Abstract
Aims
Effects of final harvest of plantations and re-conversion with different tillage intensities on quantity and distribution of organic matter in different soil fractions were assessed.
Methods
A field trial was conducted at two poplar and one willow plantation in northern Germany. Distribution of C in aboveground plant and root biomass and within various soil fractions (particulate organic matter, water-stable aggregates, microbial biomass) was determined. Directly after re-conversion, which was performed at tillage depths of 5, 15 and 30 cm, C amounts added with coarse harvest residues and changes in soil C fractions were examined.
Results
Plantation C stocks decreased in the order soil > aboveground biomass > roots. After re-conversation no change in bulk soil SOC but an increase of labile soil C was observed. Between 16 and 30 t ha−1 additional C was determined in the soil fraction of plant residues >2 mm after re-conversion. Up to 90 % SOC of the fine earth fraction was associated with macroaggregates, which increased after re-conversion despite intensive tillage with a rotary cultivator.
Conclusion
The duration of the increased macroaggregate associated C directly after soil tillage is a short term effect of the tillage. The influence of tillage depths on soil C-fractions could be observed only in some cases because of the high variability of harvest residues in the field.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The demand for woody biomass has recently led to increased interest in woody biomass production in tree plantations with fast growing tree species such as poplar (Populus spp.) and willow hybrids (Salix spp.) on former arable land. After 20 to 30 years, the productivity of trees generally decreases and the stands have to be re-established or re-converted to grass or arable land (Grogan and Matthews 2002; Kahle et al. 2010). But until now, the effects of this re-conversion have rarely been examined.
Tree plantations on former arable land lead to a non-tillage management with increased litter amounts and have the potential to increase the soil organic carbon (SOC) in the long term (Jug et al. 1999; Post and Kwon 2000; Kahle et al. 2007; Baum et al. 2009; Nair et al. 2009; Rose-Marie 2012). These investigations focused on the amount of SOC, but neglected quality and allocation of C in different organic matter fractions. Afforestation and absence of tillage generally reduced soil disturbance and increased soil aggregation (Six et al. 2000), particulate organic matter (POM) and microbial biomass C in soil (Mao and Zeng 2010). In plantations with rotation cycles exceeding 10 years, which is common in plantations producing raw material for industrial use, most C was found to be stored in the aboveground tree biomass and it appears that belowground root biomass constitute an important proportion of C-stocks (Singh 1998; Fang et al. 2007; Sartori et al. 2007). But there is still a knowledge gap on belowground biomass carbon stocks of tree plantations and almost all available studies are based on model estimations. During re-conversion, intensive soil tillage is performed for breaking up roots and coarse harvest residues after the final harvest for seedbed preparation. The incorporation of these residues increased total SOC stocks (Kirschbaum et al. 2008), but the intensive soil tillage destroyed soil aggregates and exposed organic colloids to decomposers (Post and Kwon 2000), leading to an enhanced mineralization of soil organic matter. Considering the two contrasting effects of the re-conversion of tree plantations back to arable land, more information on the impacts on SOC dynamics is needed. Studies have shown that initial C losses are mainly caused by mineralization of the labile SOC pools such as the microbial biomass, macroaggregate associated C and particulate organic matter (Okore et al. 2007; Yang et al. 2009). This led us to hypothesize that a reduction in tillage intensity reduces C loss during the re-conversion of tree plantations. In the current study, the short-term effects of the re-conversion on SOC stocks and dynamics were investigated on tree plantations with a rotation cycle of 12 years at three sites in northern Germany. The objectives of the study were (1) to examine the changes of soil properties and SOC fractions with depth under poplar and willow plantations, (2) to quantify the amount of harvest residues remaining in the soil after re-conversion, and (3) to analyse effects of tillage intensity on the distribution of SOC on different soil fractions immediately after re-conversion of tree plantations.
Material and methods
Site description
The study was conducted at three experimental sites in northern Germany. At the Georgenhof site (51°27′N, 9°0′O, 320 m a.s.l.), the studies were performed on a willow plantation and an adjacent poplar plantation. Mean annual precipitation and mean annual temperature are 740 mm and 7.9 °C, respectively. Soil type is a Stagno-Gleyic Cambisol derived from loess (FAO-WRB, 2006). Soil texture (0–30 cm) at the Georgenhof willow site is a sandy loamy silt (28 % sand, 61 % silt, 11 % clay), at the poplar site a silty loam (19 % sand, 64 % silt, 17 % clay). The poplar plantation at the third site Wachtum (52°47′N, 7°44′O, 22 m a.s.l.) has a mean annual precipitation and temperature of 815 mm and 9.0 °C, respectively. Soil type is a deep-ploughed Gleyic Podzol; soil texture is a silty sand (76 % sand, 20 % silt, 4 % clay).
