Abstract
Aims
With a high growth rate and biomass production, bamboos are frequently used for industrial applications and recently have proven to be useful for wastewater treatment. Bamboos are considered as Si accumulators and there is increasing evidence that silicon may alleviate abiotic stresses such as metal toxicity. The aim of this study was to investigate the extent of metal concentrations and possible correlations with Si concentrations in plants.
Methods
This study presents, for the first time, reference values for silicon (Si), copper (Cu) and zinc (Zn) concentrations in stems and leaves of various bamboo species grown under the natural pedo-climatic conditions of the island of Réunion (Indian Ocean).
Results
A broad range of silicon concentrations, from 0 (inferior to detection limit) to 183 mg g−1 dry matter (DM), were found in stems and leaves. Mean leaf Cu and Zn concentrations were low, i.e. 5.1 mg kg−1 DM and 15.7 mg kg−1 DM, respectively. Silicon, Cu and Zn concentrations increased over the following gradient: stem base < stem tip < leaves. Significant differences in Si, Cu and Zn contents (except Zn in the stem) were noted between bamboo species, particularly between monopodial and sympodial bamboo species, which differ in their rhizome morphology. Sympodial bamboos accumulated more Si and Cu than monopodial bamboos, in both stems and leaves, whereas sympodial bamboos accumulated less Zn in leaves than monopodial bamboos.
Conclusions
The findings of this study suggest that a genotypic character may be responsible for Si, Cu and Zn accumulation in bamboo.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bamboos are widespread plants belonging to the grass family (Poaceae). They are commonly found in temperate, tropical and subtropical regions and widely used for industrial purposes, as fresh edible shoots, making paper, building material and even in medicines. Bamboos are known for their resistance to a wide range of stress factors and their high growth rate and biomass production, with potential uses in phytoremediation. The PHYTOREM company (France) has developed BAMBOO-ASSAINISSEMENT® technology for wastewater treatment (Arfi et al. 2009). The company is currently optimizing the phytoremediation capacity of bamboos under tropical climatic conditions.
The uptake and accumulation of essential nutrients such as N, P and K are well documented in bamboos (Embaye et al. 2005; Shanmughavel and Francis 2001), but metal accumulation has been poorly documented so far. Metals are naturally present in the pedo-geochemical background of soils at various levels and many metals are essential to plants, but they may be toxic at higher concentrations. Metals accumulate in soil due to anthropogenic contamination through fertilizer and organic manure applications, industrial and municipal wastes, irrigation, and wet and/or dry deposits (Novak et al. 2004; Doelsch et al. 2010). Phytoremediation techniques have been put forward as alternatives to remediate metal contaminated soils, especially agricultural soils (McCutcheon and Schnoor 2003). One of the limitations of such technologies is the availability of plant species adapted to specific environmental conditions and, accumulating and/or tolerant to large metal or metalloid concentrations in soils (Keller 2005). Bamboos are potentially good candidates for phytoremediation because of their widespread distribution, their easy and well-known propagation mode, the broad range of species and their possible additional use as raw material. To our knowledge, there is no data available on metal concentrations in bamboos, especially under natural conditions. In this study, we focused on the case of copper (Cu) and zinc (Zn), two metals which may be present at high concentration in wastewater.
Like many Poaceae species (sugarcane, rice, wheat, etc.), bamboos are considered as Si accumulators, with Si concentrations ranging from 3 to 410 mg g−1 SiO2 (DM) (Ding et al. 2008; Li et al. 2006). The variability in Si content in bamboo may be explained by: (i) the available pool of Si in soil (Henriet et al. 2008; Jones et al. 1967); (ii) the increase in Si content during bamboo ageing (Motomura et al. 2002) and (iii) genetic variability among species. This last point has never been studied in bamboos, whereas it may be important because of the high number of bamboo species: a total of about 1030 bamboo species (77 genera) are grouped in the sub-family Bambusoideae, within the family Poaceae. In order to clarify the role of the species in the Si uptake capacity, data are thus needed on Si concentrations in bamboos of the same age and grown in similar soils.
In rice or wheat, there is increasing evidence that silicon may alleviate metal toxicity (Liang et al. 2007). Several mechanisms have been proposed to explain the role of Si in metal tolerance, such as limitation of metal uptake, reduction of root-to-shoot translocation or changes in metal allocation within the plant. In bamboos, the role of Si on metal tolerance has not been investigated. The first step before any attempt to test bamboos in contaminated soils for phytoremediation technologies is to investigate, under natural conditions, the extent of metal concentrations and possible correlations with Si concentrations in plants.
