Abstract
Background and Aims
Soil mineralization, nitrification, and dynamic changes in abundance of ammonia-oxidizing bacteria (AOB) and archaea (AOA) were studied to validate our hypothesis that soil mineralization and nitrification decreased along the chronosequence of rice cultivation.
Methods
Paddy soils with a 300, 700 and 2000-year cultivation history (P300, P700 and P2000) were selected to study net mineralization and nitrification processes. Dynamic abundance of AOB and AOA was estimated by quantifying their respective amoA gene copies.
Results
The net mineralization rate was higher for P300 than P700 and P2000. Potential nitrification (N p ) and average nitrification rates (V a ) were similar for P300 and P700 soils, but the simulated potential nitrification rate (V p ) and nitrification rate (k1) was 72 % and 88 % higher for P300 than P700, respectively. V a was about 70 % lower than for P2000 than P300 and P700. AOB amoA gene copies were higher for P300 than P700 and P2000, whereas AOA abundance did not show significant differences. AOB abundance showed a positive response to NH4 supply but AOA did not.
Conclusions
Both N mineralization and nitrification were depressed with increased cultivation time. Archaea responded to mineralization positively rather than nitrification, which suggested that readily mineralized organic matter may play an important role in AOA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice is the most important crop for more than 50 % of the world's population, and is grown on almost 155 million ha in the world (Kögel-Knabner et al. 2010). Despite its contribution to the global food supply, little is known about the longer-term effects of intensified rice cropping on soil properties that govern productivity (Olk et al. 1996). In particular, the most common yield-limiting factor is the availability of N in paddy soils (Cassman et al. 1998).
Paddy soil development is driven by the specific soil management practices that mask the soil's original character (Kirk 2004). These are regular submergence and drainage, ploughing and puddling, organic manuring and fertilization. The management-induced change of oxic and anoxic conditions results in temporal and spatial variations in reduction and oxidation reactions affecting the dynamics of soil constituents including C and N (Cheng et al. 2009). It is commonly accepted that water logging associated with rice cropping enhances accumulation of soil organic C and N (Neue et al. 1997; Lal 2002). However, Olk et al. (1996) reported that N availability deceased with intensive rice cultivation, possibly because considerable N becomes bound to lignin under water-logged conditions (Schmidt-Rohr et al. 2004), and also because of dentrification of nitrate upon flooding. Thus, the patterns of N-mineralization and nitrification are changed with duration of paddy farming.
Both ammonia -oxidizing bacteria (AOB) and ammonia oxidizing archaea (AOA) possess the amoA gene for the ammonia monooxygenase (AMO), which implies that both are key players in nitrification (Francis et al. 2005; Leininger et al. 2006; Wuchter et al. 2006; Lam et al. 2007; Nugroho et al. 2009). However, there are ambiguities concerning the relative importance of these different groups. For example, Tourna et al. (2008) suggested that AOA played a role in soil nitrification since the community structure of active AOA changed in relation to temperature during nitrification, but this was not the case for the AOB. Offre et al. (2009) also reported that of the two groups, only AOA was growing during active nitrification. However, bacteria rather than archaea were the more active ammonia oxidizers (Jia and Conrad 2009; Di et al. 2009). These contrasting results imply that the relative importance of AOB and AOA vary depending on the environmental and site conditions (Erguder et al. 2009; Wessen et al. 2010).
The hypothesis of this study is that the soil mineralization and nitrification decrease along a chronosequence of rice cultivation. Therefore, the objective of this study was to investigate soil N mineralization and nitrification processes, and AOB and AOA amoA gene abundance of AMO, for different durations of rice cultivation history.
Materials and methods
Site description and soil sampling
The study sites are located in Cixi, Zhejiang Province, P.R. China. The climate is subtropical with periodical monsoon rains. Mean annual temperature is 16.3 °C with a range from 9.3 °C to 38.5 °C, and the mean annual precipitation is 1325 mm (Cheng et al. 2009). The overall area is 433 km2 with the variation of altitude from 2.6 to 5.7 m above sea level. In late spring 2010, shortly after the harvest of the upland crops, we sampled a chronosequence of three paddy soils that have been in rice cultivation for approximately 300,700 and 2000 years (P300, P700 and P2000) (Cheng et al. 2009). Five field replicates were taken at each site from 0 - 20 cm depth, with each replicate being composed of seven individual soil cores (diameter was 5.5 cm), which were pooled and homogenized to reduce heterogeneity.
