Abstract
We assessed the effect of biochar incorporation into the soil on the soil-atmosphere exchange of the greenhouse gases (GHG) from an intensive subtropical pasture. For this, we measured N2O, CH4 and CO2 emissions with high temporal resolution from April to June 2009 in an existing factorial experiment where cattle feedlot biochar had been applied at 10 t ha−1 in November 2006. Over the whole measurement period, significant emissions of N2O and CO2 were observed, whereas a net uptake of CH4 was measured. N2O emissions were found to be highly episodic with one major emission pulse (up to 502 μg N2O-N m−2 h−1) following heavy rainfall. There was no significant difference in the net flux of GHGs from the biochar amended vs. the control plots. Our results demonstrate that intensively managed subtropical pastures on ferrosols in northern New South Wales of Australia can be a significant source of GHG. Our hypothesis that the application of biochar would lead to a reduction in emissions of GHG from soils was not supported in this field assessment. Additional studies with longer observation periods are needed to clarify the long term effect of biochar amendment on soil microbial processes and the emission of GHGs under field conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Land-use and agricultural practices affect the soil microbial carbon (C) and nitrogen (N) turnover and hence the biosphere-atmosphere exchange of greenhouse gases (GHG), namely N2O, CH4 and CO2. In Australia, the agricultural sector contributes 16% of the total emissions of GHGs, including 60% of all CH4 emissions (67.2 Mt CO2—eq.) and 85% of all N2O emissions (20.7 Mt CO2—eq.) (AGO 2007). When land-use changes involving biomass burning, soil degradation and deforestation are included in this estimate, the overall emissions account for one-third of the total national GHG release. At the same time agriculture is considered to have the highest GHG mitigation potential by reducing emissions from soil and sequestering carbon in soils via modified land-use and management.
In Australia, grasslands for the cattle and sheep industry are the principal land use covering an area of approximately 450 million hectares (AGO 2010). In the humid, subtropical zones of New South Wales, improved pastures for beef and dairy cattle production account for 40–50% of the total agricultural land use (Australian Bureau of Statistics 2009). These lands were extensively cleared of the native subtropical rainforests during the latter half of the 19th and early 20th centuries to make way for the establishment of the dairy industry (Adam 1994). The productivity of the livestock industry in this area is directly related to the use of either improved legume based pastures or N-fertilized grass pastures and the dairy industry is considered to be the largest user of N-fertilizer (Weier 1994). Annual ryegrass (Lolium multiflorum) is one of the main species for winter grazing in these systems, with high applications of N-fertilizer. Lowe et al. (2005) recommended that 50–85 kg N ha−1 month−1 be applied to annual ryegrass during the winter months to maintain high productivity. Due to the combination of high fertilizer rates, high rainfall events and temperatures throughout the year, elevated emissions of N2O can be expected.
Although high denitrification rates have been reported from subtropical pastures in Australia (Weier et al. 1993), only limited information on emissions of GHG is available for these systems. Previous studies in Australia have typically investigated GHG emissions in temperate pastoral systems (Eckard et al. 2003; Kelly et al. 2008; Livesley et al. 2008). The few studies from subtropical or tropical pastures have utilized comparatively coarse weekly or monthly gas sampling (Allen et al. 2009; Erickson et al. 2001; Keller and Reiners 1994; Veldkamp et al. 1998). To date no investigations of GHG emissions from humid subtropical pastures have been published based on high temporal resolution field studies.
A promising new approach to GHG mitigation is the application of biochar to soils. This offers the potential of sequestering carbon in the soil, since charcoal generally is resistant to rapid microbial degradation (Lehmann et al. 2006). Moreover, it has been shown that biochar amendment to soils can significantly impact soil quality and plant growth (Chan et al. 2007, 2008) and initial research also indicated the potential to reduce the emissions of GHG from soils (Yanai et al. 2007). The mechanisms responsible for the effects of biochar on soil GHG emissions are still unclear (Van Zwieten et al. 2009). Most studies have investigated the effect of biochar on soil-borne GHGs emissions in laboratory incubation studies (Clough et al. 2010; Singh et al. 2010; Van Zwieten et al. 2010) while the few studies based on field measurements have used sporadic weekly to monthly measurements (Rondon et al. 2005; Zhang et al. 2010). To date, no investigations have been published based on detailed field measurements. By using a fully automated closed chamber monitoring system we wanted to test the hypothesis that biochar amendment will reduce the emission of GHG on a field level.
The aims of this study were to (i) investigate the effect of soil biochar amendment on the emissions of soil-borne GHGs and (ii) quantify the net fluxes of N2O, CH4 and CO2 from a subtropical pasture during the winter month when high rates of fertiliser are commonly applied and consequently high fluxes of N2O can be expected.
