Abstract
Background and Aims
Recently, the truffle species Tuber lyonii Butters was found to be dominant in ectomycorrhizal (EcM) fungal communities of cultivated pecan (Carya illinoinensis (Wangenh.) K. Koch). Many truffle fungi exhibit the trait of effectively colonizing plant roots via spores. We hypothesized that T. lyonii would be well represented in the spore bank of pecan orchard soils where it is found.
Methods
We used axenically-grown pecan seedlings as trap-plants to bait for EcM associates in soils collected beneath truffle-producing pecan trees. EcM fungi on seedlings were characterized through rDNA sequencing and were compared to EcM communities of adult trees in these orchards.
Results
Tuber lyonii mycorrhizas were well formed on seedlings inoculated with truffle spores, but were limited to just a few of the trap-plants grown in field soils. We compared EcM communities of adult pecan orchard trees to those on trap-plants and found distinct communities on each, with a high degree of similarity at the ordinal but not species level.
Conclusions
Although species of Pezizales are abundant in pecan EcM communities and as propagules in their soil spore banks, only a low level of T. lyonii was detected in soil spore banks beneath orchard trees naturally colonized by T. lyonii. Other factors including land-use history or orchard management may better explain this truffle species presence and abundance in pecan EcM communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seed banks are a significant, yet stochastic, variable in plant community assembly after disturbances (Warr et al. 1993; Ward and Thornton 2000). Variation in the persistence of plant seeds (e.g. the ability of seeds to remain dormant and viable until resources become available) is ecologically important and can lead to re-colonization priority effects whereby some species quickly re-colonize the landscape and exclude other species (Robinson and Dickerson 1987). Priority effects have been shown to influence the assembly of plant (Ward and Thornton 2000), animal (Louette et al. 2008), and fungal communities (Kennedy et al. 2009; Hausmann and Hawkes 2010).
Analogous to soil seed banks, fungal spore banks are reservoirs of dormant fungal propagules (Baar et al. 1999). They are a functionally important biotic component of terrestrial ecosystems, and may remain quiescent in soil for years and possibly decades or longer (Bruns et al. 2009). Pioneer species of ectomycorrhizal (EcM) fungi are characterized by a high capacity for spore dormancy and are well-suited for dispersal (Fox 1983; Newton 1992; Visser 1995; Jumpponen et al. 1999). These fungi are often disturbance tolerant and can be important in the process of primary plant colonization (Jumpponen 2003; Nara 2006) as well as re-colonization after disturbances such as fires, hurricanes, landslides and clear-cutting (Horton et al. 1998; Izzo et al. 2005; Grubisha et al. 2007). Propagules of pioneer EcM species often remain dormant, but viable, in soil spore banks. They may activate via signals from growing roots during plant recolonization, giving a priority effect advantage (Kennedy and Bruns 2005).
Many truffle-forming fungi are pioneer species and are well-adapted to dispersal via animal mycophagy (Izzo et al. 2005; Frank et al. 2009). They are also known for establishing EcM via resistant propagules (Taylor and Bruns 1999; Frank et al. 2009). This may be one ecological explanation why truffle species such as Tuber melanosporum Vittad. and T. aestivum Vittad. have been successfully cultivated whereas cultivation of EcM fungi that are more dependent on mycelial spread for colonizing roots (e.g. Boletus edulis Bull., Cantharellus spp. and Tricholoma spp.) have generally not been successful (Yun and Hall 2004; Wedén et al. 2009). Truffle-forming fungi often fruit belowground and generally retain their spores inside sporocarps (Trappe 1975). Because mycophagy is an important agent in truffle spore dispersal (Carey et al. 2002; Frank et al. 2009) truffle spores must withstand passage through digestive tracts of animals and retain their infective abilities. Truffle spore cell walls are generally thick and resistant to changes in pH, moisture, and temperature; excellent adaptations for colonizing roots via spores (Johnson 1996).