The objectives of the present study were to: (1) determine the variability in Cu and Zn (for the first time) and Si concentrations between species by comparing 16 bamboo species grown under similar environmental conditions, i.e. a volcanic soil on the island of Réunion (Indian Ocean, France), (2) obtain reference values for Si, Cu, Zn concentrations in the different above-ground parts of bamboos and, (3) highlight the possible relationship between Si, Zn and Cu in bamboos growing in a natural soil under tropical climatic conditions.
Materials and methods
Geographical area of the study, soil description and sampling procedure
Bamboo samples were collected from a 3.5-ha bamboo nursery in Réunion (Indian Ocean island), France (Mr Perrussot, Le Guillaume, Saint Paul). 130 different species have been maintained in this nursery since 1987. The climate is both tropical and oceanic, with easterly prevailing winds. The studied site (21°03′51 S, 55°19′28 E) is on the west side of the Piton des Neiges shield volcano, 1035 m above sea level. The mean annual precipitation is 1700 mm, and the temperature ranges from 10 to 28°C, with hot and humid summers and warm and wet winters. Soil in this area is classified as a chromic Andosol developed from Piton des Neiges volcanic material (Raunet 1991).
Sixteen bamboo species belonging to six genera were sampled in the same field in October 2008. These bamboos were selected because they may be good candidates for phytoremediation under tropical conditions, due to their high biomass production and good adaptations to tropical climates. Bamboo species differ the rhizome form, i.e. the underground part from which roots and shoots grow from nodes. According to the rhizome morphology, bamboos are divided into monopodial bamboos with leptomorph rhizome systems, and sympodial bamboos with pachymorph rhizome systems (McClure 1966). These differences in rhizome systems can be regarded as adaptations to the climatic conditions from which the bamboos originated: monopodial bamboos are native to temperate climates and sympodial bamboos are native to tropical climates (Kleinhenz and Midmore 2001). Ten out of the 16 species were monopodial bamboos, i.e. Dendrocalamus giganteus, Dendrocalamus strictus, Bambusa bambos, Bambusa oldhamii, Bambusa vulgaris ‘Vittata’, Bambusa multiplex ‘Golden Goddess’, Bambusa multiplex ‘Alphonse Karr’, Bambusa tuldoides, Gigantochloa sp. ‘Malay Dwarf’ and Thyrsostachys siamensis; and six were sympodial bamboos, i.e. Phyllostachys aurea, Phyllostachys bambusoïdes ‘Castillon’, Phyllostachys bissetii, Phyllostachys flexuosa, Phyllostachys humilis and Pseudosasa japonica.
For each species, three different 1-year-old bamboo specimens were selected to sample stems and leaves. Two samples were taken from each stem: one at the third internode above the soil surface, further referred to as the “stem base”, and the second at the tip of the stem, further referred to as the “stem tip”. For each stem, one bulk leaf sample was taken. A total of 94 stem samples and 47 leaf samples were collected.
Soil material and analysis
Soil was sampled under at least one bamboo of each species. A total of 18 soil samples were obtained by collecting topsoils (0–25 cm) with a gauge auger and mixing five replicates for each sample. Only steel or plastic tools (knife, spade and shovel) were used for sampling in order to avoid heavy metal contamination.
Soil samples were air-dried, crushed and passed through a 2-mm sieve before analysis. pHwater (soil/water ratio = 1:5) was measured according to ISO 10390.
For Si and trace element analyses, a representative soil subsample was ground to 100 μm particle size before digestion. For Cu and Zn analysis, calcination at 450°C was followed by total dissolution performed using a mixture of HF, HNO3 and HClO4 (ISO 14869-1). For SiO2 analysis, complete dissolution was obtained by alkaline fusion of the soil sample in the presence of sodium peroxide (AFNOR standard BP X 30-428).
Phytoavailable fractions of Cu and Zn were estimated using an extraction method (Collin and Doelsch 2010). After shaking 50 ml of 1 mol L−1 NH4NO3 solution and 20 g dry soil sample for 2 h at 30 rpm in a room at 20 ± 2°C, the extracts were centrifuged at 1000 g for 15 min. The supernatant was filtered through a membrane unit filter (0.22 μm).
All extracts were acidified with HNO3 and stored in polyethylene bottles at 4°C before analysis. All reagents were analytical grade and only ultrapure water (Purelab Prima plus Classic from Elga Labwater) was used. All glass and plastic ware used for the experiments was previously soaked overnight in nitric acid and rinsed with ultrapure water. Three replicates were performed for each sample. Blank tubes (containing reagent but no soil) were also taken throughout each procedure.
Silicon and trace element concentrations were then determined with an inductively coupled plasma-optical emission spectrometer (ICP–OES Vista-PRO, Varian, Inc.) with an axially viewed plasma system and a charge coupled device detector. For quality control, in-house reference samples and certified samples (CRM 7001 Light Sandy Soil and CRM 7004 Loam, Analytica) were used every 10 samples and each measurement was conducted in duplicate. The detection limits were 0.025 mg.kg−1 for Cu and Zn. The measurement uncertainty was less than 10%.