The plantations were established on former arable land in 1987 (Georgenhof) and 1989 (Wachtum) for the testing of fast growing willow (Salix spp.) and poplar (Populus spp.) clones. From 1998 to the final harvest in 2010, no cutting was carried out at any site, as the plantations were used for demonstrating the production of raw material for the paper industry. The initial plant density at the Georgenhof willow site was 15,385 trees ha−1 (1.3 × 0.5 m), at the Georgenhof poplar site 10,000 trees ha−1 (1 × 1 m). At the Wachtum poplar site, clones were planted with a spacing between 2.2 × 1.2 m (3,788 trees ha−1) and 3 × 1.2 m (2,777 trees ha−1). Due to thinning and high plant mortality, the tree density before the final harvest was 1,296 trees ha−1 at the Georgenhof willow site, 1,905 trees ha−1 at the Georgenhof poplar site and 1,942 trees ha−1 at the Wachtum poplar site.
Stand level biomass
For estimating above and belowground biomass of the poplar plantations, tree height and mean diameter at 1.3 m height (dbh) of all trees without the marginal rows were measured by crosswise clipping. The trees were divided into five dbh-classes ranging from 9 to 24 cm and one tree of each class was selected for destructive sampling. The sample trees were cut at ground level and subdivided into stem until 7 cm diameter and crown. The crown was chaffed and the chaffs and the stem were weighed in the field to obtain fresh weight. Sub-samples of both compartments were dried at 105 °C until a constant weight was reached for determination of the dry matter (DM) content. For the root biomass assessment, a 1.4 × 1.4 m square centred around each sample tree was excavated to a depth of 30 cm. It was assumed that roots of surrounding trees within the sample area compensated for roots of the measured tree that were outside the sampled area. The roots were collected, washed, dried at 60 °C, divided into stump, coarse (>5 mm) and fine (<5 mm) roots, weighed and ground. Potential regression equations were developed by using the DM of the separate tree compartments (stem, crown, root fractions) as the dependent variable for calculating the aboveground and the root fraction biomass of the stands in relation to the dbh as follows:
where B is the biomass of the tree compartment or root fraction mass (kg), b0 and b1 were parameter estimates and dbh was the diameter (cm) of the stem at 1.3 m height. The dbh value of each tree was fitted into the regression equation for calculating the biomass. The values were summed and converted to a hectare basis. Due to an R 2 of between 0.7 and 0.9 the dbh was solely used as explaining variable for biomass estimation in accordance with Röhle et al. (2009). The aboveground and root biomass of the willows was estimated by the mean tree technique. Three selected trees (stem and shoots), that best represent the mean size of the plantation, were destructive sampled. Fresh weight was determined in the field and DM of chaffed sub-samples in the laboratory. Root biomass was determined as described above. For estimation of total above and belowground biomass the number of tress in the plantation was used to expand the mean tree values to an area basis.
Tree harvesting and soil sampling
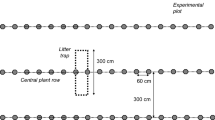
The trees were manually harvested in spring 2010 with a chain saw at the willow site and with a harvester at the two poplar sites. The harvester delimbed the trees, so that the entire crown material remained at the poplar sites. For restoring the sites, stumps and harvest residues were first mulched with a wood mulcher. Then this mulched debris, the root stumps and coarse roots were ground and tilled into the soil with a rotary cultivator. This was carried out in three strips, each with a width of 6 m at the two Georgenhof sites and 4 m at Wachtum, with tillage depths of 5 cm (shallow), 15 cm (medium) and 30 cm (deep). Soil was levelled with a rotary harrow and maize (Zea mays L.) and rye grass (Lolium perenne L.) were sown in three replicates per tillage depth in strips of 12 m.
