Abstract
The overall aim of this study was to test for inter-species variation in plant and soil responses to defoliation among a broad range of temperate grass species and life-history strategies. We used a microcosm experiment where a range of grass species differing in life history traits were subjected to different intensities of defoliation, and a range of aboveground and belowground plant and soil responses were measured. All plant attributes, including accumulated shoot biomass, root biomass and root length, showed a strong negative response to defoliation, although plant species exhibited subtle differences in the way that they responded to increased severity of defoliation. Defoliation also exerted a strong influence on soil properties, decreasing soil microbial carbon (C) and the soil microbial C:nitrogen (N) ratio, and increasing inorganic N availability and potential N mineralisation across all species. Despite the wide range in life history strategies, plant species did not differ in their influence on most of the soil variables, except for the rate of nitrate mineralisation, which was lowest under plant species that displayed the least relative detrimental responses to defoliation. Collectively, our results suggest that plant and soil responses to defoliation are reasonably consistent across a broad range of grass species, with only subtle inter-specific differences among species.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The last few decades have witnessed an increasing attention being devoted to understanding the role that grazers play in regulating soil biogeochemical processes (Bardgett and Wardle 2010). This research has shown how grazing can modify a number of ecosystem properties, all of which ultimately impact on rates of soil nutrient cycling with feedback consequences for primary production. For example, grazing-induced increases in soil compaction (Cumming and Cumming 2003), alterations of soil water regimes (Medina-Roldán et al. 2007), plant species composition (Ritchie et al. 1998; Wardle et al. 2001), and aboveground and belowground primary productivity (Milchunas and Lauenroth 1993; Burke et al. 1998) are regularly cited as drivers of soil nutrient dynamics. Also, grazing animals can affect the spatial distribution of nutrients within ecosystems as a result of their movements and patchy return of nutrients in excreta, which in turn influences vegetation patterns (de Mazancourt et al. 1998; Bardgett and Wardle 2010).
Over time scales from days to months, plant responses to biomass removal, or defoliation, act as a key mechanism by which grazing affects soil nutrient dynamics. Defoliation has been shown to alter plant allocation patterns, in terms of both root biomass (Mikola et al. 2001a) and root exudation (Paterson and Sim 1999, 2000). These changes in plant allocation can, in turn, have cascading effects on belowground food-webs, modifying microbial abundance and activity (Guitian and Bardgett 2000; Mikola et al. 2001a; Hamilton and Frank 2001) and the abundance of microbial predators (Mikola et al. 2001a; Hokka et al. 2004). All together, the effects on belowground food-webs mediated by defoliation have been linked to increases in soil nutrient mineralisation and availability (Hamilton and Frank 2001; Mikola et al. 2001a), and this is thought to contribute to the compensatory response of plant growth to herbivory (Hamilton and Frank 2001; Mikola et al. 2009).
Past studies suggest that plant and soil responses to defoliation vary across plant species and with the frequency of defoliation (Chapin and Slack 1979; Briske 1996; Guitian and Bardgett 2000; Klironomos et al. 2004; Ilmarinen et al. 2005; Gastal et al. 2010). Such differential responses of plant species to defoliation have been explained on the basis of plant functional traits which influence soil-resource dynamics, such as root exudates entering into the decomposers food-web (Holland et al. 1996; Mawdsley and Bardgett 1997; Mikola and Kytöviita 2002), and traits directly linked to the uptake of nutrients and competition for these with soil microbes such as root biomass and root length density (Chapin and Slack 1979; Oesterheld 1992). Most studies on this topic, however, have focused on a handful of plant species, and none, as far as we are aware, have comprehensively and simultaneously tested the responses of a broad range of plant species to defoliation. As a result, little is known about the differential response of grassland plant species and their associated soil microbial communities to defoliation, and this hampers our ability to draw generalizations about plant and soil responses to grazing in grassland ecosystems. Here, we redress this lack of knowledge by testing for inter-species variation in plant and soil responses to defoliation among a broad range of temperate grass species and life-history strategies. Specifically, we test the hypotheses that soil biological properties related to N cycling would be correlated with the particular response of a plant species to defoliation (i.e. soil responses could be predicted from the defoliation response of the plants). This was done by measuring aboveground and belowground plant responses of a range of common UK grass species to different intensities of defoliation, and associated changes in soil microbial biomass and rates of N cycling, in a glasshouse microcosm experiment.
