Abstract
Background and aims
Selenium is an essential micro-nutrient for animals, humans and microorganisms; it mainly enters food chains through plants. This study proposes to explore effect of inorganic Se forms on its uptake and accumulation in Zea mays.
Methods
Zea mays was grown in a controlled-atmosphere chamber for 2 weeks in a hydroponic solution of low-concentration selenium (10 μg/L (i.e.0.12 μM) or 50 μg/L (i.e. 0.63 μM) of Se). For each concentration, four treatments were defined: control (without selenium), selenite alone, selenate alone and selenite and selenate mixed.
Results
At low concentrations, selenium did not affect the biomass production of Zea mays. However, for both concentrations, Se accumulation following a selenite-only treatment was always higher than with selenate-only. Moreover, in the selenate-only treatment, Se mainly accumulated in shoots whereas in the selenite-only treatment, Se was stocked more in the roots. Interactions between selenate and selenite were observed only at the higher concentration (0.63 μM of selenium in the nutrient solution).
Conclusions
Se form and concentration in the nutrient solution strongly influenced the absorption, allocation and metabolism of Se in Zea mays. Selenate seems to inhibit selenite absorption by the roots.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For human adults, the recommended dietary allowance of selenium (Se)—an essential micro-nutrient—is estimated at between 40 and 70 μg per day (World Health Organization et al. 1996) with meat, seafood and cereals being the main sources of dietary Se in Europe. Plants, especially crop plants, play an essential role in the incorporation of Se in the terrestrial food chain. In mammals, Se plays important antioxidant, redox regulating, thyroid hormone regulating and brain and gonad protective roles though enzymes and selenoproteins which include glutathione peroxydase, thioredoxin reductase, iodothyronine deiodinase selenoprotein P and selenoprotein W. Selenium deficiency in humans can cause health disorders such as muscular dystrophy and certain cardiovascular, bone or immune system diseases and increases the risk of cancer and lymphoma (Whanger 2002). In several countries, Se intake by humans is low or deficient (i.e. < 40 μg Se/person/d). For example, Se intake reached only 15 μg Se/person/d in Saudi Arabia; values for France (36 μg Se/person/d), the UK (34 μg Se/person/d) and Brazil (33 μg Se/person/d) (Rayman 2008) were slightly better. Several solutions have been tested to raise those low values, including mineral supplements for human subjects or livestock and agronomic biofortification of crops either with Se fertilizers or through genetic biofortification (plant breeding). For example, the use of Se fertilizers in depleted soils has been widely practiced with success in Finland since 1984 (Eurola et al. 1991), and also in New Zealand and in China (Gissel-Nielsen et al. 1984).
The naturally-occurring trace element Se, chemically similar to sulfur, has two inorganic oxidized forms, namely selenite (Se(+IV)) and selenate (Se(+VI)), which are the two main forms available for plant uptake in aerobic soils. Unlike human beings, higher plants do not seem to require Se (Eshdat et al. 1997; Terry et al. 2000). However, they can accumulate and metabolize Se absorbed from their environment and growth media. Plants have been classified according to their variable absorbance capacity under natural conditions (Broadley et al. 2006; Brown and Shrift 1982; White et al. 2004) into (i) “non-accumulators” which usually contain less than 25 μg Se/g dry weight (DW), (ii) “indicators” which assimilate between 25 and 1,000 μg Se/g DW, and finally (iii) “accumulators”, which may accumulate Se to concentrations of several thousand μg/g DW. Studies of Se pathways within plants have usually been carried out on “accumulators” and “indicators” cultured in the presence of relatively high Se concentrations, i.e. between 5 and 10 μM of Se (Arvy 1993; De Souza et al. 1998; Hopper and Parker 1999; Läuchli 1993; Li et al. 2008; Liu and Gu 2009; Mazej et al. 2008; Sors et al. 2005; Terry and Zayed 1994; Terry et al. 2000; White et al. 2004; Ximenez-Embun et al. 2004; Zhang et al. 2003). Apart from these more global categories, some specific plants (i.e. rice or wheat) (Dhillon and Dhillon 2000) have also often been studied because of their essential role as staple foods. Surprisingly, selenium absorption in maize has hardly ever been studied, though the plant is on a par with wheat as one of the world’s most important crops and can account for nearly 50 % of the humans feed in some countries such as Malawi (Chilimba et al. 2011).