Plant material and analysis
Stems and leaves were washed with distilled water and dried at 60°C until constant weight. They were subsequently mixed, ground and homogenised. Sub-samples were dried at 80°C until constant weight to determine the dry weight.
The plant samples underwent dry mineralisation for Zn and Cu trace element analyses. During mineralisation, the Si content was determined by gravimetric quantification: 500 mg of dried plant material was placed in a platinum dish and gradually heated to 500°C. Silica was eliminated in the ash with HF. The Si weight was determined after cooling. The ash was dissolved in HCl and the Cu and Zn contents in the solutions were analysed by ICP-OES. For quality control, in-house reference samples and certified samples (Astrasol-Mix, Analytika) were used every 20 samples and each analysis was conducted in duplicate. The measurement uncertainty was ± 15%. The quantification limit for Si was 5 mg g−1.
Copper and Zn concentrations are expressed as mg kg−1 of dry matter (DM) and Si concentrations are expressed in mg g−1 DM SiO2 in order to have results directly comparable with the literature.
Statistical analyses
The Minitab 15.1 software package (Minitab, Inc.) was used for statistical analyses. Mean concentrations at the stem base, the stem tip and leaves were compared using a paired t-test at the 95% confidence level.
For each plant part, concentrations in the different species were analysed by ANOVA. We used one-way ANOVA at the 95% confidence level with bamboo “species” (16 levels) as the main factor, followed by Tukey’s post hoc test at the 95% confidence level to evaluate differences in Cu, Si and Zn concentration at the stem bases, the stem tips and the leaves. Differences between monopodial and sympodial bamboos were analysed using a general linear model with the type of bamboo as factor (2 levels). Mean concentrations from both types (sympodial or monopodial) were then compared using Tukey’s post hoc test at the 95% confidence level.
Results
Soil
Total Si, Cu and Zn soil concentrations are presented in Table 1. The mean total concentrations in the soil samples were 213 ± 23.3 mg g−1 for SiO2, 26.5 ± 5.8 mg kg−1 for Cu and 113 ± 18.2 mg kg−1 for Zn, and the average soil pH was 6.1 ± 0.3. Within the study framework, soil variations in Si, Cu, Zn concentration were reduced, while the coefficient of variation of concentrations within the sampled area was of 11% for Si and 21.8% for Cu and 16.2% (Table 1). The Cu NH4NO3-extractable fractions (CuNH4NO3) were below the detection limit in all soil samples and the mean Zn NH4NO3-extractable fractions (ZnNH4NO3) were 0.3 ± 0.23 mg kg−1 (Table 1).
Between plant parts
Table 2 shows the average Si, Cu, Zn concentrations at the stem base, at the stem tip and in the leaves. Within the stem, Si, Cu and Zn concentrations significantly increased from the stem base to the tip. The mean concentrations at the stem base were under quantification limit for Si, 3.5 mg Cu kg−1 and 7.0 mg Zn kg−1, whereas at the stem tip, the mean concentrations were 21 mg Si g−1, 4.5 mg Cu kg−1 and 14.8 mg Zn kg−1. The Si content at the stem tip was thus more than 4.1-fold higher than at the stem base, and this difference was greater than that noted for Cu and Zn: 1.3- and 2.1-fold, respectively. The leaf Si and Cu concentrations, i.e. 109 mg g−1 and 5.1 mg kg−1 respectively, were significantly higher than in the stem.
No correlations between Cu, Zn and Si contents were found in stems and leaves (R2 < 0.18). There was no longer any correlation between the total Si, Cu and Zn soil concentration and the Si, Cu and Zn plant concentration (R2 < 0.2), or between the ZnNH4NO3 fraction and the Zn plant concentration (R2 < 0.3) (for Cu, CuNH4NO3 fractions are below the detection limit).
Between plant species
Mean Si concentrations were significantly different between species in leaves and in the stem tips (Table 2). In our study, there was substantial variation in the Si content range between the 16 species: from 5.7 mg g−1 in Phyllostachys aurea to 56 mg g−1 in Bambusa multiplex ‘Golden Goddess’ at the stem tip, and from 82 mg g−1 in Phyllostachys bissetii to 159 mg g−1 in Dendrocalamus strictus in the leaves (Table 3). We assumed that this wide Si content range could mainly be explained by the genotypic variation since both soil and climatic conditions were similar for all the sampled species.
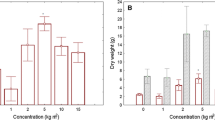
In order to explain the differences in Si content between species, we first compared Si concentrations between the two genera Phyllostachys and Bambusa (data not shown), and the Si concentration was not significantly different. However, we found significant differences between sympodial and monopodial bamboos (Fig. 1), with the Si concentrations being significantly higher in sympodial than in monopodial bamboos, both in stems and leaves.