In winter 2009/2010, before the final harvest, soil samples were taken at 0–5, 5–10, 10–15, 15–30 and 30–60 cm depth in three replicates per depth and site. Additionally the litter layer and overlying deadwood was sampled from three 1 × 1 m squares per site, oven-dried at 60 °C, weighed, ground and analysed for C and N content. Directly after rotary cultivation, the soil was sampled again in three replicates per tillage treatment at 0–5, 5–10, 10–15, and 15–30 cm depth in the shallow, in 0–15 and 15–30 cm in the medium and in 0–30 cm in the deep tillage treatment with a 10 cm diameter corer. Two samples of each depth were combined per replicate. At both dates, bulk density was determined gravimetrically in 5 cm steps, using a steel corer (diameter 4 cm). All soil samples were sieved (<2 mm). From the samples taken after cultivation, root and aboveground harvest residues >2 mm were collected from the sieve, washed and dried at 60 °C. Sub-samples of harvest residues were dried at 105 °C to constant weight for determination of the dry matter content.
Soil analysis
Soil pH was determined in 0.01 M CaCl2 at a soil/solution ratio of 1/2.5. For estimating microbial biomass C (Vance et al. 1987), two portions of 15 g field-moist sieved soil, stored at 4 °C until analysis, were extracted with and without CHCl3 fumigation with 40 ml 0.5 M K2SO4. Organic C in the extracts was measured after combustion using a Dimatoc 100 automatic analyzer (Dimatec, Essen, Germany). Microbial biomass C was calculated as the difference between fumigated and unfumigated samples using a k EC value of 0.45 (Wu et al. 1990) to account for the non-extractable part.
Water stable aggregate fractions to a depth of 30 cm were determined at both sampling dates by wet sieving, using the method described by John et al. (2005). Dried soil (40 °C) of 100 g (<2 mm) was separated in two portions of 50 g, soaked in distilled water for 10 min to allow slaking. Each mixture was poured onto a 250 μm sieve, which was moved up and down in water with 50 repetitions, taking care that the screen broke the water surface every time. The fraction >250 μm was collected, the two mixtures were combined and the sieving was repeated using a 53 μm sieve. Fine particles <53 μm in the supernatant were precipitated with 0.5 M AlCl3. All size classes were dried at 40 °C and ball-milled for C and N analysis. To obtain free particulate organic matter (fPOM), density fractions were obtained in the bulk soil before and after re-conversion according to John et al. (2005). For the soil from the two Georgenhof sites, 10 g of field-moist soil was placed in a centrifugation tube together with 30 ml sodium-polytungstate (SPT) solution (Sometu, Berlin, Germany) adjusted to a density of 1.8 g cm−3. After shaking the tube gently five times by hand, the solution was allowed to settle for 30 min and centrifuged at 3000 g for 30 min. The supernatant with floating particles was vacuum-filtered (0.45 μm) and washed with distilled water to remove SPT. The fPOM was dried for 48 h at 40 °C and ground with a mortar. For the soil from the Wachtum site, the SPT solution was adjusted to 2 g cm−3. Due to the high sand content of this site, free and aggregate-occluded POM (oPOM) was not separated. For this reason, 10 g of field moist soil was placed in a centrifugation tube together with 30 ml SPT and the suspension was shaken for 16 h at 175 rev min−1 together with ten glass beads of 5 mm diameter before centrifugation and filtration as described above.
C and N analysis in plant and soil samples
Total C and N of the soil, litter, deadwood, tree samples and harvest residues >2 mm were determined after drying at 60 °C for 24 h by dry combustion (Elementar Vario El, Heraeus, Hanau, Germany). As no significant change in soil bulk density before and after re-conversion was detected, C stocks were calculated on a volume-based method, considering the bulk density of the different soil depths. C stocks of the tree compartments were estimated by multiplying their calculated mass by the mean C concentration. C-contents of the aggregate fractions were not corrected for sand content, as analysis of the temporal variation on the individual sites was the primary aim, rather than a comparison between the sites.
Statistical analysis
All results presented are arithmetic means and are expressed on an oven-dry basis. Normality of data was tested with Shapiro-Wilk statistics and homogeneity of variance was assessed with Levene’s test. Changes in properties before and after re-conversion were calculated for a cumulative sampling depth of 30 cm by a paired t-test (P < 0.05). Differences between the willow and the poplar site Georgenhof were estimated by a t-test (P < 0.05). The effect of the tillage treatment was tested by a one-way analysis of variance (ANOVA). Mean comparisons were performed using the Tukey test at P < 0.05. All statistical analyses were performed using JMP 8.0 (SAS Inst. Inc.).