Due to the two elements’ similar chemical properties, Se has metabolic pathways that closely resemble those of sulfur (Läuchli 1993). According to many reports on the inhibition of selenate absorption by high concentrations of sulfate (Cruz-Jimenez et al. 2005; Hopper and Parker 1999; Sors et al. 2005; Terry et al. 2000) and studies on the expression of high-affinity sulfate transporter genes, selenate is absorbed by the roots via high-affinity sulfate transporters (Shibagaki et al. 2002). In contrast, no consensus exists on the absorption of selenite by the roots. While Arvy (1993) suggested a passive diffusion mechanism, Li et al. (2008) proposed an active process dependant on phosphate transporters. Whether absorbed via an active and/or a non active mechanism, the Se entering the roots has various fates in plants: (i) root-to-shoot (i.e. stems + leaves) translocation, (ii) metabolization of selenoamino-acids (sulfur substitutions by Se in amino-acids such as methionine and cysteine) which are incorporated into many proteins and (iii) volatilization as methyl organic compounds (dimethyl-selenide and dimethyl-diselenide). Previous studies have reported a Se effect on plant growth and have revealed that its uptake depends on the form and concentration of supplied Se. Indeed, 1 mg/L of Se reduces biomass production to a greater extent when supplied as selenite rather than selenate (Hopper and Parker 1999; Ximenez-Embun et al. 2004) and plants accumulate more Se from selenate than selenite (De Souza et al. 1998; Terry et al. 2000; Ximenez-Embun et al. 2004; Zhang et al. 2003). The greater mobility of selenate compared to selenite plays an important role in translocation and metabolism within the plant. When supplies as selenate, Se is mainly translocated to leaves (especially young leaves De Souza et al. 1998) where it is only weakly metabolized as selenoamino-acids; the selenate concentration in shoots represents more than 90 % of total shoot Se (De Souza et al. 1998; Hopper and Parker 1999; Li et al. 2008; Mazej et al. 2008; Terry et al. 2000; Ximenez-Embun et al. 2004; Zayed et al. 1998; Zhang et al. 2003). In fact, the reduction of selenate into selenite is rate limiting to selenate assimilation (Terry et al. 2000). However, traces of selenoamino-acids (such as selenomethionine) and selenite have been identified in plants (Li et al. 2008; Mazej et al. 2008; Ximenez-Embun et al. 2004). When supplied as selenite, Se accumulates principally in roots with little translocation but selenoamino-acid production is high (Arvy 1993; De Souza et al. 1998; Hopper and Parker 1999; Li et al. 2008; Liu and Gu 2009; Terry et al. 2000; Ximenez-Embun et al. 2004; Zayed et al. 1998). Different amino-acids such as seleno-methionine, selenomethionine Se-oxide, selenocysteine and selenomethylselenocysteine have been detected and can account for more than 40 % of the total Se in the plant (Li et al. 2008; Ximenez-Embun et al. 2004). Plants can also volatilize organic Se from their tissues at a rate which correlates with the Se concentration in the tissue. Indeed, the rate of Se-volatilization from roots is higher than from shoots (Zayed et al. 1998) and is twice as high from plants supplied with selenite rather than selenate (De Souza et al. 1998). Indeed, soil microorganisms are also known to volatilize Se (Terry and Zayed 1994).
Zea mays is not only the most widely grown cereal in the world for animal and human consumption, it also has high water requirements and therefore a high impact on the cycling and flux of nutrients within the agricultural system and the food chain. Even so, to our knowledge, little research has been conducted on the effects of the form of supplied Se on Se accumulation in maize. Since inorganic Se species are more abundant than organic species in most natural water and soil environments, in our experiments selenite and selenate were added as supplements to the plant growing solutions. Our objectives were to quantify the influence, uptake and translocation of these two inorganic chemical forms (selenate and selenite) on maize grown in a controlled hydroponic system. We were able, for the first time, to quantify the effect of the single forms (only selenite, only selenate) and co-exposure to the two forms (selenite+selenate) on the uptake and translocation of Se in different tissues of maize (roots, stems and leaves). As the Se concentrations in natural water in France are often low (from several μg/L to maximum values only rarely reaching several dozen μg/L), we set two Se concentration levels similar to current standards for drinking water in the UE and the US: 10 and 50 μg/L of Se. We therefore obtained conditions coherent with nutrient solution concentrations of Se close to those in natural media.