Material and methods
Study site
The field experiment was carried out at the Wollongbar Agricultural Institute (28°50′S, 153°25′E) in north-eastern NSW between April and June 2009. The climate is humid subtropical with a predominantly summer rainfall and an average annual precipitation of 1,800 mm. The mean daily minimum and maximum temperatures are 19.1 and 26.9°C in the summer, and 10.5 and 19.2°C in winter, respectively. The soil is a red Ferrosol (Isbell 2002) derived from basalt with a clay loam soil surface, pH of 4.6 (1:5 in CaCl2) and organic C content in unamended sites (0–10 cm) of 4.5% (Table 1). Our GHG study was superimposed on a subset of an existing factorial experiment on 5 m2 subplots using 3 replicates in an randomised block design (Sinclair et al. 2009). The GHG study compared biochar amendment with a control. The biochar was produced by Pacific Pyrolysis P/L from cattle feedlot waste using a 300 kg h−1 slow-pyrolysis unit located at Somersby, NSW. The highest temperature of treatment was 550°C, with a mean residence time of 45 min. The cattle feedlot biochar was applied at 10 t ha−1 in November 2006 and was incorporated to a depth of 10 cm. This biochar is described in more detail in (Sinclair et al. 2009); in summary, the biochar had 44% total C (Total C was measured by Dumas combustion using an Elementar vario MAX CN analyser with combustion chamber set at 900°C and oxygen flow rate of 125 mL/min), pH (CaCl2 5:1) of 9.7 and an acid neutralising capacity (method 19A1 of Rayment and Higginson (1992)), of 13% that of agricultural lime, had 73 mg/kg (Bray 1) P (method 9E2 of Rayment and Higginson (1992)). Nitrate and ammonium (method 7 C2 of Rayment and Higginson (1992)) were below level of detection (0.3 mg/kg). The biochar was also shown to have a molar H/C ratio of 0.51 (determined by Bureau Veritas International Trade Australia using Australian Standard Method AS 1038.6.1), which was similar to ratios for other slow pyrolysis biochars described in Van Zwieten et al. 2010. Amarillo pinto peanut (Arachis pintoi L.) and annual ryegrass (Lolium rigidum L.) were grown during the year, with the site receiving twice yearly applications of P (28 kg ha−1) and K 50 (kg ha−1) (K Sinclair, Wollongbar Primary Industries Institute, pers comm.). Over the winter ryegrass season, the plots received monthly applications of N-fertiliser (as urea) totalling 300 kg N ha−1. A significant response to both N and P uptake from the biochar amendment, as well as a significant increase in pasture biomass yield has been reported by Sinclair et al. (2009).
Continuous trace gas flux measurement
The soil-atmosphere exchange of N2O, CH4 and CO2 was measured with a mobile fully automated measuring system from April 19 to June 15 2009. Soil-atmosphere exchange measurements were taken from 3 subplots for each treatment within the split-plot design. Six acrylic sampling chambers (50 cm × 50 cm × 15 cm) were fixed on stainless steel frames. The lids of the chambers were opened and closed automatically with pneumatic pistons. A full measurement cycle for the GHG flux determination commenced with chamber lid closure and finished 96 min later when the lid opened. During the closure period four air samples from each chamber were taken sequentially (12 min apart) and injected towards the analytical devices. Afterwards the chambers stayed open for 96 min before a new measuring cycle was started. This enabled up to 8 single flux rates to be determined per chamber and day. Changes in N2O and CH4 concentration after chamber closure were measured with a gas chromatograph (SRI 8610C, Torrance/USA) equipped with a 63Ni electron capture detector (ECD) for N2O analysis and a flame ionisation detector (FID) for CH4 analysis. These utilized stainless steel analytical columns packed with Haysep N and Haysep Q respectively. In addition, an infrared gas analyser (LI-COR 820, LICOR, Lincoln/USA) was installed to allow measurements of CO2 concentrations in air samples. To minimize the interference of moisture vapour and CO2 on N2O measurement, an Ascarite (sodium-hydroxide-coated silica) pre-column filled was installed upstream of the ECD and changed at fortnightly intervals. Sample gas measurements were calibrated automatically by a single point calibration using certified gas standards (Air Liquide, Dellas,TX, USA) of 900 ppm CO2, 1.95 ppm CH4 and 0.5 ppm N2O. The detection limit of the system was approximately 1.0 μg N2O-N m−2 h−1 for N2O, 1.0 μg CH4-C m−2 h−1 for CH4 and 0.6 mg CO2 -C m−2 h−1 for CO2 .Sample dilution via leakage was considered negligible. Further details on the automated system and analytical conditions applied for gas analyses are found in (Breuer et al. 2000; Kiese and Butterbach-Bahl 2002). Hourly N2O, CH4 and CO2 fluxes were calculated from the slope of the linear increase or decrease in gas concentration during the chamber lid closure and corrected for air temperature, atmospheric pressure and the ratio of chamber volume to surface area as described in detail by Barton et al. (2008). The Pearson’s correlation coefficient (r2) for the linear regression was calculated and used as a quality check for the measurement. Flux rates were discarded if r2 was <0.80.
Auxiliary measurements
Soil temperature (10 cm) and chamber temperature was measured every minute in conjunction with the automatic sampling system using a PT100 probe (IMKO Germany). Soil moisture was measured in each treatment with a portable TDR probe (HydroSense CD 620 CSA). Water-filled pore space (WFPS) was calculated using the measured soil bulk density data (arithmetic means of four samples) using a particle density of 2.65 g cm−3. Additionally, at the beginning and end of the growing season, bulk soil samples were taken from each site by combining 5–10 soil cores (0–10 cm depth) and analysed for soil texture, total carbon (C %), total nitrogen (N%) (Table 1).
Statistical analysis
Statistical analysis was undertaken using SPSS 16.0 (SPSS Inc., USA). Non-normal distribution of N2O, CO2 and CH4 emissions was shown using the Kolmogorov-Smirnov test. The non-parametric pair-wise Wilcoxon test was used without any data transformation for the comparison of control and biochar treatments. The relationships between trace gas flux and soil moisture and soil temperature was investigated through linear regression. The adjusted Pearson’s regression coefficient (r2) indicated the amount of variation in trace gas flux that can be explained by changes in soil moisture or temperature.