Material and methods
Experimental set-up
A range of seven common British grass species representing a spectrum of life histories including response to grazing and defoliation (see Table 1 for a summary of RGRmax, RGRmean, C-S-R strategy, Ellenberg indicator values, and grazing and defoliation response), were grown in a glasshouse experiment during the spring of 2007 at Lancaster University, U.K. Briefly, seeds (Ermorsgate Seeds, Norfolk, UK) of the grass species Nardus stricta L., Anthoxanthum odoratum L., Festuca rubra L., Poa pratensis L., Agrostis capillaris L., Lolium perenne L., and Holcus lanatus L. (nomenclature follows Clapham et al. 1987), were germinated on a 1:1 (w/w) mixture of commercial acidic sand and Levingtons M3 growing media. The slow growing species N. stricta was sown 6 weeks before the rest of the grasses in order to reduce differences in plant size which are inherently dependent on the wide range in RGRmax employed in this study (Grime and Hunt 1975) (Table 1). The selected species represent a range of grasses with different plant traits which occur on grasslands subjected to different management intensities from infertile upland grasslands (N. stricta), Agrostis-Festuca and semi-improved grasslands (A. odoratum, F. rubra, P. pratensis, A. capillaris, H. lanatus) to highly productive pastures (L. perenne) (Rodwell 1992; Grayston et al. 2001).
Established seedlings were transplanted into pots (11 × 11 cm, height and diameter) following a similar design as that used by Guitian and Bardgett (2000). Pots’ substratum was composed of a nutrient-poor soil (N% = 0.6) coming from and acidic (pH in H2O = 4.5) soil taken from a semi-natural Festuca ovina-Agrostis capillaris grassland (National Vegetation Classification: U4b, Rodwell 1992) located in Littledale, Lancashire, UK (Bardgett et al. 2003). Two weeks later, each grass species planted in the experimental pots was randomly assigned to 1 out of 3 defoliation treatments simulating different intensities of grazing, namely: 1) control plants without defoliation (UN); 2) light defoliated plants, clipped each 2 weeks (LD); and 3) heavy defoliated plants clipped at weekly intervals (HD). The defoliation treatment was imposed over an 8-week period and consisted of the removal of aboveground tissue at 4 cm above the soil surface. Treatments were applied in a randomised block design with 4 replicates per plant species and defoliation treatment combination, yielding a total of 84 pots (7 species X 3 defoliation X 4 blocks). Plants were watered on every other day and remained in a glasshouse in Lancaster University with the following settings: 12 h light-night periods providing a mean irradiance of 430 Wm-2, mean day temperature of 22°C, and a mean night temperature of 18°C.
Measurement of aboveground variables
Before applying the defoliation treatments, initial shoot biomass was calculated in order to use this measure as a covariate for statistical analyses, and hence rule out size-specific effects of different plant species on response variables. This calculation was based on species-specific allometric relationships between leaf length and shoot biomass of a subset of grass seedlings (N. stricta, y = −6.79x1.2, R 2 = 0.87, P < 0.001, n = 17; A. odoratum, y = −6.3x1.3, R 2 = 0.97, P < 0.001, n = 6; F. ovina, y = −4.64x0.9, R 2 = 0.96, P < 0.001, n = 18; H. lanatus, y = −5.37x1.1, R 2 = 0.78, P < 0.001, n = 20; P. pratensis, y = −3.53x0.9, R 2 = 0.95, P < 0.001, n = 17; A. capillaris, y = −7.45x1.2,,R2 = 0.96, P < 0.001, n = 18; and L. perenne, y = −6.73x1.2 , R 2 = 0.93, P < 0.001, n = 17). We, then, estimated leaf length in the experimental pots (digital images analysed in JMicroVision for Linux; Roduit 2007) and initial shoot biomass was calculated based on the estimated leaf lengths using the aforementioned allometric parameters. At the end of the experiment, all aboveground plant biomass was harvested from each pot and combined with that collected from previous clippings to determine total shoot biomass (after 70°C drying for 48 h) produced over the experimental period.