Comparison of SiO2, Cu, Zn concentrations in stem bases, stem tips and leaves of monopodial bamboos (n = 18) and sympodial bamboos (n = 29). DM: dry matter. Boxes represent the median (vertical solid line), the arithmetic mean (vertical dashed line), and 25–75% percentile. Whiskers represent the 90th and 10th percentile. Significant differences were determined by a post hoc comparison of means (Tukey test after ANOVA; P < 0.05) and are indicated by different letters
For Cu, differences between species were significant both in stems and leaves (Table 2). Bambusa multiplex “Alphonse Kar” had the highest Cu concentration, with: 7.6 mg kg−1 in stem tips, while Phyllostachys bambusoides “Castillon” had the lowest concentration, with 2.0 mg kg−1 in stem tips, and 3.5 mg kg−1 in the leaves (Table 3). Grouping species by types showed that Cu concentrations were significantly higher in sympodial than in monopodial bamboo species, both in stems and leaves (Fig. 1). The range of Zn concentrations in the leaves and at the stem base was broad and significantly different between species (Table 2). For example, the Zn concentration in the stem bases ranged from 2.3 to 21.7 mg kg−1 in Gigantochloa sp. “Malay Dwarf” and Thyrsostachys siamensis, respectively (Table 3), but the influence of species with respect to concentrations in the stem tips was not significant. Unlike Cu, Zn concentrations in leaves were significantly higher in monopodial than in sympodial bamboo species. In stems, Zn concentrations were not influenced by the type of bamboo (Fig. 1).
Discussion
Phytoavailability of Si, Cu and Zn
The mean total SiO2 concentration of the soil samples (213 ± 23.3 mg g−1) was similar to the mean Si concentration previously measured in Réunion soils, i.e. 216 mg g−1 (Table 1) (Doelsch et al. 2006). This volcanic soil contains easily weatherable silicate minerals due to the presence of poorly crystalline minerals and particularly very low polymerized aluminosilicates that may contribute the phytoavailable Si pool (Basile-Doelsch et al. 2005).
The mean total Cu concentration (Table 1) was close to the mean concentration in world soil (20 mg kg−1), but lower than the mean concentration measured in a set of Réunion soils (Doelsch et al. 2006). The mean Zn concentration was also much higher than the mean soil concentration given by Kabata-Pendias and Mukherjee (2007), and slightly lower than the mean concentration calculated for Réunion soils (Doelsch et al. 2006). These larger Cu and Zn concentrations as compared to world soil concentrations could be explained by the origin of the parent material: soils formed from the Piton des Neiges volcanic material are characterised by low Cr, Cu and Ni concentrations and relatively high Zn concentrations (Doelsch et al. 2006). Indeed, these latter authors demonstrated that the natural pedogeochemical background could account for the high Cr, Cu, Ni and Zn concentrations in Réunion soils. In spite of these higher average total concentrations, the Cu and Zn NH4NO3-extractable fractions were low, which is consistent with the findings of Collin and Doelsch (2010), who demonstrated the low phytoavailability of Cu, Cr, Ni and Zn in Réunion soils. The absence of correlations between Cu and Zn total concentration in soil and Cu, Zn concentration in plants (R2 < 0.2) was thus not surprising and confirmed the low phytoavailability of these elements.
Origin of Si variation in bamboos
Silica concentrations in leaves were within the 20 to 410 mg g−1 Si range reported in several previous studies (Table 4). Silicon concentrations in stems ranged from <5 to 102 mg g−1, which is a broader range than reported in the literature presented in Table 4 (3–44 mg g−1). The mean Si concentration measured at the stem tip in the 16 species (21 mg g−1) was significantly higher than the concentration at the stem base (under the detection limit), which is out of line with the findings of Li et al. (2006). Indeed, at the stem base in the Moso bamboo stand (Phyllostachys heterocycla var. pubescens), these authors measured a concentration of 44 mg g−1 Si, whereas they measured 1.5 mg g−1 Si in the stem. However, the exact locations of the analysed samples corresponding to “base of the stem” and the “stem” were not given by Li et al. (2006), thus limiting the possibility of comparison with our results. Silicon concentrations thus varied between plant parts, with the accumulation of Si in leaves and a concentration gradient along the stem. To explain the distribution with the plant, transpiration has been proposed as the main mechanism for Si transportation and precipitation in Chinese bamboos (Ding et al. 2008). The evidence is based on the total silicon content and δ30Si values, which both increase from the stem, through the branches to the leaves. The results of this study are consistent with the hypothesis of silica being carried passively through the transpiration stream and being deposited where water is lost in largest quantities, as proposed by Ding et al. (2008). However, in an Si accumulator, it is likely that active silicon distribution mechanisms in bamboo shoots are also required (Ma and Yamaji 2008).