Results
Vertical distribution of soil properties in poplar and willow plantations
At all sites, bulk density in the upper 10 cm was low (Fig. 1a). Soil pH at Georgenhof willow and at Wachtum poplar was strongly acidic, with no clear differences between depths. With an average of 5.5, pH was higher in the upper 10 cm at Georgenhof poplar than in the depths below and in comparison with Georgenhof willow (Fig. 1b). The SOC content declined with depth in all cases. It was always lowest at Georgenhof willow (Fig. 1c) and the decline was less pronounced at Wachtum poplar. The soil C/N ratio remained roughly constant at both Georgenhof sites, but increased with depth to 21 at Wachtum (Fig. 1d). Also the microbial biomass C content generally declined with depth (Fig. 2). At 0–5 cm depth, higher microbial biomass C contents were found at both Georgenhof sites in comparison with Wachtum, whereas the reverse was observed at 20–30 cm depth.
Recovery of soil after aggregate fractionation ranged between 97 % and 104 %, whereas the proportion of C to SOC was normalized to a 100 % recovery. At all sites, aggregate fractionation showed that the C contribution to SOC increased in the order <53 μm < 250–53 μm < 250–2000 μm (Table 1). The C associated with the 250–2000 μm always decreased with depth. However, at Georgenhof poplar, less C was associated with the two smaller fractions below 10 cm and more with the 250–2000 μm fraction in comparison with Georgenhof willow. At Wachtum, a pronounced increase in the 53–250 μm occurred below 15 cm as the fine sand fraction increased (data not shown). In comparison with the mineral associated C, less C was bound in the fPOM fraction at both Georgenhof sites, with an average contribution of 2 % to SOC at the poplar and slightly above 3 % at the willow site. At Wachtum, fPOM comprised between 69 % and 83 % of SOC. In contrast to both poplar sites, POM decreased with depth at Georgenhof willow (Table 1).
Carbon stocks of plant biomass and soil
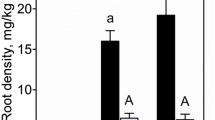
The C stocks of the aboveground biomass were 15 t ha−1 at Georgenhof willow, 71 t ha−1 at Georgenhof poplar and 50 t ha−1 at Wachtum (Table 2). The C stocks of the root biomass decreased in the order stump roots > coarse roots > fine roots. The organic surface layer was characterized by slightly decomposed litter at all sites, with absence of a humified layer. In the organic surface layer, more than 2 t C ha−1 was accumulated at Georgenhof poplar, but only 1 t C ha−1 at Georgenhof willow. The SOC stocks down to 30 cm depth contributed 79, 47 and 62 % to total C stocks at Georgenhof willow, Georgenhof poplar and Wachtum, respectively. The C/N ratio of the root fractions decreased in the order stump roots > coarse roots > fine roots at all sites, with a higher C/N for the poplar stump roots than for the willow stump roots (Table 3).
Changes in C-fractions after re-conversion
Compared with the mean C/N ratio of all three root fractions, that of the harvest residues decreased 3 to 4 fold at both poplar sites after re-conversion (Table 3). No significant change in C stocks of the mineral fine soil occurred shortly after re-conversion at 0–30 cm depth at any site or tillage treatment. Harvest residue C > 2 mm exceeded only the stocks of root C before re-conversion by 16 t C ha−1 at Georgenhof willow, 13 t C ha−1 at Georgenhof poplar and 16 t C ha−1 at Wachtum, averaging the three tillage depths (Table 4). More than 90 % of the harvest residue C accumulated in the upper 15 cm in the shallow tillage treatment at all sites. At Georgenhof willow and at Wachtum poplar, 60 % of harvest residue C was found at 0–15 cm depth and 40 % at 15–30 cm depth in the medium tillage treatment. The respective values were 80 and 20 % at Georgenhof poplar (data not shown).
Stocks of microbial biomass C increased at all sites after re-conversion, but only significantly for the shallow tillage treatment at Georgenhof poplar and for the deep tillage treatment at Wachtum. The contribution of fPOM-C to total SOC increased at both Georgenhof sites after re-conversion. At Georgenhof willow, significantly less C was associated with fPOM in the deep than in the shallow tillage treatment. At Georgenhof poplar, less fPOM-C was detected in the shallow and medium tillage treatments compared to the willow site. At Wachtum poplar, the increase in the proportion of fPOM-C was not significant after re-conversion (Table 4). Despite soil tillage during re-conversion, aggregate fractionation revealed a roughly 7 % increase of C associated with the 250–2000 μm fraction at all treatments at Georgenhof poplar, accompanied by a corresponding decrease in C associated with the 53–250 μm. In the medium tillage treatment at Georgenhof willow, the proportion of C in the 250–2000 μm fraction increased by 9 % and decreased in the smaller fractions (Table 5). As the C content of the particular fraction remained constant, the changes were only caused by the fraction mass. At Wachtum poplar, no differences in aggregate fractions were found after re-conversion. The C distribution in the fractions across all sites was not affected by the different tillage treatments (Table 5).