Measurement of belowground variables
Plant root systems were manually removed from each pot to estimate root-related variables. Total root length was estimated by species-specific allometric relationships between root length and root mass from root sub-samples. First, we scanned sub-samples of the fresh root systems in a conventional scanner (CanoScan 4200 F, Canon Inc. Tokyo Japan). Roots were submerged into water in an acrylic tray avoiding root overlapping as much as possible and scanned in a 300 dpi resolution. These digital images were analysed using an image analysis software (ImageJ, Rasband 2007) by the method described in Kimura et al. (1999), and this estimation of root length was used to model total root length using total root mass as the predictor. This approach produced highly significant parameters to estimate total root length for all species (data not included). Root dry biomass (including grass crowns) was determined by oven-drying the roots as with aboveground biomass.
Soil collected after root recovery was passed through a 2 mm mesh sieve and stored at 4°C before the analytical assays were carried out. Microbial biomass carbon (C) and N were determined by extracting 5 g of soil (fresh basis) in 25 mL of 0.5 M K2SO4 of both 24 h-CHCl3 fumigated and unfumigated soil samples (Vance et al. 1987). Soil-K2SO4 extracts were shaken for 30 min and filtered through a 1-grade Whatman paper. Microbial biomass C was calculated as the difference in total C in fumigated and unfumigated soil extracts, determined with a Shimadzu 5000A TOC analyser (Shimadzu Inc., Japan) and using an extraction efficiency factor (keC) of 0.45 (Sparling et al. 1990). Microbial biomass N was measured as the difference in total N (N-NH +4 and N-NO −3 ) produced by the persulfate digestion method (Cabrera and Beare 1993) between fumigated and unfumigated soil extracts, measured by automated continuous flow colorimetry in a Bran and Luebbe AutoAnalyser 3. The extraction efficiency factor (keN) used for N in microbial biomass was 0.54 (Brookes et al. 1985).
Potential NH +4 mineralisation rate was determined by measuring the N-NH +4 difference between KCl extracts of incubated and non-incubated soil samples (Campbell et al. 1993; Harrison and Bardgett 2010). Non-incubated samples were extracted without previous incubation, whereas incubated samples were held for a period of 14 days in sealed steriline bottles at 25°C. Extractions were made with 10 g of fresh soil extracted in 50 ml 1 M KCl, shaken for 60 min and filtered through a 1-grade Whatman paper. Ammonium mineralisation rate was calculated as (N-NH +4 t14 - N-NH +4 t0)/(14 days) (mg N-NH +4 kg dry soil−1 day−1). Nitrate mineralisation rate was determined following the same procedure as for N-NH +4 , but for N-NO −3 . Both N-NH +4 and N-NO −3 in KCl extracts were measured by automated continuous flow colorimetry as described above.
Statistical analyses
All variables were tested for normality with the Shapiro-Wilks test and normalised if necessary (Zar 1998). Data were analysed using ANOVA with plant species and defoliation as fixed experimental factors, and blocks as a random factor by using general linear mixed models (Pinheiro et al. 2008). For plant-related variables (aboveground and belowground biomass, root length, and specific root length), ANCOVA was used to test the effect of plant species and defoliation including initial shoot biomass as a covariate term and models’ estimates were corrected depending on the significance of these term (Engqvist 2005). Estimated shoot initial biomass (see aboveground variables) values were (average dry weight (mg) ± standard deviation): N. stricta (22 ± 15), F. rubra (60 ± 12), P. pratensis (160 ± 43), H. lanatus (310 ± 39), A. odoratum (373 ± 134), A. capillaris (16 ± 3), and L. perenne (165 ± 49); thus justifying its use in the ANCOVA models. All statistical tests were carried out with the R statistical package for Linux (R Development Core Team 2010).