In leaves and at the stem tips, there was marked variation in the Si content between the 16 sampled species (Table 2). This result is in good agreement with the findings of Hodson et al. (2005) who showed that variability in Si content over 735 plant species is mainly explained by genotypic variation rather than environment. However, although differences in Si content between plant families are well known (Hodson et al. 2005), genotypic variation in Si concentration within species is less documented. The effect of genotypic variation has been studied in a few other plants, particularly in other Poaceae species. For example, in 52 sugarcane genotypes grown in the same soil, the shoot Si concentration ranged from 6 to 8 mg g−1 (Deren et al. 1993). In rice, the same authors compared the Si concentration in plants from 18 cultivars grown in greenhouse experiments and in fields amended with various amounts of Si. The silica concentration in plant tissues ranged from 3 to 60 mg g−1 depending on the Si supply, but within each Si treatment the coefficient of variation due to genotypic differences was 9 to 17% (Deren et al. 1993; Deren et al. 2001). In field trials, Winslow et al. (1997) observed differences between japonica and indica rice cultivars, with mean Si concentrations in husks of 2 and 1.2 mg g−1, respectively. In a survey of about 400 barley species, the Si concentration in grain ranged from 0 (under the detection limit) to 3.8 mg g−1 in hulled barley cultivars (Feng Ma et al. 2003). However, no significant differences in Si absorption were observed in three different banana genotypes grown in hydroponic culture conditions (Henriet et al. 2006). Although genotypic differences in Si content have been found in other Poaceae species such as rice, sugarcane and wheat, this is not systematic in all species and may question the genetic mechanisms that control Si accumulation in different species.
Si concentrations were significantly higher in sympodial bamboos than in monopodial bamboos, in both stems and leaves (Fig. 1). This is consistent with findings in the literature (Table 4). For example, higher Si concentrations were reported in sympodial bamboo leaves (114 mg g−1) than in monopodial bamboos leaves (82 mg g−1) (Table 4).
There are marked morphological differences between pachymorph rhizomes (sympodial) and leptomorph rhizomes (monopodial), but no differences in root development and nutrient absorption capacity have been noted between the two types (Kleinhenz and Midmore 2001). Since these authors also reported that at least 80% of the total root biomass is located in the topsoil (0–30 cm) regardless of the species (Kleinhenz and Midmore 2001), we assume that roots of both bamboo types were within the same soil layer, so the characteristics of the soil in contact with roots were similar.
Differences in Si accumulation may be attributed to differences in the silicon uptake capacity of roots. Recently, two genes (Low silicon rice 1: Lsi1 and Lsi2) encoding silicon transporters were identified in japonica rice (Ma and Yamaji 2008). Ma et al. (2007) have shown that genotypic differences between two rice species, japonica and indica, were due to the difference in abundance of Si transporters in rice roots. Therefore, differences between bamboos species, and to a further extent between the two types of bamboo could reflect a difference in expression of genes responsible for silicon uptake.
After root uptake, Si is translocated to the shoot via the xylem. It is likely that transporters are also required for xylem Si loading and unloading and for distributing Si to the above-ground plant parts (Ma and Yamaji 2008). Genetic differences may also be expressed in the relative distribution of Si in stems and leaves. For example, Keeping et al. (2009) found that cultivar differences in sugarcane stalks could be explained by differing propensities of cultivars to deposit Si within the stalk epidermis. Active processes could partly explain differences in Si concentrations between species, but the understanding of the silica deposition process and the identification of transporters in plant shoots still need to be studied.
In the above-ground parts of bamboos, Kleinhenz and Midmore (2001) highlighted that the lifespan of leaves was substantially different between monopodial and sympodial bamboos. The canopy of monopodial species is rejuvenated every year when 2-year-old leaves are replaced by new ones. Those of sympodial species remain on culms longer, i.e. up to about 6 years. Therefore culms of sympodial bamboos of over 2-years-old contain relatively older and less productive leaves than monopodial bamboos. Motomura et al. (2002) reported that in bamboo leaves silica is continuously accumulated in the tissues throughout their life. Leaves of sympodial bamboos may therefore have a higher Si content than leaves of monopodial bamboos. However, in this study, we only sampled 1-year-old stems, so the leaf ages should all have been the same. The differences observed in our study between monopodial and sympodial bamboos were thus not related to this character.