The duration of rice cultivation at the respective sites was estimated according to well documented points in the time of sea dike construction (Cixi County Annals, abstracted information available at www.cixi.gov.cn), summarized by Cheng et al. (2009). Thus, all paddy soils developed from comparable parent materials (tidal wetlands) and under similar ecological conditions.
Incubation
For each sample, 50 g soil was added to 40 plastic bottles (250 ml). Distilled water was added to adjust the moisture content to 60 % of water-holding capacity. All bottles were covered with polyethylene film punctured with needle holes to maintain aerobic conditions, and pre-incubated at 28 °C in the dark for 7 days. After pre-incubation, half of the bottles were amended with 100 mg (NH4)2SO4 kg-1 dry soil to study nitrification, while another group without the additional N supply was prepared to study mineralization. The loss of water through evaporation was compensated by addition of distilled water every 3 d. At incubation days 0, 7, 14, 21 and 28, 4 replicate bottles of each treatment were randomly selected and the soil extracted by shaking for 1 h with 100 ml of 2 M KCl (Keeney and Nelson 1982). The resultant analyses of inorganic N forms were performed colorimetrically with a SKLAR continuous-flow analyzer (SKLAR San++, Netherland, 2003).
DNA extraction & quantitative PCR assay
At incubation days 0, 14 and 28, 4 replicate bottles of each treatment were randomly selected to extract DNA and amoA genes was performed by quantitative PCR (qPCR). The DNA was extracted for three sub-samples from 0.50 g of soil with the FastDNA Spin Kit for soil (MP Biomedicals, United States), according to the protocol of the manufacturer. The quality and quantity of the DNA extracts were determined with a spectrophotometer (Nanodrop, PeqLab, Germany), and were pooled and stored at -20 °C until use.
Quantitative PCR of amoA genes was performed to estimate the abundance of the ammonia-oxidizing bacterial and archaeal communities, respectively. The primers amoA-1 F (5’-GGGGTTTCTACTGGTGGT-3’) and amoA-2R (5’-CCCCTCKGSAAAGCCTTCTTC-3’) were used for ammonia- oxidizing bacteria generating a 491 bp fragment; Arch-amoA F (5’-STAATGGTCTGGCTTAGACG -3’) and Arch-amoA R (5’- GCGGCCATCCATCTGTATGT -3’) were used for ammonia- oxidizing archaea generating a 635 bp fragment (Francis et al. 2005). Quantification was based on the fluorescence intensity of the SYBR Green dye and reactions for each sample were carried out in a Bio-Rad CFX-96 thermal cycler. The quantification of amoA genes was performed in a total volume of 25 μl reaction mixtures by using12.5 μl of SYBR Premix Ex Taq™ as described by the suppliers (Takara Bio, Otsu, Shiga, Japan), 0.25 μl of each primer (50 μm), 1 μl of soil DNA template, with a final content of 1–10 ng in each reaction mixture,and 11 μl ddH2O. The fragments for the AOB and AOA were both amplified using an initial denaturation step at 95 °C for 3 min, followed by 35 cycles of 30s at 95 °C, 30s at 55 °C, 30s at 72 °C for AOB, and 45 s at 72 °C for AOA for the collection of fluorescence data. All reactions were finished with a melting curve starting at 65 °C with an increase of 0.5 °C up to 95 °C to verify amplicon specificity. The PCR reaction runs had an efficiency of 90 % and 94 % for the AOB and AOA, respectively. Standard curves for the AOB and AOA were obtained using serial dilutions of linearized plasmids (pGEM-T, Promega) containing cloned amoA genes amplified from environmental clones (r2 > 0.995 for both standard curves).