Results
N2O emissions
For each individual chamber, 248 separate N2O fluxes were measured over the entire field campaign. Mean N2O emissions of all 744 flux rates for the biochar and the control treatment were 35.3 μg N2O-N m−2 h−1 and 31.1 μg N2O-N m−2 h−1, respectively (Table 2). Overall mean daily N2O emissions ranged from 1.9 to 502.2 μg N2O-N m−2 h−1and a high temporal and spatial variation was observed for both treatments. There was no significant influence of the biochar amendment (when compared to the control) on net N2O flux over the entire sampling period. During individual short periods with generally low fluxes (<50 μg N2O-N m−2 h−1) N2O emissions were significantly (p < 0.05) lower from the biochar amended plots (Table 3).
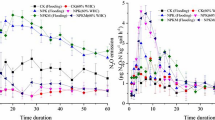
During the first week of the measurements significantly higher N2O emissions were observed from the control treatment as compared to the biochar plots (Fig. 1). But after relocation of the measuring chambers within each plot to reduce the impact of the chambers themselves on pasture growth, this effect was not apparent and from May 1 to May 11 only low N2O fluxes (<30 μg N2O-N m−2 h−1) were observed from both treatment and control with no significant treatment effect. The application of nitrogen fertilizer (50 kg N ha−1 urea) on May 5 resulted in a 2-fold increase in N2O emissions in both biochar treatment and control (Fig. 2). A major N2O emission pulse was observed from 20–23 May due to heavy rainfall (Sum: 360 mm) with WFPS exceeding 80% during this period. The mean daily emissions were 370 μg N2O-N m−2 h−1, and in individual chambers flux exceeded 700 μg N2O-N m−2 h−1. This single emission pulse accounted for 68% and 49% of the total emissions over the observation period in the biochar and the control treatment, respectively. After this emission pulse, only minor rainfall events occurred (20 mm) and WFPS remained low at less than 70%. During this dryer period, N2O fluxes ranged from 2–18 μg N2O-N m−2h−1, with slightly higher N2O emissions from the control plots (Fig. 1).
Daily rainfall, average daily soil temperature (10 cm), average daily water-filled pore space (WFPS) and daily CO2, CH4 and N2O fluxes from the biochar and control pasture plots, for the period 19 April to 15 June 2009. Error bars indicate the standard error of the means (n = 3). Connecting lines are inserted showing the data points more clearly
A positive correlation between soil moisture and N2O emissions was observed for both the biochar (r 2 = 0.23, n = 42, p < 0.01) and the control treatment (r 2 = 0.22, n = 42, p < 0.01), confirming that soil moisture was one of the main environmental factors influencing N2O emissions. There was no correlation between soil temperature and N2O emissions during the measuring period (biochar: r 2 = 0.005; control: r 2 = 0.014; n = 248, p > 0.05). However, when we excluded the extraordinarily high N2O fluxes from the emission pulse (>50 μg N2O-N m−2 h−1) a positive relationship between soil temperature and N2O fluxes was found for both the biochar (r 2 = 0.17, n = 216, p < 0.01) and the control treatment (r 2 = 0.38, n = 216, p < 0.01). A significant diurnal effect of soil temperature (10 cm) on N2O fluxes could be observed during periods with near constant WFPS and a representative example (2–7 May) is shown in Fig. 2. Depending on the daily meteorological conditions, soil temperature variation during this period ranged from 13.6°C to 23.1°C with maximum soil temperatures occurring between 13:00 and 15:00 and minimums between 3:00 and 6:00. The amplitude of the diurnal N2O variation ranged from 3.2 to 16.3 μg N m−2 hr−1 with fluxes increasing during daytime and decreasing during the night. Daily emission maxima generally occurred between 14:00 and 18:00 with minimums between 3:00 and 7:00. After the application of 50 kg N ha−1 urea on 5 May which resulted in a 2-fold increase in N2O emissions, a similar diurnal temperature effect was still observed.
CH4 uptake
The pasture soils acted as a sink for atmospheric CH4 in both treatments. Mean daily CH4 uptake rates from the pasture was found to be −6.8 μg CH4-C m−2 h−1 for the biochar plots and -7.3 μg CH4-C m−2 h−1 for the control plots. The highest uptake rates measured in individual chambers were up to -18 μg CH4-C m−2 h−1 (negative flux rates indicate soil uptake of atmospheric CH4). Over the measuring period no significant influence of the biochar amendment on CH4 fluxes could be observed.
A positive correlation between soil moisture and CH4 uptake rates was observed in both the biochar (r 2 = 0.27, n = 43, p < 0.01) and the control treatment (r 2 = 0.41, n = 43, p < 0.01), indicating that CH4 uptake is suppressed under higher soil moisture conditions. During the major rain events from 20–23 May, uptake rates decreased substantially and methane was emitted (up to 7.3 μg CH4-C m−2 h−1 in individual chambers) under the very high soil moisture conditions (Fig. 1).
There was no correlation between soil temperature and CH4 uptake rates (biochar: r 2 = 0.014; control: r 2 = 0.012; n = 284, p > 0.05). But a significant diurnal effect of soil temperature on CH4 fluxes could be observed for individual measurement days (Fig. 2). During the period from 2–5 May, highest uptake rates were generally observed early in the morning whereas lowest CH4 uptake occurred in the late afternoon. The application of N fertilizer on May 5 resulted in a reduction in CH4 uptake and during the following days no distinct diurnal pattern could be measured.
CO2 emissions
CO2 emissions arising from soil respiration could be observed during night hours only due to the confounding effects of plant photosynthesis during daylight hours inside the measuring chamber. Mean night-time CO2 emissions from the pasture ranged between 30 and 88 mg CO2 -C m−2 h−1 with no significant differences between biochar amended vs. control plots (Table 2). There was only a weak positive correlation between soil moisture and CO2 emissions in the biochar and no correlation in the control treatment (biochar: r 2 = 0.16, p < 0.05; control: r 2 = 0.03, p > 0.05, n = 41). A strong positive correlation between soil temperature and CO2 emission rates was observed in both the biochar (r 2 = 0.27, n = 41, p < 0.01) and the control treatment (r 2 = 0.41, n = 41, p < 0.01). In general, there were only minor temporal variations in CO2 emissions during the first 6 weeks of the observation period. During this time mean emissions typically ranged from 60 to 90 mg CO2 -C m−2 h−1 and followed no clear temporal trend. From 1 June, with declining soil temperature and moisture levels, CO2 fluxes decreased steadily to values of 30 mg CO2 -C m−2 h−1 (Fig. 1).