Origin of Cu and Zn variability in bamboo
Due to the lack of published Zn and Cu concentrations in bamboos, we first compared our results with another Poaceae species, i.e. sugarcane (stems), growing in Réunion on similar soils (Collin and Doelsch 2010). Copper concentrations in bamboo samples were higher than in sugarcane samples, with an average of 3.5 ± 1.0 and 4.5 ± 2.0 mg kg−1 at the stem base and tip, respectively (Table 2), whereas in sugarcane the average was 2.1 ± 0.6 mg kg−1. Zinc concentrations in bamboo stems were similar to concentrations in sugarcane stems, with an average of 7.0 ± 6.3 and 14.8 ± 16 mg kg−1 at the bamboo stem base and the tip, respectively (Table 2), and 10 ± 5.2 mg k−1 in sugarcane. We then compared leaf concentrations with data compiled for mature leaf tissue from various plant species (Kabata-Pendias and Mukherjee 2007). The concentration ranges that these authors considered normal were 5–30 mg kg−1 for Cu and 27–150 mg kg−1 for Zn. The concentration measured in bamboo leaves were within this range for Cu, with an average of 5.1 ± 1.0 mg Cu kg−1, and lower for Zn, with an average of 15.7 ± 5.6 mg Zn kg−1 (Table 2). Cu and Zn concentrations in above-ground parts of Réunion bamboos were thus relatively low, which may confirm the low phytoavailability of these elements measured by NH4NO3-extraction, as well as the nonspecific ability of bamboos to accumulate Cu and Zn.
Within the stem, Cu and Zn concentrations, similar to Si, significantly increased from the stem base to the tip (Table 2), suggesting that part of this element translocation was driven by transpiration. However, metals do not move freely in the plant. Interactions of cations with negatively charged sites (mainly with pectins) in xylem or phloem cell walls lead to decoupling of ion transport and water flow (Franco et al. 2002). In addition, most metals are complexed by organic acids, amino acids, peptides, metallothionine or phytochelatin (Cobbett and Goldsbrough 2002; Liao et al. 2000; Broadley et al. 2007). The greater Zn accumulation at the stem tip as compared to that of Cu may thus be explained by differences in the mobility of metal complexes formed within the plant.
Significant differences between species were measured for Cu in stems and leaves and for Zn in leaves (Table 2). This finding is in good agreement with the study of Broadley et al. (2007) who reported that, in a dataset of 365 species, there were substantial differences in shoot Zn content between and within genera and species. Metal absorption in plants is both active and passive, and metabolic mechanisms, such as the expression of specific transporters in root cells, is genetically controlled and may thus vary between species. For example, the expression and gene organization of proton pumping ATPase genes in the plant plasma membrane seems to differ between species (Morsomme and Boutry 2000). Moreover, the availability of elements in the soil and root uptake can be affected by plant factors such as root exudates, root surface area, root absorption ability and mycorrhization (Whiting et al. 2000; Langer et al. 2010; Keller et al. 2003). For example in rice, Zn uptake efficiency is correlated with exudation rates of low molecular weight organic anions and a substantial proportion of the phenotypic variation in Zn uptake efficiency is under genetic control (Wissuwa et al. 2006). In wheat, genotypic variations in Zn uptake may be related to the release of phytosiderophores (mugineic and avenic acids), which were shown to significantly enhance Zn bioavailability (Cakmak et al. 1996; Rengel et al. 1998; Tolay et al. 2001). Thus, these mechanisms and inherent differences in uptake, translocation and accumulation may partly explain the significant differences noted in Cu and Zn concentrations among the 16 bamboo species.
Differences between monopodial and sympodial bamboos were significant for Cu and Zn, but no information is available in the literature on any possible specific behaviour regarding metals between the two types of bamboo. Analyses of the differences with Si content were discussed above, and may also apply for Cu and Zn.
Interactions between metals and Si
No correlations between Cu, Zn and Si contents were found in stems and leaves. In the natural pedo-climatic environment of Réunion, with a soil having low NH4NO3-extractable Cu and Zn, the Si and trace metal behaviours in bamboos seem to be independent. This could be easily explained since the beneficial effects of Si are usually expressed when plants are subjected to stress conditions (Liang et al. 2007). It has been shown, for example, that Si alleviates Zn toxicity in heavy metal-tolerant Cardaminopsis halleri (Neumann and zur Nieden 2001). These authors suggested that the formation of Zn-silicate compounds in the cytoplasm may be responsible for the alleviation of the Zn toxicity. In Arabidopsis thaliana, Li et al. (2008) have shown that Si improves the resistance to Cu stress. A recent study has shown that Si modulates the expression of various genes involved in Cu tolerance in A. thaliana (Khandekar and Leisner 2011). Our study was not designed to assess the interaction between Si and metals at the cellular level, but we can assume that, with such a high Si absorption capacity in its tissues, bamboo may be able to tolerate higher metal concentrations than those present in Réunion soils. However, this would need further testing with increasing metal concentrations.
In conclusion, we report the first data on Cu and Zn concentrations in stems and leaves of various bamboo species and new data on Si in bamboos. Our results highlight the importance of the variability between species, particularly between monopodial and sympodial species for Zn, Cu, and Si contents in bamboos, suggesting that a genotypic character may be responsible for their accumulation.