Data analyses
The changes in NO -3 -N content with incubation time were modeled with a first-order reaction kinetic model, expressed as \( N = {N_0} + {N_p}\left( {1 - exp\left( { - {k_1}t} \right)} \right) \), or by a zero-order reaction kinetic model, expressed as N = N 0 + k 0 t. Where N was NO -3 -N content at incubation time t; N0 was NO -3 -N content after pre-incubation (t = 0); Np was potential nitrification; and k1 and k0 were rate constants of first- and zero-order reactions, respectively. The potential nitrification rate (V p ) was calculated from first-order kenetics as V p = k1*Np (Oorts et al. 2007). The mean actual net nitrification rate (V a ) was calculated as V a = (N28-N0)/28, here N28 was NO -3 -N content after 28 days’ incubation (t = 28).
Data (measured or calculated) were subjected to one-way ANOVA and mean values were separated using Duncan’s New Multiple Range Test at p < 0.05. All statistical analyses were performed by SPSS statistical package.
Results
Impacts of rice cultivation duration history on selected soil properties
Soil pH ranged from 6.69 to 7.07, which did not show significant differences between 300, 700 and 2000 years of rice cultivation history (Table 1). Soil organic matter (SOM) and total N showed similar trends, with the highest values observed for 2000-year soil of rice cultivation, but no significant differences between P700 and P300. Soil C to N ratio ranged from 8.17 to 8.48, and was not affected by cultivation history. Similar to soil total N, NH +4 -N was higher for P2000 than P700 and P300. However, NO -3 -N concentrations were significant higher for the 300-year history than P2000.
Impacts of rice cultivation duration history on N mineralization
After pre-incubation, mineral N contents ranged from 9.3 to 12.1 mg N kg-1 soil, and increased linearly during the first 2 weeks of incubation, then the N mineralization process slowed and mineral N concentrations deceased slightly in the last 2 weeks of incubation for all soils (Figure 1). Although the N mineralization process was similar for P300, P700 and P2000, significant differences in N mineralization rates were observed. For example, N mineralization was 0.85 mg N day-1 for P300, which was significantly higher than P700 (0.50 mg N d-1) and P2000 (0.51 mg N d-1) in the first 2 weeks of incubation (r2 > 0.999, p < 0.01).
Impacts of rice cultivation duration history on N nitrification dynamics
After pre-incubation, NH +4 -N averaged 3.8, 2.0 and 8.3 mg N kg-1 soil for P300, P700 and P2000 respectively, then 100 mg (NH4)2SO4 kg-1 soil was added to investigate nitrification dynamics. NH +4 -N increased significantly in the first week of incubation, ranging from 17.8 to 24.5 mg N kg-1 soil (F = 255.6 and 423.6 for different soils and sampling time respectively, p < 0.05). For P300 and P700, NH +4 -N concentrations decreased sharply in the second week of incubation, while NH +4 -N concentrations did not decrease for 2000-year soil. The remarkable decrease in NH +4 -N concentration for P2000 took place in the third-week of incubation. During the last week of incubation, NH +4 -N concentrations did not vary significantly for all three soils (p > 0.05). On the contrary, NO -3 -N concentrations increased as NH +4 -N concentration decreased. NO -3 -N ranged from 8.1 to 10.8 mg N kg-1 soil after pre-incubation, and kept increasing during the first 2 weeks of incubation for P300 and P700 soils (F = 25.2 and 104.9 for different soils and sampling time respectively, p < 0.05), then NO -3 -N increased slightly in the last 2 weeks of incubation (Figure 2). However, the dynamics of NO3-N for the 2000-year soil did not show similar trends as other sites. For P2000, the increase of NO -3 -N did not occur in the first 2 weeks of incubation, but took place in the third-week (F = 25.2 and 104.9 for different soils and sampling time respectively, p < 0.05), then decreased slightly in the last week.
