. Russ J Plant Physiol 52(6):779–787
Blumwald E (2000) Sodium transport and salt tolerance in plants. Curr Opin Cell Biol 12(4):431–434
Blumwald E, Aharon GS, Apse MP (2000) Sodium transport in plant cells. BBA-Rev Biomembr 1465(1–2):140–151
Breckle S-W (1975) Ionengehalte halophiler Pflanzen Spaniens. Decheniana (Bonn) 127:221–228
Breckle S-W (1976) Zur Ökologie und den Mineralstoffverhältnissen absalzender und nicht absalzender Xerohalophyten (unter besonderer Berücksichtigung von Untersuchungen an Atriplex confertifolia und Ceratoides lanata in Utah/USA). Diss Bot 35:169pp
Breckle S-W (1986) Studies on halophytes from Iran and Afghanistan.2. Ecology of halophytes along salt gradients. Proc R Soc Edinb B 89:203–215
Breckle S-W (1990) Salinity tolerance of different halophyte types. Plant Soil 148:167–175
Breckle S-W (1995) How do halophytes overcome salinity? In: Khan MA, Ungar IA (eds) Biology of salt tolerant plants. Book Graffers, Chelsea, pp 199–213
Breckle S-W (1996) Root growth and root architecture of non-halophytes under saline soil conditions. Acta Phytogeogr Suec 81:44–47
Breckle S-W (2002) Salinity, halophytes and salt affected natural ecosystems. In: Läuchli A, Lüttge U (eds) Salinity: environment -plant –molecules. Kluwer Academic Publishers, Dordrecht, pp 53–77
Breckle S-W (2003) Rehabilitation of the Aral Sea environment, Kazakhstan. In: Proceedings of the International workshop (Aleppo, May 2002): “Combating desertification – rehabilitation of degraded drylands and biosphere reserves”. UNESCO-MAB dryland series No.2, pp 47–57
Breckle S-W (2009) Is sustainable agriculture with seawater irrigation realistic? In: Ashraf M, Oztürk M, Athar H-R (eds) Salinity and water Stress. Springer, pp 187–196
Breckle S-W (2013) From Aral Sea to Aralkum – an ecological disaster or halophytes’ paradise. Prog Bot 74:351–398
Breckle S-W, Wucherer W (2012) Halophytes and salt desertification in the Aralkum Area. In: Breckle S-W, Dimeyeva L, Wucherer W, Ogar NP (ed) Aralkum – a man-made desert: the desiccated floor of the Aral Sea (Central Asia). Ecol Stud 218. Springer, pp 271–299
Breckle S-W, Freitas H, Reimann C (1990) Sampling Atriplex Bladders: a comparison of methods. Plant Cell Environ 13:871–873
Brownell PF, Crosslan C (1972) Requirement for sodium as a micronutrient by species having C4 dicarboxylic photosynthetic pathway. Plant Physiol 49(5):794–797
Chen ZH, Pottosin II, Cuin TA, Fuglsang AT, Tester M, Jha D, Zepeda-Jazo I, Zhou MX, Palmgren MG, Newman IA, Shabala S (2007) Root plasma membrane transporters controlling K+/Na + homeostasis in salt-stressed barley. Plant Physiol 145(4):1714–1725
Dajic Z (2006) Salt sterss. In: Madhava Rao KV, Raghavendra AS, Janardhan KR (eds) Physiology and molecular biology of stress tolerance in plants. Springer, Dordrecht, pp 41–99
Dimeyeva L, Breckle S-W, Wucherer W (2012) Flora of the Aralkum. In: Breckle S-W, Dimeyeva L, Wucherer W, Ogar NP (ed) Aralkum – a man-made desert: the desiccated floor of the Aral Sea (Central Asia). Ecol Stud 218, vol 218. Springer, pp 109–126
Donovan LA, Richards JH, Schaber EJ (1997) Nutrient relations of the halophytic shrub, Sarcobatus vermiculatus, along a soil salinity gradient. Plant Soil 190(1):105–117
Flowers TJ, Colmer TD (2008) Salinity tolerance in halophytes. New Phytol 179:945–963
Flowers TJ, Hajibagheri MA, Clipson NJW (1986) Halophytes. Q Rev Biol 61(3):313–337
Freitas H, Breckle S-W (1992) Importance of bladder hairs for salt tolerance of field-grown Atriplex-species from a Portuguese salt marsh. Flora 187:283–297
Frey W, Kürschner H, Stichler W (1985) Photosynthetic pathways and ecological distribution of halophytes from four littoral salt marshes (Egypt/Sinai, Saudi Arabia, Oman and Iran). Flora 177:107–130
Gautier H, Lopez-Lauri F, Massot C, Murshed R, Marty I, Grasselly D, Keller C, Sallanon H, Génard M (2009) Impact of ripening and salinity on tomato fruit ascorbate content and enzymatic activities related to ascorbate recycling. Func Plant Sci Biotech 4 (special issue 1) 66–75