Discussion
Vertical distribution of soil properties under plantations
After more than 20 years’ no-tillage management the soil physical, chemical and biological properties developed clear depth gradients. Land-use change to tree plantation management increased organic matter input, root growth and bioturbation, leading to a decrease in bulk density of the top soil, as reported by Kahle et al. (2007) and Mao and Zeng (2010). At 0–10 cm depth, the lower pH under willow than under poplar clones may be a species effect (Kahle et al. 2007), although differences in site properties cannot be excluded. At Georgenhof poplar, a deeper rooting depth was observed in comparison with Georgenhof willow, resulting in stronger translocation of alkaline cations by the litterfall into the topsoil. At Wachtum, the depth gradient of SOC was less pronounced and the C/N ratio increased with increasing depth, as peat was deep ploughed in long before the plantation was established
The high microbial biomass C content in the upper 5 cm at all sites is in accordance with studies of Makeschin (1994), Pellegrino et al. (2011) and Mao and Zeng (2010), who reported that afforestation and no-tillage resulted in larger quantities of leaf and root litter supply to the top layer than arable management. Under fast growing tree-plantations, the majority of fine roots colonize the upper 10 cm (Afas et al. 2008; Heinsoo et al. 2009) providing fresh organic matter and easy available root exudates for soil microorganisms. Different microbial biomass C contents in the upper 5 cm between the two Georgenhof sites can be attributed to higher litter input and higher soil pH at the poplar site. However, this contrasts the results of Schmitt et al. (2010), who reported higher microbial biomass C contents under willow than under poplar clones. They explained this difference by higher degradability of willow leaves, which often have a lower C/N ratio and a lower lignin content than poplar leaves (Chauvet 1987). In the current study, the C/N ratio of the litter layer did not differ between Georgenhof poplar and Georgenhof willow site.
C distribution in aggregate fractions under plantations
The contribution of aggregate fraction C to SOC decreased with decreasing aggregate size because larger aggregates are composed of small aggregates plus organic bindings (Elliott et al. 1991; Puget et al. 2000; Six et al. 2000). Another reason is that soils with permanent vegetation generally contain more macroaggregates than frequently tilled soils (Six et al. 2000; Christensen 2001; DeGryze et al. 2004). Afforestation with poplar for 10 years has been found to increase soil macro-aggregation to a level similar to that of a native forest (DeGryze et al. 2004). In contrast to the Georgenhof sites, in the sandy Wachtum soil, the 250–2000 and 53–250 μm mainly comprise mineral complexes and uncomplexed organic matter of the sand size fraction. In permanently vegetated soils, uncomplexed organic matter can significantly contribute to the organic C content of sand-sized separates (Christensen 2001). Furthermore, it is known that the amount of organic matter associated with the sand fraction is small (Christensen 2001) and that soils poor in silt and clay have a small capacity for C stabilization on mineral surfaces (Baldock and Skjemstad 2000). The high proportion of C associated with the POM at this site is with this consistent.