Recently, we found that EcM truffle-forming fungi (both Ascomycota and Basidiomycota) are prevalent in belowground EcM communities of cultivated pecan (Carya illinoinensis) in Georgia, USA (Bonito et al. 2011a). We were particularly interested to discover that the commercially valued truffle species Tuber lyonii Butters was one of the most abundant species on EcM roots of adult pecan trees. Given the reduced density of roots in pecan orchards compared to pecan trees in native mixed forests, we hypothesized that spores (meiotic propagules resistant to environmental stress) might be a more important mode for root colonization by EcM fungi in orchards, rather than mycelial (mitotic) spread which may be more adaptive in forested habitats where root densities and inoculum levels are higher (Newton 1992). We used ‘trap-plant’ assays to test whether T. lyonii is present in the spore bank of soils where the pecan truffle commonly fruits. In these bioassays, axenically grown pecan seedlings were used as bait for EcM fungi. We used soils collected under truffle-producing trees and air-dried them to select for resistant EcM fungal spores, an approach whose efficacy has previously been demonstrated (Baar et al. 1999; Rusca et al. 2006). We hypothesized that T. lyonii and other pioneer species would account for a large proportion of the EcM diversity on pecan seedlings. Finally, we compare the EcM communities of pecan trap-plants to EcM communities on adult trees in these orchards.
Materials and methods
Soil sampling
A previous study confirmed that T. lyonii was present on the roots of pecan trees at three pecan orchards in Georgia, USA: Magnolia+ (with truffle production), Magnolia− (without truffle production), and Pine Knoll+ (with truffle production) (Bonito et al. 2011a). Characteristics of these orchards and their soils have been published (Bonito et al. 2011a). Soils used in this study were collected in early fall beneath many of the same trees previously sampled, and other trees supporting the growth of truffles. From each orchard we took samples beneath five trees. Three 1-L soil samples were taken under the canopy of each tree one meter from the tree base at equidistant points (e.g. 0°, 122°, 243°) using a trowel to sample soil to a depth of ca. 10 cm. After homogenizing each of the 45 soils, passing them through a 1-mm screen to remove rocks and debris, and air drying for three months in paper bags, soils were amended with sterile sand at a 1:1 soil to sand ratio. We planted axenically raised pecan seedlings into these soil mixtures (see below).
Pecan germination and inoculations
Pecan seedlings were germinated as described by Bonito et al. (2011a,b), and the same methods for inoculating seedlings with T. lyonii spores were used except that potting media was mixed 50% with sterile sand. A total of five seedlings were inoculated with T. lyonii sporocarps as positive controls. For a comparable outlier treatment, three seedlings were inoculated with soils from a recently logged pine forest that is part of the well-characterized Calhoun Experimental Forest (Markewitz et al. 1998; Lauber et al. 2008), a long-term soil research site located in Sumter National Forest, South Carolina, USA. Five negative control seedlings were left uninoculated. All seedlings were grown in individual “cone-tainer” planting cells containing a soil volume of ca. 250 ml (Stuewe & Sons, Inc.,Tangent, OR, USA). Thus, the 58 seedlings used in this study included 45 seedling with orchard soil treatments, eight positive control seedlings, and five negative control seedlings.
Growth conditions & harvesting of ectomycorrhizas
Pecan seedlings were grown for five-months at room temperature (~ 25°C) under fluorescent lights at an intensity of ca.125 μmol photons/m2/second and 18 h of light per day. They were watered every 2–3 days with deionized water and treated once a week with 1:10 Hoagland solution (Bonito et al. 2011a,b) to maintain minimal plant nutritional requirements. Upon harvesting, attached soil was shaken off of the seedling roots, which were then cleaned by successive rounds of soaking and spraying under a fine stream of tap water.