Ghnaya T, Slama I, Messedi D, Grignon C, Ghorbel MH, Abdelly C (2007) Effects of Cd2+ on K+, Ca2+ and N uptake in two halophytes Sesuvium portulacastrum and Mesembryanthemum crystallinum: consequences on growth. Chemosphere 67(1):72–79
Grigore MN, Toma C (2008) Ecological anatomy investigations related to some halophyte species from Moldavia. Rom J Biol - Plant Biol 53:23–30
Han N, Shao Q, Lu CM, Wang BS (2005) The leaf tonoplast V-H+-ATPase activity of a C3 halophyte Suaeda salsa is enhanced by salt stress in a Ca-dependent mode. J Plant Physiol 162:267–274
Hawker JS, Walker RR (2010) The effect of sodium chloride on the growth and fruiting of Cabernet Sauvignon Vines. Am J Enol Vitic 6:498–505
Hedge IC, Akhani H, Freitag H, Kothe-Heinrich G, Podlech D, Rilke S, Uoltila P (1997) Chenopodiaceae. In: Rechinger KH (ed) Flora Iranica, vol 172. Akademische Druck- u. Verlagsanstalt, Graz
Horie T, Schroeder JI (2004) Sodium transporters in plants. Diverse genes and physiological functions. Plant Physiol 136(1):2457–2462
Keller BA (1925) Halophyten- und Xerophytenstudien. J Ecol 13:224–255
Khan MA, Weber DJ (1986) Factors influencing seed-germination in Salicornia-pacifica var. utahensis. Am J Bot 73(8):1163–1167
Kinzel H (1982) Pflanzenökologie und Mineralstoffwechsel. Ulmer, Stuttgart, p 534
Koyro H-W, Geißler N, Hussin S, Huchzermeyer B (2008) Survival at extreme locations: Life strategies of halophytes - The long way from system ecology, whole plant physiology, cell biochemistry and molecular aspects back to sustainable utilization at field sites In: Abdelly C., Oztürk M, Ashraf M, Grignon C (eds) Biosaline agriculture and high salinity tolerance. Springer, pp 2–20
Krinsley DB (1970) A geomorphological and palaeoclimatological study of the playas of Iran. U. S. Government Printing Office, Washington
Liangpeng Y, Jian M, Yan L (2007) Soil salt and nutrient concentration in the rhizosphere of desert halophytes. Acta Ecol Sin 27:3565–3571
Lianngxue L (1998) Determination of chloride in plant tissue. In: Yash KP (ed) Handbook of reference methods for plant analysis. 14. Soil and plant analysis council. CRC press, Florida, pp 111–113
Libert B, Franceschi VR (1987) Oxalate in crop plants. J Agric Food Chem 35(6):926–938
Maathuis FJM (2006) The role of monovalent cation transporters in plant responses to salinity. J Exp Bot 57(5):1137–1147
Maathuis FJM, Amtmann A (1999) K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Ann Bot 84(2):123–133
Mozafar A, Goodin JR (1970) Vesiculated hairs - a mechanism for salt tolerance in Atriplex-halimus L. Plant Physiol 45(1):62–65
Munns R, Rawson HM (1999) Effect of salinity on salt accumulation and reproductive development in the apical meristem of wheat and barley. Aust J Plant Physiol 26(5):459–464
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Ohnishi J, Kanai R (1990) Pyruvate uptake induced by a Ph Jump in Mesophyll Chloroplasts of Maize and Sorghum, Nadp-Malic Enzyme Type-C4 species. FEBS Lett 269(1):122–124
Osmond CB (1963) Oxalates and ionic equilibrium in Australian saltbushes (Atriplex). Nature 198:503–504
Osmond CB (1974) Leaf anatomy of Australian saltbushes in relation to photosynthetic pathways. Aust J Bot 22:39–44
Pandolfi C, Mancuso S, Shabala S (2012) Physiology of acclimation to salinity stress in pea (Pisum sativum). Environ Exp Bot 84:44–51
Reimann C, Breckle S-W (1988) Salt secretion in some Chenopodium species. Flora 180:289–296
Reimann C, Breckle S-W (1993) Sodium relations in Chenopodiaceae, a comparative approach. Plant Cell Environ 16:323–328
Reimann C, Breckle S-W (1995) Salt tolerance and ion relations of Salsola kali L.: difference between ssp. tragus (L.) Nyman and ssp. ruthenica (Iljin) Soo. New Phytol 130:37–45
Sahrawat KL (1987) Determination of calcium, magnesium, zinc and manganese in plant-tissue using a dilute Hcl extraction method. Commun Soil Sci Plant 18(9):947–962