Regression analysis showed that the time-dependent kinetics of net nitrification was best fitted by a first-order model for 300 and 700-year soils. Simulated parameters of nitrification were listed in Table 2. Although potential nitrification (N p ) and average nitrification rates (V a ) did not show significant difference for P300 and P700 soils, the simulated potential nitrification rates (V p ) and nitrification reaction rates (k1) were 72 % and 88 % higher for P300 than P700, respectively. However, the nitrification dynamics for P2000 did not fit first or zero-order kinetics. The average nitrification rates (V a ) during the 4-week incubation was 0.39 mg N kg-1 soil d-1, which was lower than P300 (0.69 mg N kg-1 d-1) and P700 (0.66 mg N kg-1 d-1).
pH changes in mineralization and nitrification dynamics
Soil pH ranged from 6.80 to 7.02 after pre-incubation, which did not vary between paddy ages. pH changes during 28 d mineralization and nitrification were shown in Figure 3 (a) and (b), respectively. Generally, soil pH decreased during the first 3 weeks of incubation. Statistical analysis indicated significant differences in pH between day 0 and day 21 (F = 35.3 and 261 for different soils and sampling time respectively, p < 0.05), and pH increased from day 21 to the end of the incubation. The biggest pH decrease in mineralization was 1.0 unit, whereas in nitrification it was 2.1 units (p < 0.05) (Fig. 3).
pH changes in mineralization (a) and nitrification (b) dynamics during 28 days incubation at 28 °C with soil moisture of 60 % WHC (Soils were pre-incubated in dark at 28 °C with soil moisture of 60 % WHC for 7 days. After pre-incubation, no N supply was added for mineralization study while 100 mg (NH4)2SO4 kg-1 was applied for nitrification study). Bars represent standard deviation, n = 4
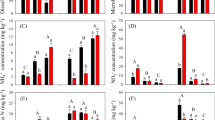
Abundance of ammonia-oxidizing bacterial (a,b) and ammonia-oxidizing archaeal (c, d) amoA gene copies in mineralization (a, c) and nitrification (b, d) dynamics during 28 days incubation at 28 °C with soil moisture of 60 % WHC (Soils were pre-incubated in dark at 28 °C with soil moisture of 60 % WHC for 7 days. After pre-incubation, no N supply was added for mineralization study while 100 mg (NH4)2SO4 kg-1 was applied for nitrification study). Bars represent standard deviation, n = 4
Abundance of ammonia-oxidizing bacterial (AOB) and ammonia-oxidizing archaeal (AOA) amoA gene copies
Abundance of AOB and AOA was estimated by quantifying their respective amoA gene copy numbers assuming that individual bacteria and archaea contain only one copy of the amoA gene. The highest level of AOB amoA gene copies were observed for 300-year soil, and the lowest was for 2000-year soil. After pre-incubation, AOB amoA gene copy numbers were 11.5, 6.92 and 1.66 * 105 copies g-1 dry soil for P300, P700 and P2000 soils, respectively, and all increased to their highest levels after 2 weeks’ incubation, then decreased sharply till the end of incubation without additional N supply (Figure 4a) (F = 142.6 and 263.4 for different soils and sampling time respectively, p < 0.05). For treatments with (NH +4 )2SO4 application, AOB amoA gene copy numbers kept increasing and their highest level were observed at the end of incubation for all soils (Figure 4b) (F = 63.4 and 87.8 for different soils and sampling time respectively, p < 0.05). Therefore, AOB amoA gene copy numbers for soils with additional ammonium application were higher than those without N supply controls at the end of incubation.
AOA amoA gene copy numbers ranged from 8.86 to 12.8 * 105 copies g-1 dry soil after pre-incubation, which did not differ significantly for soils with different rice cultivation year’s history (F = 2.67, p > 0.05). AOA dynamics during incubation showed similar trend for all soils, with or without additional N supply (Figure 4, c and d). The highest levels in AOA amoA gene copies were observed after 2 weeks’ incubation, then decreased till the end of incubation. Unlike AOB, AOA abundance did not increase by ammonium application.