Results
Aboveground responses
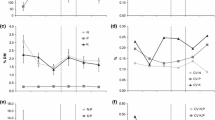
At final harvest, the extent that total accumulated shoot biomass was reduced by defoliation varied among the grass species, as shown by the significant defoliation by species interaction (F 12,57 = 7, P < 0.0001, Fig. 1a). Relative reductions in total accumulated shoot biomass resulting from defoliation were more pronounced for F. rubra, H. lanatus, A. odoratum and A. capillaris when defoliation intensity was low (72, 56, 65 and 57% reductions in shoot biomass between lightly-clipped and undefoliated controls, respectively) than under more severe defoliation (6, 20, 7 and 38% reductions in shoot biomass between heavy and light defoliation, respectively). In contrast, decreases in shoot biomass of P. pratensis and N. stricta were of a similar magnitude across the defoliation frequency treatments. Finally, shoot biomass of L. perenne increased under light defoliation but decreased moderately under heavy-defoliation frequency. Individual ANOVA’s for each grass species (note that cross-species comparisons are not valid for these analyses) showed that species roughly grouped into those whose shoot biomass significantly decreased for both defoliation frequencies (i.e., A. capillalris, H. lanatus and P. pratensis), those which were sensitive to light but not further high intensify defoliation (i.e., no difference between lightly- and heavily-defoliated plants, namely A. odoratum, F. rubra and N. stricta), and L. perenne which, although responsive to defoliation, did not show a clear separation among defoliation intensities because it yielded a very low shoot biomass in the undefoliated controls (Fig. 1a).
Plant response to different intensities of defoliation across a broad range of common grass species, including: a Total accumulated shoot biomass; b belowground biomass; c root: shoot ratio; and d total root length (as estimated from allometric equations fitted to root mass and root length data estimated by image analysis). Labels are: UN = undefoliated controls ; LC = light defoliation (defoliation each two weeks); and HD = heavy defoliation (weekly). N.st = Nardus stricta, A.od = Anthoxanthum odoratum, F.ru = Festuca rubra, P.pr = Poa pratensis, L.pe = Lolium perenne A.ca = Agrostis capillaris, H.la = Holcus lanatus. All values are back-transformed means ± 1 SE. In the case of aboveground biomass, values are adjusted means ± 1 adjusted SE as determined by ANCOVA with biomass in the beginning of experiment being the significant covariate
Belowground responses
Root biomass decreased strongly in response to defoliation across all plant species (F 2,55 = 140.5, P < 0.0001, Fig. 1b). Unlike shoot biomass, the decrease in root biomass in response to defoliation was similar for all species (species X defoliation interaction effect, F 12,55 = 1.5, P = 0.17), and all species showed a high sensitivity to light defoliation (an average 71% reduction between light defoliation and controls). Post-hoc tests showed that there were no differences between the means for the light- and heavy-frequency defoliation for A. odoratum, F. rubra, and N. stricta (data not shown). Plant species significantly differed in root biomass (F 6,55 = 18, P < 0.0001, values for the individual q statistics of the Tukey’s test not included) in the order H. lanatus > A. odoratum > A. capillaris, N. stricta = L. perenne > P. pratensis > F. rubra.
We detected a significant defoliation by species interaction for the root to shoot mass ratio (R:S) (F 12,55 = 3, P = 0.004) which provides additional evidence that plant species responded differently across the two defoliation frequencies (Fig. 1c). No clear relationship could be observed for the reductions in R:S in response to defoliation or in relation to species life history strategy, at least based on published values of RGRmax.