Materials and methods
Seed germination and culture conditions
First, dried Zea mays subsp. mays (L.) seeds were germinated on glass balls. Two weeks after germination, the seedlings were transplanted into a hydroponic system in a plastic container (20 L) filled with a modified Hoagland nutrient solution. In the Plexiglas chamber, irradiance was provided by two 400-W metal halide discharge lamps (Growth spectra, MH400W, E40) over an eight-hour photoperiod. The change in the biomass of the plants was monitored by recording the leaf area five times during the experiments.
The modified Hoagland solution was composed of KNO3 (3 mM), Ca(NO3)2.4 H20 (2.72 mM), NH4NO3 (2 mM), NaCl (0.2 mM), KH2PO4 (0.98 mM), MgSO4.7 H20 (0.70 mM), (NH4)6Mo7O24.4H2O (0.04 μM), H3BO3 (24 μM), MnSO4 (13 μM), ZnSO4 (6 μM), CuSO4 (1.5 μM) and FeEDDHA (6 %) (48 μM). Two weeks after the transfer of the seedlings, the original nutrient solution was replaced with seven new nutrient solutions supplemented with:
-
1-
SeVI-T50: 0.63 μM of SeVI with Na2SeO4 (50 μg/L of Se)
-
2-
SeIV-T50: 0.63 μM of SeIV with Na2SeO3 (50 μg/L of Se)
-
3-
SeIV+VI-T50: Mixed with 0.32 μM of SeVI with Na2SeO4 and 0.32 μM of SeIV with Na2SeO3 (50 μg/L of Se)
-
4-
SeVI-T10: 0.12 μM of SeVI with Na2SeO4 (10 μg/L of Se)
-
5-
SeIV-T10: 0.12 μM of SeIV with Na2SeO3 (10 μg/L of Se)
-
6-
SeIV+VI-T10: Mixed with 0.06 μM of SeVI with Na2SeO4 and 0.06 μM of SeIV with Na2SeO3 (10 μg/L of Se)
-
7-
C-T: two control treatments without Se
The experiment was maintained for a further 2 weeks following Se addition. After 2 weeks of growth under Se exposure, the plants (five for each treatment) were harvested and the roots rinsed 3 times with deionized water to remove all traces of nutrient solution. The Se concentration in this rinse water fell below the detection threshold of CRC-ICP-MS. The leaves, roots and stems (consisting of stalk and leaf sheaths) were then separated and weighed. The plant samples were freeze-dried, ground with an automatic agate mortar and pestle and weighed (dry weight or DW).
Element concentration
A suitable amount of powdered plant tissue (around 100 mg DW) was digested in 2 ml of HNO3 (70 %) and 1 ml H2O2 (30 %). Selenium concentrations in the digested tissues were obtained by inductively coupled plasma mass spectroscopy (CRC-ICP-MS, Thermo X Series II, LOD: 50 ng/L) and blank and reference material (White clover, BCR402) were included in each batch of samples.
LOD for Se in tissues is 0.01 μg/g. In the C-T plants, the median value of Se concentrations is 0.1 (0.09; 0.11) μg/g which remains very much lower for all Se concentrations in plants treated with Se.
Calculation of leaf area
Measuring leaf area is a non-destructive method of monitoring plant growth during experiments. The length and width of all the leaves on all the plantlets were measured to assess growth on the day of the transplant into the Plexiglas chamber; thereafter, measurements were taken weekly. Leaf area was calculated with the following formula (Fakorede et al. 1977):
Statistical analysis
Processing statistics are used to adjust the size of statistical samples; in our study, the number of individuals was less than 30 (five plants). We therefore used non-parametric statistical hypothesis tests. We determined the significance of the effect of treatment conditions with a Kruskal and Wallis Test or Mann and Whitney Test (bidirectional test with alpha equal to 0.05) and calculated the probability P of the difference between groups being random. P values of less than 5 % were considered statistically different. In the figures below, the results of the statistical tests are represented by the letters a, b and c.
Results
Biomass production and tolerance toward selenium
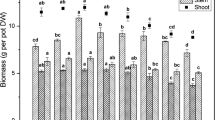
To monitor the canopy biomass for each of the seven treatments (C-T, SeIV-T, SeVI-T and SeIV+VI-T), leaf area was calculated (Fig. 1). In all treatment conditions, the change in leaf area was exponential and never reached a plateau. Whatever form (selenite or/and selenate) or concentration (10 or 50 μg/L) supplied, Se had no statistically negative effect on leaf area. Biomass production is presented in Fig. 2. The dry weights of the maize plants at the end of the experiment were not significantly different. Similarly, no difference in dry weights of the roots, stems or leaves was found among all the treatment conditions (data not shown).