Molecular methods and data analyses
Washed roots were observed under a stereo-microscope. Three individual ectomycorrhizal root tips from different parts of the root system and representing the observed EcM morphotype diversity were selected from each seedling. The EcM roots were photographed with a Nikon Coolpix 990 digital camera and placed in 2X CTAB buffer. DNA was extracted with a CTAB-chloroform protocol as described by Bonito et al. (2010). Both ITS and LSU rDNA regions were amplified with a combination of forward primers ITS1f or 5.8SR and reverse primers ITS4 or LR3. Three EcM roots were sequenced from each seedling. Representative sequences of each molecular operational taxonomic unit (mOTU) have been accessioned in Genbank as JN569340-JN569360 (Table 1).
Molecular OTUs, approximates of EcM fungal species, were conservatively assigned at a 97% similarity level for ITS (Smith et al. 2007a,b; Peay et al. 2008) and a 99% similarity level for LSU rDNA (Bonito et al. 2010). They were identified to the genus or family level by comparison against the NCBI BLAST nucleotide database. Rank abundance was calculated across all pecan orchard samples.
We were interested in the phylogenetic placement of taxa belonging to the Pezizales because they were well-represented in our dataset. BLAST results only provided limited taxonomic resolution, therefore, sequences from these taxa were aligned with the software MUSCLE (Edgar 2004) to sequences of taxa with high sequence affinity (based on BLAST similarity scores). Alignments were checked by eye in MacClade 4.0.6 (Maddison and Maddison 2002) and ambiguously aligned regions were excluded. Heuristic searches based on maximum parsimony and 5000 bootstrap replicates were carried out with PAUP* 4.0 (Swofford 2002).
Because ectomycorrhizal communities within these pecan orchards were previously sampled, proportions of shared taxa between trap-plant seedlings and adult orchard trees were calculated at both species and ordinal levels. Each individual EcM root is assumed to represent a discrete individual arising from a single meiospore, therefore each EcM tip was treated as an independent sample unit. Ectomycorrhizal communities within fields and age class (adults vs. seedlings) were compared with Shannon diversity and abundance-based species estimators; to assess β-diversity, Bray-Curtis dissimilarity values for EcM communities between fields and age class (adults vs. seedlings) were calculated and visualized through principle coordinate analysis in QIIME (Caporaso et al. 2010).
Results
Plant survival and formation of ectomycorrhizas
Pecan seedlings in this study had a moderately high germination rate (75%) and survival rate (88%). After five-months, the roots of all seedlings were visually assessed under the stereoscope. Most seedlings had developed EcM, although individual seedlings were generally colonized by only one to three EcM morphotypes (Fig. 1).
Molecular results
Tuber lyonii was detected on only three trap-plants and in soils of two orchards. It did not appear to be particularly common on any of the root systems. In contrast, T. lyonii colonized at least 50% of the root tips of seedlings that had been inoculated with T. lyonii spores. There identification was confirmed through DNA sequencing of individual EcM.
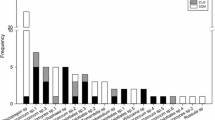
From 135 sequenced EcM root tips we recovered 21 mOTUs of EcM fungi (Table 1). Two mOTUs were only found in the outlier forest soils of the Calhoun experimental forest in South Carolina. Most mOTUs were clearly assignable to known EcM fungal groups (Table 1). More than half of the EcM taxa on trap-plants were members of the order Pezizales (Ascomycota) (Fig. 2). Five of these taxa belong to a lineage of Pezizaceae that is currently known only from environmental sampling of EcM roots (Fig. 3 and Healy, Bonito & Smith unpublished data). Aside from Sphaerosporella, all of the Pezizales ECM morphotypes exhibited limited extramatrical hyphae growth and are characterized as contact and short-distance exploration EcM types (Fig. 1) (Agerer 2001). More than one quarter of the species belonged to the closely-related genera Hymenogaster and Hebeloma (Strophariaceae, Agaricales) (Fig. 4). These taxa had moderate extrametrical hyphae and are considered short-distance exploration and medium-fringe EcM types (Fig. 1) (Agerer 2001). Nearly half of the mOTUs were shared between sites (Fig. 5a). The six most abundant mOTUs, representing 77% of all sequences, were recovered from multiple orchards (Fig. 4). The two most commonly detected mOTUs represented 49% of the sequences and belonged to an unresolved Pezizaceae species (Fig. 4) and a Hymenogaster species. These two mOTUs were recovered from soils of all three orchards (Table 1; Fig. 4). The greatest species richness (13 mOTUs) was found on seedlings grown in Pine Knoll soils although seven of these taxa were also recovered from seedlings grown in Magnolia+ soils (Fig. 5a).