Discussion
N2O emissions
The mean N2O emissions were 30–35 μg N2O-N m−2 h−1 (corresponding to 2.7–3.1 kg N2O-N ha−1yr−1). This is higher than the 0.5 kg N2O-N ha−1yr−1 reported from extensively grazed subtropical pasture systems (Dalal et al. 2003; Denmead et al. 2000; Weier et al. 1991) and the 0.5–1.6 kg N2O-N ha−1yr−1 measured from a range of well established, unfertilized tropical pastures (Erickson et al. 2001; Keller and Reiners 1994; Mosier and Delgado 1997; Neill et al. 2005). But our measurements were lower than N2O flux measured in intensively managed temperate dairy pastures in Australia (4–13 kg N2O-N ha−1yr−1) (Dalal et al. 2003; Eckard et al. 2003; Phillips et al. 2007). However, it should be noted that we only measured emissions over a 2 month period in winter when high rates of fertiliser are commonly applied and consequently high fluxes of N2O can be expected. In order to fully capture seasonal and interannual variations of N2O emissions from subtropical pasture systems in Australia more year round measurements are required.
N2O emissions were found to be highly episodic with one major emission pulse accounting for 68% and 49% of the total emissions in the biochar and the control treatment, respectively (Fig. 1). This pulse emission occurred after heavy rainfall and the application of N fertilizer. This is in good agreement with previous reports that found highest N2O emissions following rainfall/irrigation shortly after N application (Hyde et al. 2006; Phillips et al. 2007). Various studies reported soil moisture content as one of the key regulators in gaseous N emissions from both temperate (Dobbie and Smith 2003; Ruzjerez et al. 1994; Smith et al. 1998) and tropical pastures (Keller and Reiners 1994; Veldkamp et al. 1998). Water content controlls the level of microbiological activity and the pathway of nitrogen loss (aerobic nitrification vs. anaerobic denitrification). In our study, peak N2O emissions occurred under soil moisture contents ranging from 78% to 83% WFPS, indicating that these pulse emissions were primarily a result of enhanced denitrification activity. During the rest of the measuring period, soil moisture typically varied from 43% to 73% WFPS. At WFPS below 65–75%, nitrification is typically the major source of N2O emissions, with optimum rates occurring between 60–70% (Bollmann and Conrad 1998; Linn and Doran 1984; Weier and Macrae 1993). This suggests that during periods with generally low fluxes (<50 μg N2O-N m−2 h−1) N2O emissions were predominantly produced through nitrification. However, this does not mean that at a WFPS below 70%, nitrification is the only source of N2O, and it is likely that nitrification and denitrification and/or nitrifier-denitrification were occurring simultaneously within aerobic and anaerobic microsites in the soil system (Livesley et al. 2008; Wrage et al. 2001). In many laboratory studies a strong positive correlation between soil temperature and N2O emissions has been observed (Kiese and Butterbach-Bahl 2002; Smith et al. 2003). Field studies in subtropical and tropical ecosystems often could only establish a weak or nonexistent relationship between N2O flux and soil temperature. In tropical Australia, Breuer et al. (2000) reported no influence of temperature and whilst Kiese and Butterbach-Bahl (2002) demonstrated a significant influence in a laboratory experiment, however observed no influence during their field campaign using the same soil. This weak correlation is most likely related to the small daily and seasonal temperature fluctuation in tropical climates, and the overlaying effect that changes in WFPS override any obvious influence of temperature variations. This is supported by our results which only showed a significant correlation between soil temperature and N2O emissions when we excluded the extraordinarily high N2O fluxes from the emission pulse, indicating that the N2O emission pulse was primarily triggered by rapid changes in soil moisture.
Over short periods when soil moisture conditions were non-limiting and near constant a clear diurnal N2O response to daily temperature fluctuations could be observed (Fig. 2). Highest fluxes were generally observed in the late afternoon/early night and the diurnal amplitude in N2O emissions was approximately 2-fold. This agrees well with the work of Livesley et al. (2008) who observed 2-fold N2O flux variation in response to diurnal temperature from 5–15°C changes in a temperate pasture system in Australia. Moreover, Scheer et al. (2008) reported a similar diurnal temperature effect in irrigated cotton when soil moisture conditions and inorganic nitrogen content were not limiting. However, other studies in agricultural systems did not observe a correlation between diurnal patterns of soil temperature and N2O flux (Ginting and Eghball 2005; Lessard et al. 1996), indicating that this diurnal emission patterns can only be observed under certain field conditions when other parameters such as WFPS and/or the availability of mineral nitrogen are not limiting. The diurnal temperature effect demonstrates that daily point measurements are often insufficient to represent the N2O daily flux rates, and emphasises the need for automated trace gas measurements with sub-daily resolution.