Comparison of selenite and selenate uptake and accumulation in Zea mays
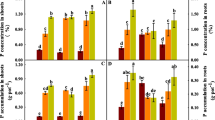
Se concentrations in the dry samples from a given plant were analyzed separately for each tissue type (roots, stems and leaves), the sum of which gave the total Se concentration for that plant. The median Se concentration of the plants is presented in Fig. 3. For both Se nutrient solution concentrations, Se plant concentrations for the SeIV-T (selenite treatments 2 and 5) were higher (188 % and 223 % in 10 μg/L and 50 μg/L respectively) than for the SeVI-T (selenate treatments 1 and 4). Figure 4 shows that Se concentrations in the different plant tissues strongly depended on the Se-forms supplied. Se concentrations in the roots and stems only were also higher for the SeIV-T compared to the SeVI-T. However, notwithstanding the inorganic form of Se supplied, Se concentrations in the leaves remained similar (around 0.9 μg/g in 10 μg/L treatments and 3.3 μg/g in 50 μg/L treatments). Translocation factors (concentration ratio of shoots to roots) (Table 1) were lower when the plants were treated with selenite than with selenate. The allocation of Se in plants is summarized in Fig. 5. For both SeVI-T and SeIV-T, Se allocations were independent of the Se concentrations in the nutrient solution. For the SeIV-T, Se content in roots was around 63 % of total plant Se whereas only 16 % and 20 % was found in leaves and stems respectively. However, for the SeVI-T, the allocation of Se among the 3 tissue types differed dramatically: the Se amount in leaves represented about 50 % of total plant Se compared to about 25 % in roots and stems.
Selenium concentrations (in μg/g DW) in whole maize plants in 6 different treatments (excluding controls): SeVI-T (in gray); SeIV-T (in dark gray); SeIV+VI-T (in white). A theoretical average ( ) was calculated from measured values in SeIV-T and SeVI-T. a, b, a* and b*: results of the Kruskal and Wallis test
) was calculated from measured values in SeIV-T and SeVI-T. a, b, a* and b*: results of the Kruskal and Wallis test
Selenium concentrations (in μg/g DW) in roots, stems or leaves of maize plants in 6 different treatments (excluding controls): SeVI-T (in gray); SeIV-T (in dark gray); SeIV+VI-T (in white A theoretical average ( ) was calculated from measured values in SeIV-T and SeVI-T. a, b, a* and b*: results of the Kruskal and Wallis test
) was calculated from measured values in SeIV-T and SeVI-T. a, b, a* and b*: results of the Kruskal and Wallis test
Se plant concentrations were significantly higher for the SeVI-T50, and SeIV-T50 than for the SeVI-T10 and SeIV-T10 (3.4 and 3.8 times respectively). Likewise, Se concentrations in different tissues increased with external Se concentrations (Fig. 6). In roots enriched with selenite, the rate of accumulation is about eight times faster compared to roots enriched with selenate, whereas in shoots, the rate of accumulation is similar in plants enriched with selenate and selenite.
Interaction between selenate and selenite
Se plant concentration (Fig. 3) for the SeIV-T treatments was higher (22 % in the 10 μg/L treatment and 111 % in the 50 μg/L treatment) than for the mixtures SeIV+VI-T. Inversely, Se plant concentrations for the SeIV+VI-T were higher (136 % in the 10 μg/L treatment and 53 % in the 50 μg/L treatment) than for to SeVI-T. Se plant concentrations are therefore best described as SeIV-T>SeIV+VI-T>SeVI-T whatever the concentration of Se supplied. As was the case for total plant concentrations, Se concentrations in roots and stems (Fig. 4) were higher for the SeIV-T than for the SeIV+VI-T, which in turn was higher than for the SeVI-T. On the other hand, leaf Se concentrations were similar for the SeIV+VI-T, SeVI-T and SeIV-T for both concentrations supplied. Translocation factors (Table 1) are best described as SeVI-T>SeIV+VI-T>SeIV-T for both concentrations of supplied Se. Se allocation (Fig. 5) for the SeIV+VI-T was similar to SeIV-T, Se being mainly stored in the roots; however, root Se content represented 61 % for the SeIV+VI-T10 and only 44 % for the SeIV+VI-T50.