Ordinal level comparisons between ectomycorrhizal communities of trap-plant pecan seedlings and adult pecan trees. Ectomycorrhizal species of Pezizales were well represented on both seedling and adult trees. Although many of the dominant orders of ectomycorrhizal fungi were similar on seedlings and adult trees, the orders Russulales, Sebacinales, and Eurotiales (i.e. Elaphomyces sp.) were only detected on adult trees
One of 18 most parsimonious trees showing the phylogenetic placement of the ten Pezizalean taxa detect as ectomycorrhizas on pecan trap-plants. These taxa belong to five lineages within the Pezizales believed to have gained the ectomycorrhizal habit independently, including a lineage only known through environmental sequences. Lineages labeled on the right. The analysis included 27 taxa, 618 characters (217 of which were parsimony-informative), and was mid-point rooted. Bootstrap support values >70 are deemed significant and values based on 5000 bootstrap relicates are shown above nodes with thickened branches
Rank abundance of ectomycorrhizal species / molecular operational taxonomic units (mOTUs) detected on pecan trap-plant seedlings. Nineteen mOTUs were detected on pecan seedlings grown in air-dried soils from orchards. The two most abundant EcM species on seedlings (e.g. Pezizaceae sp1 & Hymenogaster sp2) were recovered from all three assayed soils
Venn diagram comparing ectomycorrhizal molecular operational taxonomic units (mOTUs) recovered from (A) seedlings grown in three different pecan orchard soils and (B) trap-plant seedlings and adult orchard trees. The gray circles denote the two orchards where T. lyonii was detected on ectomycorrhizas of adult trees. Although the Magnolia+ and Magnolia− orchards are closest to each other in distance, more fungal species were shared between the Pine Knoll+ and Magnolia+ orchard soils. Note the low amount of overlap at the species-level between seedling trap-plants and adult trees
Only four of the 19 ectomycorrhizal taxa on seedlings in this experiment were previously sequenced from adult trees in the same orchards (Fig. 5b, Bonito et al. 2011a). Ordination analysis (Fig. 6) showed ectomycorrhizal communities on seedlings clustered tightly together in species space. This seedling fungal community was distinct from ectomycorrhizal communities of adult trees (Fig. 6). Lower Shannon diversity values were generally observed for seedlings compared to adults (Table 2).
Seedlings inoculated with outlier soils from a recently logged pine forest had low levels of EcM colonization and poor growth and survival. Cenococcum geophilum was the most common species on these seedlings, and the taxa Pyronemataceae sp3 and Pezizaceae sp5 were also detected (Table 1). No ectomycorrhizas were observed on the three surviving negative-control seedlings. These seedlings were grown only in sand and had stunted growth and visual signs of nutrient deprivation.
Discussion
In a previous study we found that the commercially valuable truffle T. lyonii is dominant on EcM roots in pecan orchards (Bonito et al. 2011a). Our hypothesis that T. lyonii is a major component of the resistant EcM propagule community in pecan orchards is not supported by the results of the trap-plant experiment presented here. We predicted that T. lyonii would account for a large portion of the EcM fungal community on pecan bait seedlings grown in soils from under trees colonized by T. lyonii, because most Tuber species are well-adapted for EcM colonization by spores (Parlade et al. 1996; Mamoun and Oliver 1997). Although T. lyonii was detected on seedlings grown in two out of the three orchard soils, it was only detected on a total of three seedlings. Thus, T. lyonii may not be as common in the spore bank in these orchard soils as we predicted or perhaps was out-competed by other EcM taxa. It may be that T. lyonii spore deposits are patchy within pecan orchards or our soil samples contained few or no viable T. lyonii spores at the time of sampling. It is important to note that seedlings directly inoculated with spores from T. lyonii sporocarps readily colonized EcM roots, indicating that this truffle has the capacity to colonize seedlings via spores when inoculum is sufficient and conditions are favorable. Tuber lyonii may also be able to colonize new roots via mycelial spread. However, given that their EcM generally lack emanating hyphae and rhizomorphs (traits adaptive for colonizing newly formed roots) we predict that they are not particularly effective at colonizing roots in this mode (Agerer 2001). Some Tuber species have been shown to produce asexual mitospores (Urban et al. 2004) . Such spores could have a role in dispersal and ectomycorrhizal formation, but it is not yet known whether the T. lyonii life cycle includes an asexual phase. Future studies should also account for the effects of ectomycorrhizal competition on the colonization of roots by T. lyonii.