CH4 uptake
The soil at our site predominantly acted as a net sink for atmospheric methane. Mean CH4 uptake rates of−6.7 μg CH4-C m−2 h−1 (biochar) and -7.3 μg CH4-C m−2 h−1 (control) (corresponding to 0.59–0.64 kg CH4-C ha−1yr−1) are comparable to those measured in other subtropical or tropical pasture systems (Allen et al. 2009; Mosier and Delgado 1997; Verchot et al. 2000). Mosier and Delgado (1997) measured average uptake rates of −5.8 μg CH4-C m−2 h−1 from different tropical soils in Western Cost Rica with no significant differences across sites. However, little data is available for subtropical/tropical pasture systems and CH4 fluxes ranging from −58 to +70 μg CH4-C m−2 h−1 have been reported for different subtropical/tropical pasture sites (Dalal et al. 2008). The uptake rates are also within the range of different pasture system in temperate climates (Mosier et al. 1991; van der Weerden et al. 1999). In Australia, Livesley et al. (2008, 2009) measured uptake rates between −6.3 and -8.6 μg CH4-C m−2 h−1 in a sheep grazed pasture in Victoria and −5.97 μg CH4-C m−2 h−1 in a clover-grass pasture in Western Australia. Generally, observed rates of CH4 consumption were higher in temperate compared to tropical grasslands. In a review study on soil CH4 fluxes from different ecosystems Dalal et al. (2008) reported seven times greater CH4 uptake rates from temperate (−55 μg CH4-C m−2 h−1) compared to tropical (−8 μg CH4-C m−2 h−1) grasslands.
A significant positive correlation of soil moisture content and CH4 uptake has often been reported, since the magnitude of CH4 uptake by soils is largely controlled by diffusion of atmospheric methane into the soil (Ball et al. 1997; Koschorreck and Conrad 1993). In our study, we found CH4 uptake rates suppressed under high soil moisture conditions during major rainfalls from 20–23 May (Fig. 1). CH4 emissions were observed when WFPS exceeded 80%, indicating that the high soil moisture content created anaerobic conditions in the subsoil in such way that the soil became a net source of CH4. However, the soil-atmosphere exchange of CH4 is the result of simultaneously occurring production and consumption processes in soils and it has been shown that both CH4 production and CH4 oxidation can occur simultaneously in wet soil (Khalil and Baggs 2005).
We found only a weak correlation between soil temperature and CH4 oxidation with CH4 oxidation decreasing with increasing temperature. However, during the first week of the measurements we could clearly identify a response of CH4 uptake to the diurnal temperature fluctuations (Fig. 2) with highest uptake rates during the night and early in the morning when soil temperature was low. This is in contrast to other studies who found a positive correlation of net CH4 uptake with temperature (Butterbach-Bahl and Papen 2002; Dunfield et al. 1993; Wu et al. 2010). It remains unclear what caused this contrasting observations since microbial activity and hence CH4 uptake rates are generally expected to increase with soil temperatures increasing from 10°C to 25°C. We presume that CH4 uptake was influenced by diurnal effects of root respiration and/or other microbial soil processes which affected the availability of oxygen in the soil.
The application of urea on 5 May resulted in an approximate 30% decrease of CH4 uptake rates. This agrees well with observation from other studies where soil NH +4 status has frequently been reported to inhibit soil CH4 oxidation (Steudler et al. 1989; Veldkamp et al. 2001). This effect is commonly explained by CH4 oxidation and ammonium oxidation both competing for O2 and ammonia competitively binding to the methane monooxygenase (MMO) enzyme (Hutsch 1998). However, it has been noted that this may be an oversimplification since other studies showed no effect of fertilizer application on soil CH4 oxidation or even a stimulation of CH4 consumption in N limited soils (Bodelier and Laanbroek 2004; Glatzel and Stahr 2001; Veldkamp et al. 2001). Soil N content and fertilizer application can affect CH4 oxidation via various soil physicochemical and biological factors and alter the competition for N and C between plants methanotrophs and other microbial communities. Forest soils with high deposition of atmospheric nitrogen or agricultural soils with high fertiliser input seem to be the most prevalent systems where a N-based inhibition is expected to occur (Bodelier and Laanbroek 2004).
CO2 emissions
Average CO2 emission of 68 mg CO2-C m−2 h−1 (corresponding to 16.3 kg CO2-C ha−1 day−1) measured in the pasture system was greater than the 9.9 kg CO2-C ha−1 day−1 reported in a sub-humid subtropical pasture in central Queensland (Kaur et al. 2005) and values generally reported for global grasslands (4.1–5.1 kg CO2-C ha−1 day−1) (Raich and Schlesinger 1992), though data from tropical and subtropical pastures is sparse. The high CO2 emissions from this site could also be an indication of high pasture productivity and soil microbial turnover and potentially high rates of mineralisation and de/nitrification, which is reflected in the generally high GHG fluxes. Soil temperature was the most important environmental variable influencing CO2 flux which is in accordance with other studies on grasslands (Raich and Schlesinger 1992; Wu et al. 2010). However, there was only a weak correlation between soil moisture and CO2 emissions, which has often been reported as a key factor controlling soil respiration in pastures soils (Salimon et al. 2004; Wu et al. 2010). Soil moisture levels at our site were always in an optimal range (50–80% WFPS) for soil respiration so that it was never significantly limited by the soil water content. This is in agreement with other studies who reported enhanced CO2 emissions after the first rewetting after a prolonged dry period but only small increases after subsequent wetting events (Fierer and Schimel 2002; Wu et al. 2010). Therefore, we presume that the gradual decrease of soil CO2 emissions toward the end of the measurements was mainly because of a decrease in soil temperature rather than moisture limitation.