For both Se concentrations in the nutrient solution, a theoretical value of Se (TV) was calculated (average and its variation) for the SeVI-T and SeIV-T treatments based on actual measured plant or tissues concentrations. This TV represented the concentration of Se in plants when there was no interaction between selenite and selenate and was compared to the co-exposure treatment results. In SeIV+VI-T10 plants, Se concentration did not differ from the TV, but in the SeIV+VI-T50 plants, it was 34 % lower than the TV. Such a feature is also observed in roots and stems: Se concentrations for the SeIV+VI-T10 were equal to the TVs; whereas for the SeIV+VI-T50, the concentrations were lower than the TVs (52 % and 30 % in roots and stems respectively).
Total plant Se concentration for the SeIV+VI-T50 was only 2.2 times higher compared to the Se concentration for the SeIV+VI-T10. This enrichment factor of SeIV+VI-T is significantly lower than in SeIV-T and SeVI-T plants. In SeIV+VI-T roots, the rate of accumulation was about four times slower than in SeIV-T roots but about twice faster than in SeVI-T roots; whereas in shoots, the rate of accumulation was similar in SeIV+VI-T and SeIV-T or SeVI-T plants (Fig. 6).
Discussion
Metabolic pathways of selenium in Zea mays
Leaf area characterizes the canopy of an ecosystem which is the preferred site for exchange between plants and the atmosphere. Biomass production is directly associated with light interception by the canopy, which is also related to leaf area (Gitelson et al. 2003; Maddonni and Otegui 1996). Changes in leaf area are characterized by: 1) a linear increase associated to the growth stage and biomass production by the plant, and 2) a stationary stage associated to the reproductive stage of plant during which biomass production is slowed, sometimes sharply (Gitelson et al. 2003; Maddonni and Otegui 1996). In this study, the maize plants were harvested during the growth stage before any decrease in biomass production. Based on the exponential change of leaf area and dry biomass measured in the present experimentations, we found that neither selenate nor selenite is harmful to plant growth at concentrations not exceeding 0.63 μM. Our results are similar to previous studies which showed that at low Se concentrations (2 μM of selenite), and whatever form of Se supplied, the biomass production of strawberry clover and perennial ryegrass (Hopper and Parker 1999) were not affected. However, at higher concentrations (Hopper and Parker 1999; Ximenez-Embun et al. 2004), both selenite and selenate decrease biomass production, i.e. 20 % for white lupine and 40 % for sunflowers in a selenite treatment (12 μM of selenite) (Ximenez-Embun et al. 2004) and 10 % for strawberry clover in a selenate treatment (10 μM of selenate) (Hopper and Parker 1999).
In this discussion, Se volatilization has not been taken into account. In fact, in another experiment (data not presented) with 12 μM of Se in the nutrient solution (i.e. 50 or 100 times higher than the concentration used in the present study), we found that Zea mays does not significantly volatize Se.
Selenium accumulation in plants depends on the species of Se available. In the literature, results on Se metabolism in plants are not homogenous. With 20 μM of Se added, Se accumulation from selenate was two to four times higher than from selenite in Indian mustard, broccoli, sugarbeet, white lupine and sunflowers (De Souza et al. 1998; Ximenez-Embun et al. 2004; Zayed et al. 1998). Zayed et al. (1998) and Li et al. (2008) found a similar accumulation for two Se forms in rice (supplied with 20 μM of Se) and wheat (supplied with 10 μM of Se). According to Zhang et al. (2003), Se accumulation from selenite is higher than from selenate in soybean, independent of Se concentrations in the nutrient solution. According to Li et al. (2008), this discrepancy is mainly due to differences in phosphate concentrations in the nutrient solution: high concentrations of phosphate (i.e. 100 μM) must have suppressed the selenite uptake. However, in our study, though phosphate concentrations were as high as in most hydroponic experiments (0.95 mM of phosphate), Se accumulation in plants supplied with selenite was higher than with selenate. Our results clearly show that at low concentrations of Se in the hydroponic solution, selenite is more accumulated than selenate in Zea mays, without any detrimental effects on plant growth. Maize, like ferns (Srivastava et al. 2007) and cereals, varies in its ability to accumulate Se from different Se forms.