Total root length (TRL) decreased in response to defoliation (Fig. 1d), and this reduction varied among the plant species with defoliation frequency as shown by the species by defoliation interaction (F 12,52 = 2, P < 0.05). A capillaris and P. pratensis showed similar reductions in TRL across the two defoliation frequencies (A. capillaris = 71% reduction between the undefoliated control and light defoliation, and 55% between light defoliation and the heavy defoliation treatment; P. pratensis = 65 and 57% for the same treatments respectively). On the other hand, the reduction in TRL of L. perenne was higher when defoliation frequency increased (33% reduction between undefoliated controls and light defoliation and 55% reduction between light and heavy defoliation respectively). A. odoratum, F. rubra, H. lanatus and N. stricta were relatively insensitive to heavy defoliation, displaying only a low reduction in TRL from the low to the high intensity treatment. Individual ANOVA’s for each grass species (data not shown) showed basically the same patterns as those already described, i.e., a group of species which responded consistently negative to increasing frequency of defoliation (i.e., A. capillaris, H.lanatus, L. perenne and P. pratensis) and another which was sensitive to low, but not further high intensity defoliation (as shown by the means differences between these treatments, A. odoratum, F. rubra, and N. stricta). Since we used root mass in our species-specific estimations of TRL, statistical analysis of specific root length would produce more or less the same qualitative results as those for root mass. However, results among species can portray some insight on the extent of the costs species incurred in terms of root production (with lower values indicating higher construction costs). Specific root length followed the sequence L. perenne (110 ± 10) < F. rubra (137 ± 11) < P. pratensis (141 ± 14) < N. stricta (143 ± 22) < H. lanatus (169 ± 14) < A. odoratum (172 ± 11) < A. capillaris (172 ± 12 mg−1 respectively).
We used principal component analysis (PCA) on transformed response variables (aboveground and belowground biomass, root to shoot ratio and total root length) to summarize plant species responses to defoliation. The first PCA axis explained 78% of the variance in the data and was associated with defoliation frequency, with belowground plant attributes (i.e. root mass and total root length) having the highest loads on this first component (data not shown).
Soil responses
When data were integrated across all species, light and heavy frequency defoliation reduced microbial biomass C in soil (F 2,53 = 7.5 P < 0.01, Fig. 2a). Microbial biomass C was reduced from 2040 ± 147 (mean ± 1 SE) mg C kg-1 in soil of control plants to 1480 ± 71 and 1465 ± 102 mg C kg−1 in soil of plants under moderate and heavy defoliation frequency respectively and this reduction in microbial C was correlated to reductions in root biomass caused by defoliation (r = 0.36, t 74 = 3.5, P = 0.001). Post-hoc analyses showed, however, that microbial C means across defoliation frequencies were significantly different only for H. lanatus and A capillaris, and for A. odoratum between the undefoliated and the clipped-treatments (Fig. 2a). In contrast, defoliation did not affect microbial biomass N (F 2,54 = 1.5, P = 0.26, Fig. 2b); hence, across all plant species, microbial C:N ratio was reduced by 20.0 and 25.6% under moderate and heavy defoliation, respectively, when compared with the undefoliated control (F 2,53 = 8.5, P < 0.001, Fig. 2c).