Effect of biochar amendment on soil GHG emissions
Assessment of the net emissions showed that there was no influence of the biochar amendment, however, some reductions were observed during certain periods of the sampling. The hypothesis that the application of biochar would lead to a reduction in emissions of GHG from Ferrosol under pasture was not confirmed in this field experiment. This is in contrast to other studies (laboratory based) where significant reductions in GHG emissions after the addition of biochar to soils were reported (Rondon et al. 2005; Singh et al. 2010; Spokas et al. 2009; Yanai et al. 2007). In a recent laboratory study Clough et al. 2010 found no impact of wood biochar on N2O emissions from a urine amended pasture soil and even elevated emissions from the biochar treatments for the first 30 d of incubations. These contrasting findings clearly show that the effect of biochar on soil borne GHGs is not understood yet and that different biochar types in combination with different soils can yield varying results. Moreover, so far these experiments were mainly conducted as short term laboratory studies and care must be taken when extrapolating laboratory findings to field scenarios. In our current study, the significantly lower N2O emissions from the biochar amended plots during short periods of time where WFPS was below 75% and the significant increase of plant N and P uptake in the biochar plots (Sinclair et al. 2009) shows that biochar can potentially affect C and N transformations in the soil. In contrast, using the same soil type, Van Zwieten et al. (2010) showed the greatest influence of biochar on reduction of N2O emissions during flooding of soil in a longer-term laboratory incubation. Therefore we assume that under certain soil and management conditions biochar amendment could potentially mitigate GHG emissions from soils. Clearly, more studies are needed to investigate if biochar has the potential to mitigate N2O emissions at field scale as has been indicated by laboratory incubations. Moreover, long-term studies are necessary to understand the long-lasting effect of biochar amendment on soil GHG emissions, and its response to seasonal and annual climatic variations.
Conclusion
To our knowledge this is the first study to report on the effect of soil biochar amendment on the emission of soil-borne GHGs based on high resolution field measurements. Using a fully automated closed chamber monitoring system we quantified emissions of N2O, CH4 and CO2 from an intensive subtropical pasture with and without biochar amendment. The hypothesis that the application of biochar would lead to a reduction in emissions of GHG from soils did not hold. This demonstrates that conclusions drawn from microcosm incubation studies cannot be automatically applied on a field scale. This study also confirmed that intensive pastures on acidic Ferrosols in Northern NSW in Australia can be a significant source of GHGs due to substantial emissions of N2O following fertilizer application. However, more long-term studies that fully capture seasonal and interannual variations of GHG emissions are necessary in order to develop accurate greenhouse gas budgets for these subtropical pasture systems in Australia.
References
Adam P (1994) Australian Rainforests. Oxford University Press
AGO (2007) National greenhouse gas inventory 2005. Australian Greenhouse Office, Commonwealth of Australia, Canberra
AGO (2010) National Greenhouse Account, National Inventory Report 2008, Volume 2. Australian Greenhouse Office, Commonwealth of Australia, Canberra
Allen DE, Mendham DS, Bhupinderpal S, Cowie A, Wang W, Dalal RC, Raison RJ (2009) Nitrous oxide and methane emissions from soil are reduced following afforestation of pasture lands in three contrasting climatic zones. Aust J Soil Res 47:443–458
Australian Bureau of Statistics (2009) Land Management and Farming in Australia, 2007–08
Ball BC, Dobbie KE, Parker JP, Smith KA (1997) The influence of gas transport and porosity on methane oxidation in soils. J Geophys Res Atmos 102:23301–23308
Barton L, Kiese R, Gatter D, Butterbach-Bahl K, Buck R, Hinz C, Murphy DV (2008) Nitrous oxide emissions from a cropped soil in a semi-arid climate. Glob Change Biol 14:177–192
Bodelier PLE, Laanbroek HJ (2004) Nitrogen as a regulatory factor of methane oxidation in soils and sediments. FEMS Microbiol Ecol 47:265–277
Bollmann A, Conrad R (1998) Influence of O2 availability on NO and N2O release by nitrification and denitrification in soils. Glob Change Biol 4:387–396
Breuer L, Papen H, Butterbach-Bahl K (2000) N2O emission from tropical forest soils of Australia. J Geophys Res Atmos 105:26353–26367
Butterbach-Bahl K, Papen H (2002) Four years continuous record of CH4-exchange between the atmosphere and untreated and limed soil of a N-saturated spruce and beech forest ecosystem in Germany. Plant Soil 240:77–90
Chan KY, Van Zwieten L, Meszaros I, Downie A, Joseph S (2007) Agronomic values of greenwaste biochar as a soil amendment. Aust J Soil Res 45:629–634
Chan KY, Van Zwieten L, Meszaros I, Downie A, Joseph S (2008) Using poultry litter biochars as soil amendments. Aust J Soil Res 46:437–444
Clough TJ, Bertram JE, Ray JL, Condron LM, O’Callaghan M, Sherlock RR, Wells NS (2010) Unweathered wood biochar impact on nitrous oxide emissions from a bovine-urine-amended pasture soil. Soil Sci Soc Am J 74:852–860