Many factors can influence spore-bank composition and the reasons for the low colonization by T. lyonii on seedlings are unclear. We air-dried our soils for three month to select for resistant EcM propagules. This treatment seems to have successfully selected spore bank fungi given that the EcM community on seedlings was dominated by taxonomic groups known to germinate and readily establish via spores (e.g. Strophariaceae, Thelephoraceae, Pezizales) (Baar et al. 1999; Nara 2009). These included putative truffle species (e.g. Pezizaceae sp1, Hymenogaster sp1) as well as taxonomic groups of EcM fungi (e.g. Thelephoraceae, Pyronemataceae) common to disturbed ecosystems. Previous spore bank studies in natural ecosystems have also found that spores of truffle (e.g. Tuber, Rhizopogon) and non-truffle fungi (e.g. Hebeloma, Thelephora) persist in soil and are effective at colonizing seedlings (Baar et al. 1999; Taylor and Bruns 1999; Lilleskov and Bruns 2005; Nara 2009).
The commonly detected EcM taxa appear to be successful competitors and widely distributed in the spore banks of pecan orchards. It is possible that these taxa may have outcompeted T. lyonii in our bioassays. Although competition with other species may partially explain the poor performance of T. lyonii on seedlings, abiotic factors may also have played a role. Variables such as moisture, heat, pH and nutrient availability act as ecological filters and can alter the viability and assembly of fungi from spore banks (Peay et al. 2009).
EcM community comparisons between trap-plants in this study and adult trees in the same orchards from a previous study (Bonito et al. 2011a) showed that EcM communities were very similar at the ordinal level (Fig. 2). However, there were few fungal species in common between the two studies (4 species or 6.6%—Fig. 5). One exception, Pezizaceae sp1., was common on seedlings and adult trees, suggesting it is a widely dispersed symbiont that is common in Georgia pecan fields. Taylor and Bruns (1999) found a similarly low species overlap when they compared EcM communities of Pinus muricata trees before a wildfire and seedling trap-plants sampled after the fire. We hypothesize that differences between adult and seedling EcM communities are due largely to adaptive traits of the EcM fungi themselves; some fungi are better at colonizing EcM roots via spores while other taxa colonize roots more effectively through mycelial spread (Agerer 2001; Kjoller 2006). Although seedlings and adult plants are presumed to be functionally equivalent, seedlings fix less carbon than adult trees and have fewer roots available for EcM colonization. These factors undoubtedly constrain the amount of carbon and niche space available for EcM fungal symbionts and may alter EcM community dynamics and resource allocation to seedlings (Simard et al. 1997).