Responses to different intensities of defoliation across a broad range of common grass species of soil microbial biomass carbon (a); soil microbial nitrogen (b); and the microbial C: N ratio (c). UN = undefoliated controls; LD = light-defoliation (defoliation each two weeks); HD = heavy defoliation (weekly). N.st = Nardus stricta, A.od = Anthoxanthum odoratum, F.ru = Festuca rubra, P.pr = Poa pratensis, L.pe = Lolium perenne A.ca = Agrostis capillaris, H.la = Holcus lanatus. All values are means ± 1 SE
Soil N availability was also affected by defoliation treatments (Fig. 3a). Across all plant species, heavy defoliation increased soil NH +4 (F 2,54 = 3, P = 0.05) and total inorganic N (F 2,54 = 3, P = 0.05) concentrations by 35% and 29% respectively compared to the undefoliated control. Mean comparison tests showed significant differences in N stricta and A. capillaris between undefoliated controls and the defoliated treatments but not in the rest of the species. Soil NO −3 concentrations were not significantly affected by defoliation (F 2,54 = 3, P > 0.05, Fig. 3b), although potential net nitrification rate increased by almost 200% under heavy defoliation (F 2,54 = 3.5, P < 0.05) relative to the undefoliated controls (Fig. 3c). The rate of net NO3 − mineralisation also varied significantly among plant species (F 6,54 = 3.5, P < 0.01, Fig. 3c), being greatest in soil planted with N. stricta (2.9 ± 1.9 mg NO −3 kg soil−1 day−1) and lowest in soil of A. capillaris (0.21 ± 0.22 mg NO −3 kg soil−1 day−1). The sequence of NO −3 mineralisation after post-hoc tests was, in descending order, N. stricta, L. perenne, F. rubra, P. pratensis, H. lanatus, A. odoratum, and A. capillaris. Some plant species at the fast-growing end of the life history gradient had the lowest values of net NO −3 mineralisation, but the overall trend was erratic (non significant species X defoliation interaction). Post-hoc tests showed that mean differences in NO −3 mineralisation between heavy defoliation and the other treatments were significant for A. odoratum, F. rubra, H. lanatus and P. pratensis. Neither net potential NH +4 nor total N mineralisation rates (Fig. 3d) were affected by the experimental treatments and grass species identity did not, in general, influence soil variables, aside potential net NO −3 mineralisation rate as already stated.
Soil responses to different intensities of defoliation across a broad range of common grass species, including ammonium availability (a), mineral soil N (ammonium + nitrate) (b), net potential nitrate mineralisation rate (c) and the total N mineralisation rate (ammonium + nitrate) (d); UN = undefoliated controls; LD = light defoliation (defoliation each two weeks); HC = heavy defoliation (weekly). N.st = Nardus stricta, A.od = Anthoxanthum odoratum, F.ru = Festuca rubra, P.pr = Poa pratensis, L.pe = Lolium perenne A.ca = Agrostis capillaris, H.la = Holcus lanatus. All values are means ± 1 SE
Discussion
In this study, we tested whether plant responses to defoliation vary across a range of grassland species representing a broad range in life-history strategies, and whether soil microbial and biogeochemical responses to defoliation were related to such inter-species variation. We detected a general detrimental effect of defoliation on shoot and root growth among all plant species tested, and subtle inter-specific differences in the response to defoliation frequency. In particular, A. capillaris and H. lanatus showed consistently higher absolute values in shoot and root biomass and root length, and relatively lower losses in root attributes across defoliation treatments (the relative change in biomass in Ferraro and Oesterheld 2002), but their performance was reduced when defoliation was intensified (from light to heavy defoliation frequencies). The same reductions in response to defoliation were observed in P. pratensis and L. perenne, but these later species displayed low absolute values in plant measures. Yet another group of species, A. odoratum, F. rubra and N. stricta, showed no additional decreases in performance between light and heavy defoliation in most of our plant measures, which we interpret here as a sign of resistance to defoliation. Abundance of N stricta is often high in grazed grasslands (Welch 1986), which is attributed to its unpalatable shoot tissue (Massey et al. 2007), and Hartley and Amos (1999) reported that defoliation (a less severe regime than that used here) did not cause reductions in root length of N. stricta plants, adding evidence that this species is resistant to defoliation. There is evidence of F. rubra showing compensatory growth responses to grazing as well (Berg et al. 1997; Van der Graaf et al. 2005). Several other studies have likewise found declines in root productivity in response to defoliation (Guitian and Bardgett 2000; Mikola et al. 2001a), although evidence is mixed. For example, fenced exclusion studies on Serengeti grasslands show that mammalian grazers do not necessarily inhibit root biomass and productivity (McNaughton et al. 1998), and in a global literature synthesis Milchunas and Lauenroth (1993) reported both enhancements and reductions in root biomass as a result of herbivore exclusion. Moreover, in a meta-analysis on graminoids, Ferraro and Oesterheld (2002) showed that the effects of defoliation were less acute on root than on shoot biomass. Nevertheless, our analysis showed that root mass and root length are important attributes in describing inter-specific differences in response to defoliation. Root length is a measure of plant foraging scale (Kembel and Cahill 2005) and therefore it might be associated with the response of grasses to defoliation.