Discussion
Effects of prolonged rice cultivation on N mineralization and nitrification
Present results clearly supported the hypothesis that both N mineralization and nitrification were depressed along prolonged paddy cultivation. Soil TN did not differ significantly for P300 and P700. Roth et al. (2011) reported that TN increased along prolonged paddy management from land embankment (P0) and reached a maximum for a 300-year site under paddy-wheat rotation (P300). The maximum indicated a steady-state equilibrium in which additional N input was balanced by N loss (Roth et al. 2011). However, the 2000 year-old paddy (P2000) showed a significantly elevated N content than P300 and P700. This may be a result of high rates of fertilizer-N application and organic input from roots and stubble under intensive cultivation. Similar results were also reported for paddy soils at the same site (Cheng et al. 2009). However, an increase in TN did not necessarily coincide with an increase in readily mineralizable organic N. During the four-week incubation, the highest mineral N fraction (NH4 and NO3) of TN decreased with prolonged paddy management (0.82 %, 0.69 % and 0.49 % for P300, P700 and P2000, respectively). The lower mineral N fraction for P2000 means lower available N pools compared to P300 and P700. Furthermore, soil net N mineralization rate was 70 % higher for P300 than P700 and P2000 during the first 2 weeks of incubation. The results indicated that SON pools changed and the fraction of readily mineralized organic N decreased with prolonged paddy management. Olk et al. (1996) reported that N utilization efficiency deceased with intensive rice cultivation and concluded that the bioavailability of the soil organic N (SON) had declined. Schmidt-Rohr et al. (2004) also found that N bound to lignin accounted for the reduced bioavailability of N under water-logged conditions.
Additional NH +4 -N was added to investigate nitrification dynamics for soils with different rice cultivation duration history. Results showed nitrification dynamics differed from N mineralization. The N mineralization process was similar for P300, P700 and P2000, however, nitrification dynamics for P2000 showed different pattern compared to P300 and P700. For P300 and P700, strong nitrification occurred the first 2 weeks of incubation, whereas obvious nitrification took place in the third-week of incubation for P2000. This was clearly demonstrated by a decrease in NH4 + -N concentrations and an increase in NO -3 -N concentrations.
The nitrification process in soils could be simulated by zero- or first-order kinetics (Mary et al. 1998; Pansu et al. 2003; Cheng et al. 2004; Oorts et al. 2007; Jiang et al. 2011). In the present study, results the time-dependent kinetics of net nitrification was best fitted by a first-order kinetic model for P300 and P700. This indicated that the substrate (NH +4 -N) is less than the capacity of catalyst, and the rate of nitrification was proportion to the first power of substrate concentration. Since additional NH +4 -N supply was the same (100 mg (NH4)2SO4 kg-1 soil), actual net average nitrification rates (V a ) and potential nitrification (N p ) did not vary between P300 and P700 soils. This result was also supported by the decrease in NH +4 -N concentrations and the increase in NO -3 -N concentrations that did not differ significantly for P300 and P700 during the 4-week incubation. However, results may be different when the bioavailable-N supply is sufficient. Simulated potential nitrification rate (V p ) and nitrification reaction rate (k 1 ) were 72 % and 88 % higher for P300 than P700 respectively, with simulated results also supported by amoA gene abundance. Highest level of AOB amoA gene copies were observed for 300-year soil which were significant higher than P700, and the lowest was for 2000-year soil during the whole incubation. Bannert et al. (2011) also reported AOB amoA gene copies were more than 5 times higher for P300 than P2000. Nitrification dynamics for P2000 did not fit first or zero-order kinetics, and nitrification rates during 4-week incubation was significant lower for P2000 than P300 and P700, suggesting that soil nitrification deceased with prolonged paddy management.
Response of AOA and AOB dynamics to mineralization and nitrification
Similar dynamic patterns were observed in both AOB and AOA abundance during mineralization and nitrification processes for soils with different duration of paddy management. All paddy soils collected in this study were developed from comparable parent materials (tidal wetlands) and under the same ecological conditions, and soil N accumulation were reported to reach steady-state condition after about 300 years of rice cultivation (Cheng et al. 2009; Roth et al. 2011; Bannert et al. 2011). Although soil organic C and total N was higher for P2000, soil C to N ratio was similar to P300 and P700. Therefore, it is possible that microbial community involved in N turnover was similar for soils with different duration of paddy management.