References
Arfi V, Bagoudou D, Korboulewsky N, Bois G (2009) Initial efficiency of a bamboo grove-based treatment system for winery wastewater. Desalination 246(1–3):69–77
Basile-Doelsch I, Amundson R, Stone WEE, Masiello CA, Bottero JY, Colin F, Masin F, Borschneck D, Meunier JD (2005) Mineralogical control of organic carbon dynamics in a volcanic ash soil on La Reunion. Eur J Soil Sci 56(6):689–703
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A (2007) Zinc in plants. New Phytol 173(4):677–702
Cakmak I, Sari N, Marschner H, Ekiz H, Kalayci M, Yilmaz A, Braun HJ (1996) Phytosiderophore release in bread and durum wheat genotypes differing in zinc efficiency. Plant Soil 180(2):183–189
Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53:159–182
Collin B, Doelsch E (2010) Impact of high natural soilborne heavy metal concentrations on the mobility and phytoavailability of these elements for sugarcane. Geoderma 159(3–4):452–458
Deren CW, Glaz B, Snyder GH (1993) Leaf-tissue silicon content of sugarcane genotypes grown on everglades histosols. J Plant Nutr 16(11):2273–2280
Deren CW, Datnoff LE, Snyder GH, Korndorfer GH (2001) Plant genotype, silicon concentration, and silicon-related responses. Stud Plant Sci 8:149–158
Ding TP, Zhou JX, Wan DF, Chen ZY, Wang CY, Zhang F (2008) Silicon isotope fractionation in bamboo and its significance to the biogeochemical cycle of silicon. Geochim Cosmochim Acta 72(5):1381–1395
Doelsch E, Van de Kerchove V, Saint Macary H (2006) Heavy metal content in soils of Reunion (Indian Ocean). Geoderma 134(1–2):119–134
Doelsch E, Masion A, Moussard G, Chevassus-Rosset C, Wojciechowicz O (2010) Impact of pig slurry and green waste compost application on heavy metal exchangeable fractions in tropical soils. Geoderma 155(3–4):390–400
Embaye K, Weih M, Ledin S, Christersson L (2005) Biomass and nutrient distribution in a highland bamboo forest in southwest Ethiopia: implications for management. Forest Ecol Manag 204(2–3):159–169
Feng Ma J, Higashitani A, Sato K, Takeda K (2003) Genotypic variation in silicon concentration of barley grain. Plant Soil 249(2):383–387
Franco CR, Chagas AP, Jorge RA (2002) Ion-exchange equilibria with aluminum pectinates. Colloid Surface Physicochem Eng Aspect 204(1–3):183–192
Henriet C, Draye X, Oppitz I, Swennen R, Delvaux B (2006) Effects, distribution and uptake of silicon in banana (Musa spp.) under controlled conditions. Plant Soil 287(1):359–374
Henriet C, Bodarwe L, Dorel M, Draye X, Delvaux B (2008) Leaf silicon content in banana (Musa spp.) reveals the weathering stage of volcanic ash soils in Guadeloupe. Plant Soil 313(1–2):71–82
Hodson MJ, White PJ, Mead A, Broadley MR (2005) Phylogenetic variation in the silicon composition of plants. Ann Bot 96(6):1027–1046
Jones LHP, Handreck KA, Norman AG (1967) Silica in soils, plants, and animals. In: Advances in Agronomy, vol 19. Academic Press, pp 107–149
Kabata-Pendias A, Mukherjee AB (2007) Trace elements from soil to human. Springer
Keeping MG, Kvedaras OL, Bruton AG (2009) Epidermal silicon in sugarcane: cultivar differences and role in resistance to sugarcane borer Eldana saccharina. Environ Exp Bot 66(1):54–60
Keller C (2005) Efficiency and limitations of phytoextraction by high biomass plants. In: Trace elements in the environment. CRC Press, pp 611–630
Keller C, Hammer D, Kayser A, Richner W, Brodbeck M, Sennhauser M (2003) Root development and heavy metal phytoextraction efficiency: comparison of different plant species in the field. Plant Soil 249(1):67–81
Khandekar S, Leisner S (2011) Soluble silicon modulates expression of Arabidopsis thaliana genes involved in copper stress. J Plant Physiol 168(7):699–705
Kleinhenz V, Midmore DJ (2001) Aspects of bamboo agronomy. In: Sparks DL (ed) Advances in agronomy, vol 74. Academic, New York, pp 99–153
Langer I, Syafruddin S, Steinkellner S, Puschenreiter M, Wenzel WW (2010) Plant growth and root morphology of Phaseolus vulgaris L. grown in a split-root system is affected by heterogeneity of crude oil pollution and mycorrhizal colonization. Plant Soil 332(1–2):339–355