Distribution of C stocks in long-term managed tree plantations
The C stocks of 70 t ha−1 for the aboveground biomass and 8.6 t ha−1 for the roots at Georgenhof poplar are in range with the results of Fang et al. (2007) who reported 72 t C ha−1 for the aboveground biomass and 9.5 t C ha−1 for the roots. However, their study was conducted in an 10-year-old poplar plantation with a planting density of 1,111 plants ha−1. This is almost half of the plant density of the Georgenhof poplar site (1,905 plants ha−1) where aboveground biomass has grown for 12-years from 23 year-old root stumps. A higher mean temperature (14 °C) at the study site of Fang et al. (2007) may be reason for the higher growth rate compared to this study. In the study of Sartori et al. (2007), aboveground biomass accumulated 62 % of total C and coarse roots >5 mm 12 % in an 11-year-old poplar plantation. At Georgenhof poplar and Wachtum poplar, 39 and 24 % of total C were stored in the aboveground biomass as well as 2.5 and 1.7 % C in the coarse roots, respectively. Crow and Houston (2004) observed that more than 70 % of poplar and willow roots were <5 mm in diameter at root stump ages of between 3 and 9 years. In contrast, the more than 20-year-old root stumps of the current study had more coarse roots. Generally, data for root biomass vary widely in the literature due to different sampling methods as well as plantation characteristics such as age, tree density, clones, rotation cycles and soil characteristics, making a comparison between studies difficult (Vogt et al. 1998; Nair et al. 2009). A loss of fine root mass might have occurred during root excavation and further sample preparation, leading to an underestimation of the fine root biomass. An annual amount of between 3 and 4 t leaf biomass ha−1 was reported for an 18-year-old poplar plantation (Meiresonne et al. 2006), equivalent to approximately 1.5–2 t C ha−1 a−1 deposited as litter on the soil surface. This amount is in accordance with the C stocks of 1.4 and 1.7 t ha−1 in the litter layer at Georgenhof poplar and Wachtum poplar. In summary, between 60 and 102 t C ha−1 are stored in the litter layer, root biomass and soil down to 30 cm and will be affected by the deep tillage treatment during re-conversion of the plantations.
Short-term effects of soil tillage and harvest residue input on soil C fractions
Directly after re-conversion, C stocks of harvest residues increased over all tillage treatments by roughly 14 t C ha−1 at the two poplar sites. This increase was mainly caused by incorporation of 12 t C ha−1 crown material, remaining after delimbing of the trees and the tree stumps. At Georgenhof willow, little aboveground biomass remained due to motor-manual harvesting. The differences between C stock in the former willow plantation, which consisted of only roots, and the harvest residues after re-conversion were possibly caused by different sampling methods: excavation was used before harvest and coring after harvest, which may have resulted in a large underestimation of the willow root mass. Woody residues left after harvest in native forests temporarily increased C in the soil by up to 18 % for 4 to 18 years (Johnson and Curtis 2001). Consequently, woody harvest residues may be able to contribute to the formation of soil organic matter, mitigating C losses after re-conversion.
At both poplar sites, the C/N ratio of the harvest residues decreased after re-conversion in comparison with the weighted mean of C/N ratio in the three root fractions of the former plantations, despite the incorporation of large amounts of crown material with a high C/N ratio. This observation was caused by the underestimation of the fine root biomass with a lower C/N ratio or of the coarse harvest residues (>10 cm diameter) with a wide C/N ratio. At the Georgenhof willow site, the C/N ratio of root and harvest residues differed only slightly before and after re-conversion, as only little aboveground biomass remained after harvest.
The SOC associated fPOM is considered to be mostly determined by the C input (Six et al. 1998). For this reason, the increased amounts of fPOM-C after re-conversion reflects the large input of C with chopped root and aboveground harvest residues at both Georgenhof sites. At the willow site, more fPOM-C was determined after re-conversion in the shallow and medium tillage treatment in comparison with the poplar site, despite similar yields of root and harvest residue C. However, more fPOM-C was already found in the upper 15 cm at the willow site before re-conversion, additionally explaining the higher amounts of fPOM-C in the shallow than in the deep tillage treatment. fPOM represents a very labile SOC pool and soil tillage during subsequent arable use increase C-effluxes from this fraction (Okore et al. 2007; Yang et al. 2009).
A decrease of macroaggregates directly after re-conversion was assumed, as soil physical disturbance generally induces a loss of C-rich macroaggregates (250–2000 μm) and the release of relatively stable microaggregates (Six et al. 1999). In contrast to this assumption, an increase of C associated with macroaggregates was observed at both Georgenhof sites after tillage, presumably caused by the incorporation and binding of microaggregates by the harvest residues. As reflected by the increase in fPOM-C after re-conversion, parts of the harvest residues were shredded to particles <2 mm diameter during tillage. Fresh organic residues provide a C source for microbial activity and the production of microbial-derived binding agents induces the formation of macroaggregates (Six et al. 1999; DeGryze et al. 2005; Cosentino et al. 2006). An increase of microbial biomass C was detected after tillage, but no significant correlation between macroaggregates and microbial biomass C was found. It is assumed that the microbial biomass will decrease again when labile sources of the incorporated harvest residues and easily available C and N sources in the soil are exhausted.