AOA amoA gene copies were greater than AOB in the present paddy soils, and AOA abundance did not differ significantly for soils with different rice cultivation year’s history. Furthermore, AOA did not response to additional NH +4 supply during incubation for all soils. These results agreed well with previous studies (Leininger et al. 2006; He et al. 2007; Chen et al. 2008; Shen et al. 2008; Jia and Conrad 2009; Bannert et al. 2011). However, AOA amoA gene copies increased significantly when mineralization took place, and decreased to original levels when mineralization stopped. This indicated that archaeal AMO responded to mineralization positively more so than nitrification, and further suggested that readily mineralized organic matter may play an important role in AOA abundance. In support of this hypothesis, Wessen et al. (2010) found that AOA community size was negatively correlated to soil organic C content and the C: N ratio, and archaeal amoA transcript levels were stimulated by plant derived organic substrates (Chen et al. 2008). Bacterial AMO is a multifunctional enzyme, oxidizing methane, carbon monoxide and a range of organic compounds (Prosser and Nicol 2008). Although little is currently known of the alternative substrates for archaeal AMO, the significant differences in cell metabolic and biochemical processes may have consequences for functional diversity. The abundance of archaeal AMO may result not from ammonia oxidation but, for example, from mixotrophic or heterotrophic growth (Prosser and Nicol 2008; Wessen et al. 2010).On the contrary, AOB abundance showed positive response to ammonium supply for all soils with different duration of rice cultivation. Similar responses were also observed in acid (He et al. 2007) and alkaline soils (Shen et al. 2008), suggesting that nitrification was driven by bacteria rather than archaea in the present rice-based ecosystem.
Conclusions
N mineralization was depressed along prolonged paddy cultivation, probably because that SON pools changed and the fraction of readily mineralized organic N decreased along prolonged paddy management. Nitrification was also found depressed along prolonged paddy cultivation, possibly because abundance of ammonia-oxidizing bacterial decreased with continuing paddy use. The archaeal amoA responded to mineralization more positively than nitrification, suggesting that readily mineralized organic matter may play an important role in AOA AMO abundance.
References
Bannert A, Mueller-Niggemann C, Kleineidam K (2011) Comparison of lipid biomarker and gene abundance characterizing the archaeal ammonia-oxidizing community in flooded soils. Biol Fertil Soils 2011(7):839–843
Cassman KG, Peng S, Olk DC, Ladha JK, Reichardt W, Dobermann A, Singh U (1998) Opportunities for increased nitrogen-use efficiency from improved resource management in irrigated rice systems. Field Crop Res 56:7–39
Chen XP, Zhu YG, Xia Y (2008) Ammonia-oxidizing archaea: important players in paddy rhizosphere soil. Environ Microbiol 10:1978–1987
Cheng W, Tsuruta H, Chen G, Yagi K (2004) N2O and NO production in various Chinese agricultural soils by nitrification. Soil Biol Biochem 36:953–963
Cheng YQ, Yang LZ, Cao ZH, Yin S (2009) Chronosequential changes of selected pedogenic properties in paddy soils as compared with non-paddy soils. Geoderma 151:31–41
Di HJ, Cameron KC, Shen JP, Winefield CS, O'Callaghan M, Bowatte S, He JZ (2009) Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat Geosci 2:621–624
Erguder TH, Boon N, Wittebolle L (2009) Environmental factors shaping the ecological niches of ammonia—oxidizing archaea. FEMS Microbiol Rev 33:855–869
Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB (2005) Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc Natl Acad Sci USA 102:14683–14688
He J, Shen J, Zhang L (2007) Quantitative analyses of the abundance and composition of ammonia – oxidizing bacteria and ammonia—oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ Microbiol 9:2364–2374
Jia ZJ, Conrad R (2009) Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ Microbiol 11:1658–1671
Jiang X, Ma Y, Yuan J, Wright AL (2011) Soil surface electrochemical property effects on abundance of ammonia-oxidizing bacterial and ammonia-oxidizing archaeal community, NH +4 activity and net nitrification in an acid soil. Soil Biol Biochem 43:2215–2221