As for the distribution of Se within the tissues of the plants, at low concentrations of Se in the nutrient solution, our data show that the activity of root absorption is eight times lower with selenate than with selenite. The mechanism of selenate absorption via sulfate transporters has been well documented. Two types of transporters are involved in the process of root sulfate absorption: high affinity transporters (Km for sulfate of 7–10 μM), and low affinity transporters (Km for sulfate 100 μM). Studies on the over-expression of high-affinity or low-affinity transporter genes have demonstrated that only the over-expression of high transporters increases selenate accumulation in Indian mustard (Terry et al. 2000). These results support the view that selenate uptake is mediated by high-affinity sulfate transporters in plants. This expression gene is strongly up regulated in plants deprived of external sulfate: low sulfate conditions and/or the presence of selenate in the nutrient solution induced an increase in high-affinity transporter gene expression (Terry et al. 2000; Yoshimoto et al. 2002). In our experiment, Zea mays was grown in normal nutrient conditions (0.72 mM sulfate); soil solutions typically contain about 0.5 mM sulphate (Hopper and Parker 1999). The mechanisms of selenite uptake have not been documented as well as for selenate and are less well understood. Several studies have suggested that plant uptake of selenite is via passive diffusion (Arvy 1993; Sors et al. 2005); but this hypothesis has been refuted by Li et al. (2008) and Hopper and Parker (1999) who support the view that selenite uptake is active but is inhibited by phosphate and metabolic inhibitors. In the present study, despite the high phosphate concentration in nutrient solution (0.95 mM of phosphate), selenite uptake remains higher than selenate.
For leaves, the results are totally different: for both inorganic forms of Se supplied, the Se concentrations in the leaves are similar and vary only with Se concentration levels in the nutrient solution. Similar results have been reported by Zhang et al. (2003). These data provide evidence for the singularity of Se pathways in Zea mays supplied with low Se concentrations: there appears to be a nearly exclusive form of Se which is translocated into leaf blades, probably an organic form metabolized in the roots and/or leaf sheaths. Selenate is less metabolized into an organic Se form than selenite. Indeed, the reduction of selenate into selenite is the rate-limiting step in selenate metabolism in plants (De Souza et al. 1998; Li et al. 2008; Terry et al. 2000). In Zea mays at low concentrations of Se supplied, organoselenium compounds, which are less toxic (Terry et al. 2000), are metabolized and stored in roots and/or leaf sheaths more effectively when plants treated with selenite as opposed to selenate. According to the literature (De Souza et al. 1998; Li et al. 2008; Terry et al. 2000), Se translocation from root to shoot was higher in plants enriched with selenate. In fact, in selenate supplied plants, the translocation factor varied between 1.5 and 17 but in selenite supplied plants, it was consistently less than 0.5. In the literature, this has been explained by the high mobility of selenate compared to selenite resulting in high Se levels in leaves. However, in this study, Se allocation was mainly controlled or limited by root absorption.
Interactions between selenite and selenate in Zea mays
Interactions between selenite and selenate depend on the Se concentration in the nutrient solution. With 0.12 μM of Se added, total plant accumulation, and more particularly root absorption, were not affected by either Se form. With a higher concentration of Se supplied (0.63 μM), selenate inhibited the accumulation of selenite. The selenium concentrations in roots as well as the rate of Se accumulation show that this decrease was mainly due to the inhibition of root selenite absorption. This can be explained by the metabolism of selenate in plants : the reduction of the selenate into selenite is a limiting step (Pilon-Smits et al. 1999). At 0.12 μM for both Se forms supplied, the saturation threshold of the enzyme is not reached; so the rate of reduction is high: selenate is reduced into selenite and then to organoselenium compounds. At 0.63 μM for both Se forms supplied, the enzyme may not completely reduce selenate uptake by the roots; in this case, selenate would have a negative effect (direct and/or indirect) on root selenite absorption.
This result is not consistence with Li et al. (2008) who conclude that the presence of selenite decreases selenate uptake and xylem transport. The two studies cannot be compared; in fact, Li et al. (2008) used high concentrations of Se supplied (10 μM) which therefore induced higher Se accumulation in plants with supplied selenate than with supplied selenite.
Selenium diet
Our findings indicate that even at low concentrations and with only slight increases in Se in the nutrient solution, Se content in maize (leaves, steams and roots) could be enhanced without toxic effect. Such slight changes should therefore be taken into account to improve Se density in mammals’ diets via the food chain.