Schirmer U, Breckle S-W (1982) The role of bladders for salt removal in some Chenopodiaceae (mainly Atriplex species). In: Sen DN, Rajpurohit KS (eds) Contributions to the ecology of halophytes: tasks for vegetation science. Junk, Den Haag, pp 215–231
Shabala S, Demidchik V, Shabala L, Cuin TA, Smith SJ, Miller AJ, Davies JM, Newman IA (2006) Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol 141(4):1653–1665
Soil and Plant Analysis Council I (2000) Soil analysis: handbook of reference methods. CRC Press, Boca Raton
Steiner M (1939) Die Zusammensetzung des Zellsaftes bei höheren Pflanzen in ihrer ökologischen Bedeutung. Erg Biol 17:151–254
Teakle NL, Bazihizina N, Shabala S, Colmer TD, Barrett-Lennard EG, Rodrigo-Moreno A, Läuchli AE (2013) Differential tolerance to combined salinity and O2 deficiency in the halophytic grasses Puccinellia ciliata and Thinopyrum ponticum: the importance of K + retention in roots. Environ Exp Bot 87:69–78
Uchiyama Y, Sugimura Y (1985) Salt-excreting function of vesiculated hairs of Atriplex nummularia. Jpn J Crop Sci 54(1):39–46
Ushakova SA, Kovaleva NP, Gribovskaya TV, Dolgushev VA, Tikhomirova NA (2005) Effect of NaCl concentration on productivity and mineral composition of Salicornia europaea as a potential crop for utilization NaCl in LSS. In: Space life sciences: gravity-related effects on plants and spaceflight and man-made environments on biological systems, vol 36. Adv Space Res. pp 1349–1353
Veste M, Sartorius U, Breckle SW (2008) Ion relations of plants and soil patterns. In: Breckle S-W, Yair A, Veste M (eds) Ecological studies, arid dune ecosystems, vol 200. Ecological studies. Springer, Berlin, pp 353–365
Walker RR, Kriedemann PE, Maggs DH (1979) Growth, leaf physiology and fruit development in salt-stressed guavas. Aust J Agric Res 30:477–488
Walter H, Lieth H (1967) Klimadiagram-Weltatlas. VEB Gustav Fischer Verlag, Jena
Wang SM, Wan CG, Wang YR, Chen H, Zhou ZY, Fu H, Sosebee RE (2004) The characteristics of Na+, K+ and free proline distribution in several drought-resistant plants of the Alxa Desert, China. J Arid Environ 56(3):525–539
Welkie GW, Caldwell MM (1970) Leaf anatomy of species in some dicotyledon families as related to the C3 and C4 pathways of carbon fixation. Can J Bot 48:2135–2146
Wiebe HH, Walter H (1972) Mineral ion composition of halophytic species from Northern Utah. Am Midl Nat 87(1):241–245
Wucherer W, Veste M, Bonilla OH, Breckle S-W (2005) Halophytes as useful tools for rehabilitation of degraded lands and soil protection. In: Proceeding International forum on ecological construction of the western Baijing, Baijing, pp 87–94
Wucherer W, Breckle S-W, Kaverin VS, Dimeyeva L, Zhamantikov K (2012) Phytomelioration in the Northern Aralkum. In: Breckle S-W, Dimeyeva L, Wucherer W, Ogar NP (ed) Aralkum – a man-made desert: the desiccated floor of the Aral Sea (Central Asia). Ecol Stud 218, vol 218. Springer, pp 343–386
Acknowledgments
This paper is the result of a research project supported by Iranian National Science Foundation (INSF) under Project No. 842951 and “Geobotanical Studies in Different Parts of Iran VI” supported by the Research Council University of Tehran under project number 6104037/1. The soil and water analysis were carried out in part in the Plant Physiology Laboratory of the School of Biology, Laboratory of Geology of the School of Geology and the Soil and Water Research Institute, Ministry of Jihade Agriculture. We thank the directors and staffs of these laboratories in particular Dr. V. Niknam and Dr. K. Bazargani for their generous help and the useful comments by two anonymous referees.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Frans J.M Maathuis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 121 kb)
Rights and permissions
About this article
Cite this article
Matinzadeh, Z., Breckle, SW., Mirmassoumi, M. et al. Ionic relationships in some halophytic Iranian Chenopodiaceae and their rhizospheres. Plant Soil 372, 523–539 (2013). https://doi.org/10.1007/s11104-013-1744-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1744-7