Keeney DR, Nelson DW (1982) Nitrogen: Inorganic forms. In: Page AL et al. (eds.) Methods of soil analysis. Part 2. Chemical and microbiological properties, 2nd ed. SSSA Book Ser. 5. SSSA, Madison, WI, pp 643-698
Kirk G (2004) The biogeochemistry of submerged soils. Wiley, Chichester
Kögel-Knabner I, Amelung W, Cao ZH (2010) Biogeochemistry of paddy soils. Geoderma 157:1–14
Lal R (2002) Soil carbon sequestration in China through agricultural intensification, and restoration of degraded and desertified ecosystems. Land Degral Dev 13:469–478
Lam P, Jensen MM, Lavik G, McGinnis BM, Schubert CJ, Amann R, Thamdrup B, Kuypers MMM (2007) Linking crenarchaeal and bacterial nitrification to anammox in the Black Sea. Proc Natl Acad Sci USA 104:7104–7109
Leininger S, Urich T, Schloter M (2006) Archaea predominate among ammonia—oxidizing prokaryotes in soils. Nature 442:806–609
Mary B, Recous S, Robin D (1998) A model for calculating nitrogen fluxes in soil using 15N tracing. Soil Biol Biochem 30:1963–1979
Neue HU, Gaunt JL, Wang ZP (1997) Carbon in tropical wetlands. Geoderma 79:163–185
Nugroho RA, Röling WFM, van Straalen NM, Verhoef HA (2009) Changes in nitrification and bacterial community structure upon crossinoculation of Scots pine forest soils with different initial nitrification rates. Soil Biol Biochem 41:243–250
Offre P, Prosser JI, Nicol GM (2009) Growth of ammonia—oxidizing archaea in soil microcosms is inhibited by acetylene. FEMS Microbiol Ecol 70:99–108
Olk DC, Cassman KG, Randall EW (1996) Changes in chemical properties of organic matter with intensified rice cropping in tropical lowland soil. Eur J Soil Sci 47:293–303
Oorts K, Laurent F, Mary B, Thiebeau P, Labreuche J, Nicolardot B (2007) Experimental and simulated soil mineral N dynamics for long-term tillage systems in northern France. Soil Till Res 94:441–456
Pansu M, Thuriès L, Larré-Larrouy MC, Bottner P (2003) Predicting N transformations from organic inputs in soil in relation to incubation time and biochemical composition. Soil Biol Biochem 35:353–363
Prosser JI, Nicol GW (2008) Relative contributions of archaea and bacteria to aerobic ammonia oxidation in the environment. Environ Microbiol 11:2931–2941
Roth PJ, Lehndorff E, Cao ZH, Zhuang S, Bannert A, Wissing L, Schloter M, Kögel-knabner I, Amelung W (2011) Accumulation of nitrogen and microbial residues during 2000 years of rice paddy and non-paddy soil development in the Yangtze River Delta, China. Glob Chang Biol 17:3405–3417
Schmidt-Rohr K, Mao JD, Olk DC (2004) Nitrogen bonded aromatics in soil organic matter and their implications for a yield decline in intensive rice cropping. Proc Natl Acad Sci USA 101:6351–6354
Shen JP, Zhang LM, Zhu YG, Zhang JB, He JZ (2008) Abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea communities of an alkaline sandy loam. Environ Microbiol 10:1601–1611
Tourna M, Freitag TE, Nicol GW (2008) Growth, activity and temperature responses of ammonia—oxidizing archaea and bacteria in soil microcosms. Environ Microbiol 5:1357–1364
Wessen E, Hallin S, Philippot L (2010) Differential responses of bacterial and archaeal groups at high taxonomical ranks to soil management. Soil Biol Biochem 10:1759–1765
Wuchter C, Abbas B, Coolen MJL, Herfort L, van Bleijswijk J, Timmers P, Strous M, Teira E, Herndl GJ, Middelburg JJ, Schouten S, Sinninghe Damsté JS (2006) Archaeal nitrification in the ocean. Proc Natl Acad Sci USA 103:12317–12322
Acknowledgements
We thank Dr. Yigang Luo, University of Florida, for reviewing this manuscript. We wish to acknowledge useful suggestions by the reviewers and the editor. This research was financially supported by the joint Sino-German research project (GZ518), Program for New Century Excellent Talents in University (NCET-08-0817) and the special fund for agro-scientific research in the public interest of China(201203030).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Dan Murphy.
Rights and permissions
About this article
Cite this article
Jiang, X., Liu, W., Liu, Q. et al. Soil N mineralization, nitrification and dynamic changes in abundance of ammonia-oxidizing bacteria and archaea along a 2000 year chronosequence of rice cultivation. Plant Soil 365, 59–68 (2013). https://doi.org/10.1007/s11104-012-1377-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1377-2