Following root uptake, the Se is redistributed in varying degrees to the aerial parts of the plant which are often of greater interest in the food chain. Our study shows that selenite supplementing is the most effective way to accumulate Se in the shoots (part of the maize plant consumed as forage). Moreover, according to the literature (Li et al. 2008; Terry et al. 2000; Ximenez-Embun et al. 2004), selenoamino-acid production is high (about 40 % of the total Se in the plant). These organic Se forms are more efficiently assimilated by animals and humans than inorganic forms (Rayman 2008). However, interactions between selenite and selenate exist and influence their absorption. Their assimilation also greatly depends on the Se concentrations supplied. Maize ranks first among cereals produced worldwide with 824 million tons in 2010/2011 (Le Stum 2011); maize plants are consumed as grains or used as forage for animals. It therefore seems crucial to investigate the possible enrichment of Zea mays to counteract Se deficiencies in mammal’s diets.
To this end, the hydroponics experiment provided a useful starting point without extrapolating results from hydroponics culture to field conditions. Nevertheless, to date the rare results on selenium accumulation in maize realized in field, concluded that Se accumulation in Zea mays is generally weak, i.e. between 50 and 500 ng/g, in Se poor soils or with fly-ash amendment (Chilimba et al. 2011; Mbagwu 1983). In the present study, even with the low Se concentration chosen in the nutrient solution, maize could accumulate more than 500 ng/g in all the vegetative tissues of the plants.
Conclusion
At low concentrations of Se supplied (i.e. 0.12 μM and 0.63 μM of Se), plant biomass production does not decrease. Se plant concentrations are higher with selenite rather than selenate added to the nutrient solution. In the selenate supplied experiment, Se was principally translocated to leaves whereas in the selenite supplied experiment, it mainly accumulated in the roots. Moreover, at 0.63 μM of Se, selenate seems to inhibit selenite root absorption. The difference between our results and the literature can mainly be explained by the difference in Se concentrations in the nutrient solution used: typically, supplied Se concentrations are high; whereas in our study, we kept Se concentrations close to levels typically found in soil solutions.
Abbreviations
- DW:
-
Dry weight
- CRC-ICP-MS:
-
Collision/reaction cell—Inductively coupled plasma mass spectrosmetry
- LOD:
-
Limit of detection
- SD:
-
Standard deviation
- FeEDDHA:
-
Iron- Ethylenediaminedi-Q-hydroxyphenlyacetic acid
References
Arvy M (1993) Selenate and selenite uptake and translocation in bean plants (Phaseolus vulgaris). J Exp Bot 44:1083–1087
Broadley MR, White PJ, Bryson RJ, Meacham MC, Bowen HC, Johnson SE, Hawkesford MJ, McGrath SP, Zhao FJ, Breward N, Harriman M, Tucker M (2006) Biofortification of UK food crops with selenium. Proc Nutr Soc 65:169–181
Brown TA, Shrift A (1982) Selenium: toxicity and tolerance in higher plants. Biol Rev 57:59–84
Chilimba A, Young S, Black C, Rogerson K, Ander E, Watts M, Lammel J, Broadley M (2011) Maize grain and soil surveys reveal suboptimal dietary selenium intake is widespread in Malawi. Sci Rep 72
Cruz-Jimenez G, Peralta-Vodea JR, de la Rosa G, Meitzner G, Parsons JG, Gardea-Torresdey JL (2005) Effect of sulfate on selenium uptake and chemical speciation in Convolvulus arvensis L. Environ Chem 2:100–107
De Souza MP, Pilon-Smits EAH, Mel Lytle C, Hwang S, Tai J, Honma TSU, Yeh L, Terry N (1998) Rate-Limiting steps in selenium assimilation and volatilization by Indian Mustard. Plant Physiol 117:1487–1494
Dhillon K, Dhillon S (2000) Selenium accumulation by sequentially grown wheat and rice as influenced by gypsum application in seleniferous soil. Plant Soil 227:243–248
Eshdat Y, Holland D, Faltin Z, Ben-Hayyim G (1997) Plant glutathione peroxidases. Physiol Plant 100:234–249
Eurola MH, Ekholm PI, Ylinen ME, Koivistoinen PE, Varo PT (1991) Selenium in Finnish foods after beginning the use of selenate supplemented fertilizers. J Sci Food Agric 56:57–70
Fakorede MAD, Mulamba NN, Mock JJ (1977) A comparative study of methods used for estimating leaf area of mays (Zea mays L.) from non destructive measurements. Maydica 22:37–46