It is important to note that over half of the species detected in this study belonged to the Pezizales and species in this order were also common in EcM communities on adult trees (Fig. 2, Bonito et al 2011a). For reasons that are not yet clear, the mature trees were heavily colonized by Tuber species whereas seedlings were more often colonized by EcM symbionts in the Pezizaceae and Pyronemataceae. Ectomycorrhizal associations of Pezizales have not been well studied, but we do know that there are at least seven divergent Pezizales EcM lineages and most appear to be well-adapted to disturbed habitats (Tedersoo et al. 2010). Although species of Pezizales are generally not dominant in EcM communities of mesic forests (Tedersoo et al. 2006), they are often frequent and diverse after fires (Warcup 1990; Fujimura et al. 2005), at forest edges (Dickie and Reich 2005), in seasonally dry forests and woodlands (Gehring et al. 1998; Smith et al. 2007a,b), at high-altitude sites (Bidartondo et al. 2001), and in EcM studies of seedlings (Warcup 1991; Peay et al. 2009). All of these habitats and ecological scenarios have several key aspects in common: 1) exposed soil with scant or absent litter, 2) lower than average EcM host root density, 3) neutral or basic soil pH, and 4) physical disturbance of the soil surface. We expect that these factors are important for the success of EcM Pezizales in general and may help to explain the high proportion of Pezizales found on EcM roots of pecan plants. Pezizales may respond favorably to the manicured habitats of pecan orchards, where trees are uniformly spaced, soils are regularly disturbed and limed and leaf litter is minimized (Sparks 1976; Bonito et al. 2011a). The reason for the common occurrence of Tuber species on adult pecan trees with relatively low success on trap-plant seedlings is still unclear. More research will be needed to determine the causes for this phenomenon and to more fully elucidate the dynamics of T. lyonii in spore banks.
Abbreviations
- EcM:
-
ectomycorrhizal
References
Agerer R (2001) Exploration types of ectomycorrhizae—a proposal to classify ectomycorrhizal mycelial systems according to their patterns of differentiation and putative ecological importance. Mycorrhiza 11:107–114
Baar J, Horton TR, Kretzer AM, Bruns TD (1999) Mycorrhizal colonization of Pinus muricata from resistant propagules after a stand-replacing wildfire. New Phytol 143:409–418
Bidartondo MI, Baar J, Bruns TD (2001) Low ectomycorrhizal inoculum potential and diversity from soils in and near ancient forests of bristlecone pine (Pinus longaeva). Can J Bot 79:293–299
Bonito G, Isikhuemhen OS, Vilgalys R (2010) Identification of fungi associated with municipal compost using DNA-based techniques. Bioresour Technol 101:1021–1027
Bonito G, Brenneman T, Vilgalys R (2011a) Ectomycorrhizal fungal diversity in orchards of cultivated pecan (Carya illinoinensis; Juglandaceae). Mycorrhiza 21:601–612
Bonito G, Trappe JM, Donovan S, Vilgalys R (2011b) The Asian black truffle Tuber indicum can form ectomycorrhizas with North American host plants and complete its life cycle in non-native soils. Funct Ecol 4:83–93
Bruns TD, Peay KG, Boynton PJ, Grubisha LC, Hynson NA, Nguyen NH, Rosenstock NP (2009) Inoculum potential of Rhizopogon spores increases with time over the first 4 yr of a 99-yr spore burial experiment. New Phytol 181:463–470
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Tumbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) Qiime allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Carey AB, Colgan W, Trappe JM, Molina R (2002) Effects of forest management on truffle abundance and squirrel diets. NW Sci 76:148–157
Dickie IA, Reich PB (2005) Ectomycorrhizal fungal communities at forest edges. J Ecol 93:244–255
Edgar RC (2004) Muscle: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5:1–19