Conclusions
The input of crown material and tree stumps after the final harvest increased total SOC stocks. However, these quantities of harvest residues will only appear in plantations that have prolonged rotation periods. Re-conversion did not affect SOC of the bulk soil, which was not expected in such a short time, but increased microbial biomass C and POM-C. The increase of C associated with macroaggregates after re-conversion showed that freshly supplied organic matter increased macroaggregate formation. However, the duration of this effect is not known as intensive soil tillage during subsequent arable use may disrupt macroaggregates again, enhancing C turnover. The incorporation of the harvest residues may mitigate C losses with progressive decomposition as residue carbon enters the soil C-pool. Periodical sampling during the following years may give deeper insight into the SOC-dynamics after re-conversion. No influence of tillage intensity for re-conversion was detected on SOC stocks, suggesting that shallow tillage with a rotary cultivator is sufficient. However, it is not known whether shallow tillage is sufficient for preventing regeneration of trees from chopped stump roots. More information is also necessary on how the accumulation of harvest residues with high C/N ratios in the surface soil affect subsequent crop growth.
References
Afas NA, Marron N, Zavalloni C, Ceulemans R (2008) Growth and production of a short-rotation coppice culture of poplar-IV: Fine roots characteristics of five poplar clones. Biomass Bioenerg 32:494–502
Baldock JA, Skjemstad JO (2000) Role of the soil matrix and minerals in protecting natural organic materials against biological attack. Org Geochem 31:697–710
Baum C, Leinweber P, Weih M, Lamersdorf N, Dimitriou I (2009) Effects of short rotation coppice with willows and poplar on soil ecology. Landbauforschung—vTI Agric For Res 59:183–196
Chauvet E (1987) Changes in the chemical composition of alder, poplar and willow leaves during decomposition in a river. Hydrobiologia 148:35–47
Christensen BT (2001) Physical fractionation of soil and structural and functional complexity in organic matter turnover. Eur J Soil Sci 52:345–353
Cosentino D, Chenu C, Le Bissonnais Y (2006) Aggregate stability and microbial community dynamics under drying—wetting cycles in a silt loam soil. Soil Biol Biochem 38:2053–2062
Crow P, Houston PJ (2004) The influence of soil and coppice cycle on the rooting habit of short rotation poplar and willow coppice. Biomass Bioenerg 26:497–505
DeGryze S, Six J, Paustian K, Morris SJ, Paul EA, Merckx R (2004) Soil organic carbon pool changes following land-use conversions. Global Change Biol 10:1120–1132
DeGryze S, Six J, Brits C, Merckx R (2005) A quantification of short-term macroaggregate dynamics: influences of wheat residue input and texture. Soil Biol Biochem 37:55–66
Elliott ET, Palm CA, Reuss DA, Monz CA (1991) Organic matter contained in soil aggregates from a tropical chronosequence: correction for sand and light fraction. Agric Ecosyst Environ 34:443–451
Fang C, Xue J, Tang L (2007) Biomass production and carbon sequestration potential in poplar plantations with different management patterns. Environ Manag 85:672–679
Grogan P, Matthews R (2002) A modelling analysis of the potential for soil carbon sequestration under short rotation coppice willow bioenergy plantations. Soil Use Manag 18:175–183
Heinsoo K, Merilo E, Petrovits M, Koppel A (2009) Fine root biomass and production in a Salix viminalis and Salix dasyclados plantation. Est J Ecol 58:27–37
John B, Yamashita T, Ludwig B, Flessa H (2005) Storage of organic carbon in aggregate and density fractions of silty soils under different types of land use. Geoderma 128:63–79
Johnson DW, Curtis PS (2001) Effects of forest management on soil C and N storage: meta-analysis. Forest Ecol Manag 140:227–238
Jug A, Makeschin F, Rehfuss KE, Hofmann-Schielle C (1999) Short-rotation plantations of balsam poplars, aspen and willows on former arable land in the Federal Republic of Germany. III. Soil ecological effects. Forest Ecol Manag 121:85–99
Kahle P, Hildebrand E, Baum C, Boelcke B (2007) Long-term effects of short rotation forestry with willows and poplar on soil properties. Arch Agron Soil Sci 53:673–682
Kahle P, Baum C, Boelcke B, Kohl J, Roland U (2010) Vertical distribution of soil properties under short-rotation forestry in Northern Germany. J Plant Nutr Soil Sci 173:737–746