Gissel-Nielsen G, Gupta UC, Lamand M, Westermarck T (1984) Selenium in soils and plants and its importance in livestock and human nutrition. Adv Agron 37:397–460
Gitelson AA, Vina A, Arkebauer TJ, Rundquist DC, Keydan G, Leavitt B (2003) Remote estimation of leaf area index and green leaf biomass in maize canopies. Geophys Res Lett 30:1248–1251
Hopper JL, Parker DR (1999) Plant avaibility of selenite and selenate as influenced by the competing ions phosphate and sulfate. Plant Soil 210:199–207
Läuchli A (1993) Selenium in plants: uptake, functions, and environmental toxicity. Bot Acta 106:455–468
Le Stum H (2011) Association Générale des Producteurs de Blé et autres céréales. IOP Publishing AGPB. http://www.agpb.fr/fr/chiffre/recolte_monde.asp. Accessed 13 december 2011
Li HF, McGrath SP, Zhao FJ (2008) Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol 178:92–102
Liu K, Gu Z (2009) Selenium accumulation in different brown rice cultivars and its distribution in fractions. J Agric Food Chem 57:695–700
Maddonni GA, Otegui ME (1996) Leaf area, light interception, and crop development in maize. Field Crops Res 48:81–87
Mazej D, Osvald J, Stibilj V (2008) Selenium species in leaves of chicory, dandelion, lamb’s lettuce and parsley. Food Chem 107:75–83
Mbagwu J (1983) Selenium concentrations in crops grown on low-selenium soils as affected by fly-ash amendment. Plant Soil 74:75–81
Pilon-Smits E, Hwang S, Lytle C, Zhu Y, Tai J, Bravo R, Chen Y, Leustek T, Terry N (1999) Overexpression of ATP sulfurylase in india Mustard leads to increased selenate uptake, reduction, and tolerance. Plant Physiol 119:123–132
Rayman MP (2008) Food-chain selenium and human health: emphasis on intake. Br J Nutr 100:254–268
Shibagaki N, Rose A, McDermott JP, Fujiwara T, Hayashi H, Yoneyama T, Davies JP (2002) Selenate-resistant mutants of Arabidopsis thaliana identify Sultr1;2, a sulfate transporter required for efficient transport of sulfate into roots. Plant J 29:475–486
Sors TG, Ellis DR, Salt DE (2005) Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth Res 86:373–389
Srivastava M, Ma QL, Cotruvo JA (2007) Uptake and distribution of selenium in different fern species. Int J Phytoremediat 7:33–42
Terry N, Zayed A (1994) Selenium volatilization by plants. In: Frankenberger JR, Benson S (eds) Selenium in the environment. Marcel Dekker, New York, pp 343–368
Terry N, Zayed AM, De Souza MP, Tarun AS (2000) Selenium in higher plants. Annu Rev Plant Physiol Plant Mol Biol 51:401–432
Whanger PC (2002) Selenocompounds in plants and animals and their biological significance. J Am Coll Nutr 21:223–232
White PJ, Bowen HC, Parmaguru P, Fritz M, Spracklen WP, Spiby RE, Meacham MC, Mead A, Harriman M, Trueman LJ, Smith BM, Thomas B, Broadley MR (2004) Interactions between selenium and sulfur nutrition in Arabidopsis thaliana. J Exp Bot 55:1927–1937
World Health Organization, Food and Agriculture Organisation and International Atomic Energy Agency expert group (1996) Selenium. In: WHO (ed) Trace elements in human nutrition and health. WHO, Geneva, pp 105–122
Ximenez-Embun P, Alonso I, Madrid-Albarran Y, Camara C (2004) Establishment of selenium uptake and species distribution in lupine, india mustard and sunflower plants. J Agric Food Chem 52:832–838
Yoshimoto N, Takahashi H, Smith FW, Yamaya T, Saito K (2002) Two distinct high-affinity sulfate transporters with different inducibilities mediate uptake of sulfate in Arabidopsis roots. Plant J 29:465–473
Zayed A, Lytle CM, Terry N (1998) Accumulation and volatilization of different chemical species of selenium by plants. Planta 206:284–292
Zhang Y, Pan G, Chen J, Hu Q (2003) Uptake and transport of selenite and selenate by soybean seedlings of two genotypes. Plant Soil 253:437–443
Aknowledgments
The authors gratefully acknowledge Victoria Moore from CFPPA Le Chesnoy, Amilly (France) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Yong Chao Liang.
Rights and permissions
About this article
Cite this article
Longchamp, M., Angeli, N. & Castrec-Rouelle, M. Selenium uptake in Zea mays supplied with selenate or selenite under hydroponic conditions. Plant Soil 362, 107–117 (2013). https://doi.org/10.1007/s11104-012-1259-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1259-7