Fox FM (1983) Role of basidiospores as inocula of mycorrhizal fungi of birch. Plant Soil 71:269–273
Frank JL, Anglin S, Carrington EM, Taylor DS, Viratos B, Southworth D (2009) Rodent dispersal of fungal spores promotes seedling establishment away from mycorrhizal networks on Quercus garryana. Botany 87:821–829
Fujimura KF, Smith JE, Horton TR, Weber NS, Spatafora JW (2005) Pezizalean mycorrhizas and sporocarps in ponderosa pine (Pinus ponderosa) after prescribed fires in eastern Oregon, USA. Mycorrhiza 15:79–86
Gehring CA, Theimer TC, Whitham TG, Keim P (1998) Ectomycorrhizal fungal community structure of pinyon pines growing in two environmental extremes. Ecology 79:1562–1572
Grubisha LC, Bergemann SE, Bruns TD (2007) Host islands within the California northern channel islands create fine-scale genetic structure in two sympatric species of the symbiotic ectomycorrhizal fungus Rhizopogon. Mol Ecol 16:1811–1822
Hausmann NT, Hawkes CV (2010) Order of plant host establishment alters the composition of arbuscular mycorrhizal communities. Ecology 91:2333–2343
Horton TR, Cázares E, Bruns TD (1998) Ectomycorrhizal, vesicular-arbuscular and dark septate fungal colonization of bishop pine (Pinus muricata) seedlings in the first 5 months of growth after wildfire. Mycorrhiza 8:11–18
Izzo AD, Meyer M, Trappe JM, North M, Bruns TD (2005) Hypogeous ectomycorrhizal fungal species on roots and in small mammal diet in a mixed-conifer forest. Forest Sci 51:243–254
Johnson CN (1996) Interactions between mammals and ectomycorrhizal fungi. Trends Ecol Evol 11:503–507
Jumpponen A (2003) Soil fungal community assembly in a primary successional glacier forefront ecosystem as inferred from rDNA sequence analyses. New Phytol 158:569–578
Jumpponen A, Trappe JM, Cázares E (1999) Ectomycorrhizal fungi in Lyman lake basin: a comparison between primary and secondary successional sites. Mycologia 91:575–582
Kennedy PG, Bruns TD (2005) Priority effects determine the outcome of ectomycorrhizal competition between two Rhizopogon species colonizing Pinus muricata seedlings. New Phytol 166:631–638
Kennedy PG, Peay KG, Bruns TD (2009) Root tip competition among ectomycorrhizal fungi: are priority effects a rule or an exception? Ecology 90:2098–2107
Kjoller R (2006) Disproportionate abundance between ectomycorrhizal root tips and their associated mycelia. FEMS Microbiol Ecol 58:214–224
Lauber CL, Strickland MS, Bradford MA, Fierer N (2008) The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol Biochem 40:2407–2415
Lilleskov EA, Bruns TD (2005) Spore dispersal of a resupinate ectomycorrhizal fungus, Tomentella sublilacina, via soil food webs. Mycologia 97:762–769
Louette G, De Meester L, Declerck S (2008) Assembly of zooplankton communities in newly created ponds. Freshwat Biol 53:2309–2320
Maddison D, Maddison W (2002) Macclade: analysis of phylogeny and character evolution. Sunderland: Sinauer Associates. 4.0
Mamoun M, Oliver JM (1997) Mycorrhizal inoculation of cloned hazels by Tuber melanosporum: effect of soil disinfestation and co-culture with Festuca ovina. Plant Soil 188:221–226
Markewitz D, Richter DD, Allen HL, Urrego JB (1998) Three decades of observed soil acidification in the Calhoun experimental forest: has acid rain made a difference? Soil Sci Soc Am J 62:1428–1439
Nara K (2006) Ectomycorrhizal networks and seedling establishment during early primary succession. New Phytol 169:169–178
Nara K (2009) Spores of ectomycorrhizal fungi: ecological strategies for germination and dormancy. New Phytol 181:245–248
Newton AC (1992) Towards a functional classification of ectomycorrhizal fungi. Mycorrhiza 2:75–79
Parlade J, Pera J, Alvarez IF (1996) Inoculation of containerized Pseudotsuga menziesii and Pinus pinaster seedlings with spores of five species of ectomycorrhizal fungi. Mycorrhiza 6:237–245
Peay KG, Kennedy PG, Bruns TD (2008) Fungal community ecology: a hybrid beast with a molecular master. Biosci 58:799–810