Kirschbaum MUF, Harms B, Mathers N, Dalal R (2008) Soil carbon and nitrogen changes after clearing mulga (Acacia aneura) vegetation in Queensland, Australia: Observations, simulations and scenario analysis. Soil Biol Biochem 40:392–405
Makeschin F (1994) Effects of energy forestry on soils. Biomass Bioenerg 6:63–79
Mao R, Zeng D (2010) Changes in soil particulate organic matter, microbial biomass and activity following afforestation of marginal agricultural lands in a semi-arid area of northeast China. Environ Manag 46:110–116
Meiresonne L, Schrijver AD, De Vos B (2006) Nutrient cycling in a poplar plantation (Populus trichocarpa × Populus deltoids ‘Beaupre’) on former agricultural land in northern Belgium. Can J For Res 37:41–155
Nair PKR, Kumar BM, Nair VD (2009) Agroforestry as a strategy for carbon sequestration. J Plant Nutr Soil Sci 172:10–23
Okore IK, Tijani-Eniola H, Agboola AA, Aiyelari EA (2007) Impact of land clearing methods and cropping systems on labile soil C and N pools in the humid zone Forests of Nigeria. Agr Ecosyst Environ 120:250–258
Pellegrino E, Di Bene C, Tozzini C, Bonari E (2011) Impact on soil quality of a 10-year-old short-rotation coppice poplar stand compared with intensive agricultural and uncultivated systems in a Mediterranean area. Agric Ecosyst Environ 140:245–254
Post W, Kwon K (2000) Soil carbon sequestration and land-use change: processes and potential. Glob Chang Biol 6:317–328
Puget P, Chenu C, Balesdent JB (2000) Dynamics of soil organic matter associated with particle-size fractions of water-stable aggregates. Eur J Soil Sci 51:595–605
Röhle H, Hartmann KU, Steinke C, Murach D (2009) Leistungsvermögen und Leistungserfassung von Kurzumtriebsbeständen. In: Reeg T, Bennmann A, Konold W, Murach D, Spiecker H (eds) Anbau und Nutzung von Bäumen auf landwirtschaftlichen Flächen. Weinheim, Wiley-VHC, pp 41–54
Rose-Marie R (2012) The potential of willow and poplar plantations as carbon sinks in Sweden. Biomass Bioenerg 36:86–95
Sartori FLR, Ebinger MH, Eaton JA (2007) Changes in soil carbon and nutrient pools along a chronosequence of poplar plantations in the Columbia Plateau, Oregon, USA. Agr Ecosyst Environ 122:325–339
Schmitt AK, Tischer S, Elste B, Hofmann B, Christen O (2010) Auswirkung der Energieholzproduktion auf physikalische, chemische und biologische Bodeneigenschaften auf einer Schwarzerde im Mitteldeutschen Trockengebiet. J für Kulturpflanzen 62:189–199
Singh B (1998) Biomass production and nutrient dynamics in three clones of Populus deltoides planted on Indogangetic plains. Plant Soil 203:15–26
Six J, Elliott ET, Paustian K, Doran JW (1998) Aggregation and soil organic matter accumulation in cultivated and native grassland soils. Soil Sci Soc Am J 62:1367–1377
Six J, Elliott ET, Paustian K (1999) Aggregate and soil organic matter dynamics under conventional and no-tillage systems. Soil Sci Soc Am J 63:1350–1358
Six J, Elliott ET, Paustian K (2000) Soil macroaggregate turnover and microaggregate formation: a mechanism for C sequestration under no-tillage agriculture. Soil Biol Biochem 32:2099–2103
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Vogt KA, Vogt DJ, Bloomfield J (1998) Analysis of some direct and indirect methods for estimating root biomass and production of forests at an ecosystem level. Plant Soil 200:71–89
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of soil microbial biomass C—an automated procedure. Soil Biol Biochem 22:1167–1169
Yang Y, Guo J, Chen G, Yin Y, Gao R, Lin C (2009) Effects of forest conversion on soil labile organic carbon fractions and aggregate stability in subtropical China. Plant Soil 323:153–162
Acknowledgements
We gratefully thank Gabriele Dormann, Andrea Gerke and Wolfgang Funke for their technical assistance and help with the experiment. This work was funded by the Hessian Ministry of environment, energy, agriculture and consumer protection and Volkswagen AG.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Zucong Cai.
Rights and permissions
About this article
Cite this article
Toenshoff, C., Stuelpnagel, R., Joergensen, R.G. et al. Carbon in plant biomass and soils of poplar and willow plantations—implications for SOC distribution in different soil fractions after re-conversion to arable land. Plant Soil 367, 407–417 (2013). https://doi.org/10.1007/s11104-012-1481-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1481-3