Li Z-J, Lin P, He J-Y, Yang Z-W, Lin Y-M (2006) Silicon’s organic pool and biological cycle in moso bamboo community of Wuyishan Biosphere Reserve. J Zhejiang Univ Sci B 7(11):849–857
Li J, Leisner M, Frantz J (2008) Alleviation of copper toxicity in Arabidopsis thaliana by silicon addition to hydroponic solutions. J Am Soc Hort Sci 133(5):670–677
Liang YC, Sun WC, Zhu YG, Christie P (2007) Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environ Pollut 147(2):422–428
Liao M, Hedley M, Woolley D, Brooks R, Nichols M (2000) Copper uptake and translocation in chicory (Cichorium intybus L. cv. Grasslands Puna) and tomato (Lycopersicon esculentum Mill. cv. Rondy) plants grown in NFT system. II. The role of nicotianamine and histidine in xylem sap copper transport. Plant Soil 223(1):245–254
Lux A, Luxova M, Abe J, Morita S, Inanaga S (2003) Silicification of bamboo (Phyllostachys heterocycla Mitf.) root and leaf. Plant Soil 255:85–91
Ma JF, Takahashi E (2002) Soil, fertilizer and plant silicon research in Japan. Elsevier Science
Ma JF, Yamaji N (2008) Functions and transport of silicon in plants. Cell Mol Life Sci 65(19):3049–3057
Ma JF, Yamaji N, Tamai K, Mitani N (2007) Genotypic Difference in Silicon Uptake and Expression of Silicon Transporter Genes in Rice. Plant Physiol 145(3):919–924
McClure FA (1966) The bamboos. A fresh perspective. Harvard University Press, Massachusetts
McCutcheon SC, Schnoor JL (2003) Phytoremediation—Transformation and control of contaminants. Wiley Inter-science, USA
Meunier J-D, Colin F, Alarcon C (1999) Biogenic silica storage in soils. Geology 27:835–838
Morsomme P, Boutry M (2000) The plant plasma membrane H + -ATPase: structure, function and regulation. Biochim Biophys Acta Biomembr 1465(1–2):1–16
Motomura H, Mita N, Suzuki M (2002) Silica accumulation in long-lived leaves of Sasa veitchii (Carriere) Rehder (Poaceae-Bambusoideae). Ann Bot 90(1):149–152
Neumann D, zur Nieden U (2001) Silicon and heavy metal tolerance of higher plants. Phytochem 56(7):685–692
Novak JM, Watts DW, Stone KC (2004) Copper and zinc accumulation, profile distribution, and crop removal in coastal plain soils receiving long-term, intensive applications of swine manure. Trans Am Soc Agric Eng 47(5):1513–1522
Raunet M (1991) Le milieu physique et les sols de l’île de la Réunion. Conséquences pour la mise en valeur agricole. CIRAD IRAT, Montpellier
Rengel Z, Romheld V, Marschner H (1998) Uptake of zinc and iron by wheat genotypes differing in tolerance to zinc deficiency. J Plant Physiol 152(4–5):433–438
Shanmughavel P, Francis K (2001) Bioproductivity and nutrient cycling in bamboo and acacia plantation forests. Biores Tech 80(1):45–48
Tolay I, Erenoglu B, Romheld V, Braun HJ, Cakmak I (2001) Phytosiderophore release in Aegilops tauschii and Triticum species under zinc and iron deficiencies. J Exp Bot 52(358):1093–1099
Whiting SN, Leake JR, McGrath SP, Baker AJM (2000) Positive responses to Zn and Cd by roots of the Zn and Cd hyperaccumulator Thlaspi caerulescens. New Phytol 145(2):199–210
Winslow MD, Okada K, CorreaVictoria F (1997) Silicon deficiency and the adaptation of tropical rice ecotypes. Plant Soil 188(2):239–248
Wissuwa M, Ismail AM, Yanagihara S (2006) Effects of zinc deficiency on rice growth and genetic factors contributing to tolerance. Plant Physiol 142(2):731–741
Acknowledgments
This work was financially supported by the Direction Générale des Entreprises, Direction Générale de la Compétitivité, de l’Industrie et des Services, région Réunion, région PACA in the frame of the research program RUN INNOVATION II and by the Association Nationale de la Recherche et de la Technologie (CIFRE grant).
The authors thank Mr Alexandre Perrussot, Mr Gregory Bois and MmeVeronique Arfi for their assistance and fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jian Feng Ma.
Rights and permissions
About this article
Cite this article
Collin, B., Doelsch, E., Keller, C. et al. Distribution and variability of silicon, copper and zinc in different bamboo species. Plant Soil 351, 377–387 (2012). https://doi.org/10.1007/s11104-011-0974-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-0974-9