Peay KG, Garbelotto M, Bruns TD (2009) Spore heat resistance plays an important role in disturbance-mediated assemblage shift of ectomycorrhizal fungi colonizing Pinus muricata seedlings. J Ecol 97:537–547
Robinson JV, Dickerson JE (1987) Does invasion sequence affect community structure. Ecol 68:587–595
Rusca TA, Kennedy PG, Bruns TD (2006) The effect of different pine hosts on the sampling of rhizopogon spore banks in five eastern Sierra Nevada forests. New Phytol 170:551–560
Simard SW, Perry DA, Smith JE, Molina R (1997) Effects of soil trenching on occurrence of ectomycorrhizas on Pseudotsuga menziesii seedlings grown in mature forests of Betula papyrifera and Pseudotsuga menziesii. New Phytol 136:327–340
Smith ME, Douhan GW, Rizzo DM (2007a) Ectomycorrhizal community structure in a xeric Quercus woodland based on rDNA sequence analysis of sporocarps and pooled roots. New Phytol 174:847–863
Smith ME, Douhan GW, Rizzo DM (2007b) Intra-specific and intra-sporocarp ITS variation of ectomycorrhizal fungi as assessed by rDNA sequencing of sporocarps and pooled ectomycorrhizal roots from a Quercus woodland. Mycorrhiza 18:15–22
Sparks D (1976) Soil pH and the pecan. 67th Annual Report of the Northern Nut Growers Association, pp. 93-99
Swofford DL (2002) Paup* phylogenetic analysis using parsimony (*and other methods). Sunderland, Massachusetts. Sinauer Associates. 4.0
Taylor DL, Bruns TD (1999) Community structure of ectomycorrhizal fungi in a Pinus muricata forest: minimal overlap between the mature forest and resistant propagule communities. Mol Ecol 8:1837–1850
Tedersoo L, Hansen K, Perry BA, Kjoller R (2006) Molecular and morphological diversity of pezizalean ectomycorrhiza. New Phytol 170:581–596
Tedersoo L, May TW, Smith ME (2010) Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 20:217–263
Trappe JM (1975) Generic synonyms in the Tuberales. Mycotaxon 2:109–122
Urban A, Neuner-Plattner I, Krisai-Greilhuber I, Haselwandter K (2004) Molecular studies on terricolous microfungi reveal novel anamorphs of two Tuber species. Mycol Res 108:749–758
Visser S (1995) Ectomycorrhizal fungal succession in jack pine stands following wildfire. New Phytol 129:389–401
Warcup JH (1990) Occurrence of ectomycorrhizal and saprophytic discomycetes after a wild fire in a eucalypt forest. Mycol Res 94:1065–1069
Warcup JH (1991) The fungi forming mycorrhizas on eucalypt seedlings in regeneration coupes in Tasmania. Mycol Res 95:329–332
Ward SA, Thornton IWB (2000) Chance and determinism in the development of isolated communities. Glob Ecol Biogeogr 9:7–18
Warr SJ, Thompson K, Kent M (1993) Seed banks as a neglected area of biogeographic research—a review of literature and sampling techniques. Progr Phys Geogr 17:329–347
Wedén C, Pettersson L, Danell E (2009) Truffle cultivation in Sweden: results from Quercus robur and Corylus avellana field trials on the island of Gotland. Scand J Forest Res 24:37–53
Yun W, Hall IR (2004) Edible ectomycorrhizal mushrooms: challenges and achievements. Can J Bot 82:1063–1073
Acknowledgements
The authors thank the willingness and cooperation of the Magnolia and Pine Knoll pecan farms for participating in this study. We thank Bernadette O’Reilley and Allen Lowrance for field assistance. Thanks to Connie Robertson for curating truffle collections for the Duke herbarium. Bill Bunn was kind to provide pecans for this study. We appreciate the constructive feedback given by two anonymous reviewers. This study was made possible through NSF funding to RV and GB (DBI-0710213). The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Thom W. Kuyper.
Rights and permissions
About this article
Cite this article
Bonito, G., Smith, M.E., Brenneman, T. et al. Assessing ectomycorrhizal fungal spore banks of truffle producing soils with pecan seedling trap-plants. Plant Soil 356, 357–366 (2012). https://doi.org/10.1007/s11104-012-1127-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1127-5