Dalal RC, Wang W, Robertson GP, Parton WJ (2003) Nitrous oxide emission from Australian agricultural lands and mitigation options: a review. Aust J Soil Res 41:165–195
Dalal RC, Allen DE, Livesley SJ, Richards G (2008) Magnitude and biophysical regulators of methane emission and consumption in the Australian agricultural, forest, and submerged landscapes: a review. Plant Soil 309:43–76
Denmead OT, Leuning R, Jamie I, Griffth DWT (2000) Nitrous oxide emissions from grazed pastures: measurements at different scales. Chemosphere Glob Change Sci 2:301–312
Dobbie KE, Smith AK (2003) Nitrous oxide emission factors for agricultural soils in Great Britain: the impact of soil water-filled pore space and other controlling factors. Glob Change Biol 9:204–218
Dunfield P, Knowles R, Dumont R, Moore TR (1993) Methane production and consumption in temperate and sub-arctic peat soils—response to temperature and Ph. Soil Biol Biochem 25:321–326
Eckard RJ, Chen D, White RE, Chapman DF (2003) Gaseous nitrogen loss from temperate perennial grass and clover dairy pastures in South-Eastern Australia. Aust J Agric Res 54:561–570
Erickson H, Keller M, Davidson EA (2001) Nitrogen oxide fluxes and nitrogen cycling during postagricultural succession and forest fertilization in the humid tropics. Ecosystems 4:67–84
Fierer N, Schimel JP (2002) Effects of drying-rewetting frequency on soil carbon and nitrogen transformations. Soil Biol Biochem 34:777–787
Ginting D, Eghball B (2005) Nitrous oxide emission from no-till irrigated corn: temporal fluctuation and wheel traffic effects. Soil Sci Soc Am J 69:915–924
Glatzel S, Stahr K (2001) Methane and nitrous oxide exchange in differently fertilised grassland in southern Germany. Plant Soil 231:21–35
Hutsch BW (1998) Methane oxidation in arable soil as inhibited by ammonium, nitrite, and organic manure with respect to soil pH. Biol Fertil Soils 28:27–35
Hyde BP, Hawkins MJ, Fanning AF, Noonan D, Ryan M, O’Toole P, Carton OT (2006) Nitrous oxide emissions from a fertilized and grazed grassland in the South East of Ireland. Nutr Cycl Agroecosyst 75:187–200
Isbell RF (2002) The Australian soil classification. CSIRO, Melbourne
Kaur K, Kapoor KK, Gupta AP (2005) Impact of organic manures with and without mineral fertilizers on soil chemical and biological properties under tropical conditions. J Plant Nutr Soil Sci-Zeitschrift Fur Pflanzenernahrung Und Bodenkunde 168:117–122
Keller M, Reiners WA (1994) Soil atmosphere exchange of nitrous-oxide, nitric-oxide, and methane under secondary succession of pasture to forest in the atlantic lowlands of Costa Rica. Glob Biogeochem Cycles 8:399–409
Kelly KB, Phillips FA, Baigent R (2008) Impact of dicyandiamide application on nitrous oxide emissions from urine patches in northern Victoria, Australia. Aust J Exp Agric 48:156–159
Khalil MI, Baggs EM (2005) CH4 oxidation and N2O emissions at varied soil water-filled pore spaces and headspace CH4 concentrations. Soil Biol Biochem 37:1785–1794
Kiese R, Butterbach-Bahl K (2002) N2O and CO2 emissions from three different tropical forest sites in the wet tropics of Queensland, Australia. Soil Biol Biochem 34:975–987
Koschorreck M, Conrad R (1993) Oxidation of atmospheric methane in soil—measurements in the field, in soil cores and in soil samples. Glob Biogeochem Cycles 7:109–121
Lehmann J, Gaunt J, Rondon M (2006) Bio-char sequestration in terrestrial ecosystems—a review. Mitig Adapt Strateg Glob Change 11:395–419
Lessard R, Rochette P, Gregorich EG, Pattey E, Desjardins RL (1996) Nitrous oxide fluxes from manure-amended soil under maize. J Environ Qual 25:1371–1377
Linn DM, Doran JW (1984) Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils. Soil Sci Soc Am J 48:1264–1272
Livesley SJ, Kiese R, Graham J, Weston CJ, Butterbach-Bahl K, Arndt SK (2008) Trace gas flux and the influence of short-term soil water and temperature dynamics in Australian sheep grazed pastures of differing productivity. Plant Soil 309:89–103
Livesley SJ, Kiese R, Miehle P, Weston CJ, Butterbach-Bahl K, Arndt SK (2009) Soil-atmosphere exchange of greenhouse gases in a Eucalyptus marginata woodland, a clover-grass pasture, and Pinus radiata and Eucalyptus globulus plantations. Glob Chang Biol 15:425–440
Lowe KF, Fulkerson WJ, Walker RG, Armour JD, Bowdler TM, Slack K, Knight RI, Moody PW, Pepper P (2005) Comparative productivity of irrigated annual ryegrass (Lolium multiflorum) pasture receiving nitrogen, grown alone or in a mixture with white (Trifolium repens) and Persian (T. resupinatum) clovers. Aust J Exp Agric 45:21–39
Mosier AR, Delgado JA (1997) Methane and nitrous oxide fluxes in grasslands in western Puerto Rico. Chemosphere 35:2059–2082
Mosier A, Schimel D, Valentine D, Bronson K, Parton W (1991) Methane and nitrous oxide fluxes in native, fertilized and cultivated grasslands. Nature Publishing Group
Neill C, Steudler PA, Garcia-Montiel DC, Melillo JM, Feigl BJ, Piccolo MC, Cerri CC (2005) Rates and controls of nitrous oxide and nitric oxide emissions following conversion of forest to pasture in Rondonia. Nutr Cycl Agroecosyst 71:1–15
Phillips FA, Leuning R, Baigenta R, Kelly KB, Denmead OT (2007) Nitrous oxide flux measurements from an intensively managed irrigated pasture using micrometeorological techniques. Agric For Meteorol 143:92–105
Raich JW, Schlesinger WH (1992) The global carbon-dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus Ser B-Chem Phys Meteorol 44:81–99
Rayment GE, Higginson FR (1992) Australian laboratory handbook of soil and water chemical methods. Inkata press ISBN 0 909605 68 8
Rondon M, Ramirez JA, Lehmann J (2005) Greenhouse Gas Emissions Decrease with Charcoal Additions to Tropical Soils. In: Proceedings of the 3rd USDA Symposium on Greenhouse Gases and Carbon Sequestration, Baltimore, USA, March 21–24, 2005, pp 208
Ruzjerez BE, White RE, Ball PR (1994) Long-term measurement of denitrification in 3 contrasting pastures grazed by sheep. Soil Biol Biochem 26:29–39
Salimon CI, Davidson EA, Victoria RL, Melo AWF (2004) CO2 flux from soil in pastures and forests in southwestern Amazonia. Glob Change Biol 10:833–843
Scheer C, Wassmann R, Kienzler K, Ibragimov N, Eschanov R (2008) Nitrous oxide emissions from fertilized irrigated cotton (Gossypium hirsutum L.) in the Aral Sea Basin, Uzbekistan: Influence of nitrogen applications and irrigation practices. Soil Biol Biochem 40:290–301
Sinclair K, Slavich P, Van Zwieten L, Downie A (2009) Productivity and nutrient availability on a ferrosol: Biochar, lime and fertiliser. In: 1st Asia Pacific Biochar Conference, Gold Coast 17–20 May, 2009, pp 79. ISBN 978 0 7347 1973 7
Singh BP, Hatton BJ, Singh B, Cowie AL, Kathuria A (2010) Influence of Biochars on Nitrous Oxide Emission and Nitrogen Leaching from Two Contrasting Soils. J Environ Qual 39
Smith KA, Thomson PE, Clayton H, McTaggart IP, Conen F (1998) Effects of temperature, water content and nitrogen fertilisation on emissions of nitrous oxide by soils. Atmos Environ 32:3301–3309
Smith KA, Ball T, Conen F, Dobbie KE, Massheder J, Rey A (2003) Exchange of greenhouse gases between soil and atmosphere: interactions of soil physical factors and biological processes. Eur J Soil Sci 54:779–791
Spokas KA, Koskinen WC, Baker JM, Reicosky DC (2009) Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere 77:574–581
Steudler PA, Bowden RD, Melillo JM, Aber JD (1989) Influence of nitrogen fertilization on methane uptake in temperate forest soils. Nature 341:314–316
van der Weerden TJ, Sherlock RR, Williams PH, Cameron KC (1999) Nitrous oxide emissions and methane oxidation by soil following cultivation of two different leguminous pastures. Biol Fertil Soils 30:52–60
Van Zwieten L, Singh BP, Joseph S, Kimber S, Cowie A, Chan Y (2009) Biochar reduces emissions of non-CO2 GHG from soil. In: Lehmann J Joseph S (eds) Biochar for environmental management. Earthscan Publications, pp 227–249
Van Zwieten L, Kimber S, Morris S, Downie AE, Berger E, Rust J, Scheer C (2010) Influence of biochars on flux of N2O and CO2 from Ferrosol. Aust J Soil Res 48:555–568
Veldkamp E, Keller M, Nunez M (1998) Effects of pasture management on N2O and NO emissions from soils in the humid tropics of Costa Rica. Glob Biogeochem Cycles 12:71–79
Veldkamp E, Weitz AM, Keller M (2001) Management effects on methane fluxes in humid tropical pasture soils. Soil Biol Biochem 33:1493–1499
Verchot LV, Davidson EA, Cattanio JH, Ackerman IL (2000) Land-use change and biogeochemical controls of methane fluxes in soils of eastern Amazonia. Ecosystems 3:41–56
Weier KL (1994) Nitrogen use and losses in agriculture in subtropical Australia. Fertil Res 39:245–257
Weier KL, Macrae IC (1993) Net mineralization, net nitrification and potentially available nitrogen in the subsoil beneath a cultivated crop and a permanent pasture. J Soil Sci 44:451–458
Weier KL, MacRae IC, Myers RJK (1991) Seasonal variation in denitrification in a clay soil under a cultivated crop and a permanent pasture. Soil Biol Biochem 23:629–635
Weier KL, Macrae IC, Myers RJK (1993) Denitrification in a clay soil under pasture and annual crop—estimation of potential losses using intact soil cores. Soil Biol Biochem 25:991–997
Wrage N, Velthof GL, van Beusichem ML, Oenema O (2001) Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol Biochem 33:1723–1732
Wu X, Yao Z, Bruggemann N, Shen ZY, Wolf B, Dannenmann M, Zheng X, Butterbach-Bahl K (2010) Effects of soil moisture and temperature on CO2 and CH4 soil atmosphere exchange of various land use/cover types in a semi-arid grassland in Inner Mongolia, China. Soil Biol Biochem 42:773–787
Yanai Y, Toyota K, Okazaki M (2007) Effects of charcoal addition on N2O emissions from soil resulting from rewetting air-dried soil in short-term laboratory experiments. Soil Sci Plant Nutr 53:181–188
Zhang ALC, Gengxing P, Lianqing L, Qaiser H, Xuhui Z, Jinwei Z, David C (2010) Effect of biochar amendment on yield and methane and nitrous oxide emissions from a rice paddy from Tai Lake plain, China. Agriculture, Ecosystems & Environment In Press, Corrected Proof
Acknowledgements
The authors would like to acknowledge Dr Peter Slavich and Ms Katrina Sinclair from Industry and Investment NSW. This work was undertaken on a field site established as a component an ACIAR funded project, ‘Improving the utilisation of water and soil resources for tree crop production in coastal areas of Vietnam and NSW’. We also thank three anonymous reviewers for valuable comments on an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Klaus Butterbach-Bahl.
Rights and permissions
About this article
Cite this article
Scheer, C., Grace, P.R., Rowlings, D.W. et al. Effect of biochar amendment on the soil-atmosphere exchange of greenhouse gases from an intensive subtropical pasture in northern New South Wales, Australia. Plant Soil 345, 47–58 (2011). https://doi.org/10.1007/s11104-011-0759-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-0759-1