In general, the detrimental response of plant growth to defoliation was mirrored in the soil biological properties measured, although inter-specific differences were not detected. Across all plant species, defoliation was found to reduce microbial biomass and its C: N ratio, and to increase NH +4 availability and the rate of NO −3 mineralisation. The negative response of microbial biomass C to defoliation that we observed contrasts sharply with results of a number of experimental studies, which have found that defoliation stimulates soil microbes (Mawdsley and Bardgett 1997; Bardgett et al. 1998; Mikola et al. 2001b). Increases in microbial biomass following defoliation have been attributed to the stimulation of root exudation (Holland 1995; Holland et al. 1996; Mawdsley and Bardgett 1997; Hamilton et al. 2008), and this stimulation of root exudates as a result of clipping has been reported for some of the plant species used in our experiment, namely L. perenne and F. rubra (Paterson and Sim 1999, 2000). Our findings indicate, however, that defoliation caused soil microbes to become limited by C, as evidenced by the decline in the microbial C:N ratio, which is indicative of increase in C relative to N limitation (Kaye and Hart 1997). This decline is also likely to be related to the reduction in root biomass across all species as a result of defoliation, a view supported by the positive correlation of root biomass with microbial biomass C. The lack of effect that increasing the intensity of defoliation had on microbial biomass C is likely explained by the relatively large effect that light defoliation had on plant performance relative to the undefoliated controls. The decline in microbial biomass C could also be partly due to increased predation by soil animals, given that previous studies have shown that defoliation enhances the abundances of microbial-feeding faunal groups in soil (Mikola et al. 2001a; b). However, in our study, it is most likely that reductions in root C allocation under defoliation, and hence C supply to soil, is the main cause of the consistent decline in microbial biomass across all defoliated plant species (Mikola et al. 2001a; Bazot et al. 2005; Hamilton et al. 2008; Sankaran and Augustine 2004).
Defoliation increased NH +4 and total inorganic N availability, as well as the potential rates of NO −3 mineralisation across all species tested. A number of studies have documented a stimulatory effect of defoliation (Hamilton and Frank 2001; Mikola et al. 2001a; Ayres et al. 2007) and ungulate grazing (Seagle et al. 1992; Hamilton et al. 2008) on soil N availability and mineralisation, and this response is thought to be a key mechanism contributing to compensatory growth in grazed grassland (Owen 1980; Ritchie et al. 1998; Hamilton and Frank 2001; Bardgett and Wardle 2003). Such enhanced soil N mineralisation has been attributed to a variety of mechanisms, including the return of N-rich plant litter and animal wastes to soil (Day and Detling 1990; de Mazancourt et al. 1998), and the stimulation of microbial activity and N mineralisation in the root zone due to enhanced root exudation in defoliated plants (Hamilton and Frank 2001; Mikola et al. 2001a, b). In our experiment, however, we propose that the defoliation-induced increase is soil N availability and mineralisation was due to the previously mentioned switch to C limitation of the microbial biomass, as indicated by the reduction in microbial C:N across all defoliated plants. Indeed, it is well established that under conditions when microbial growth is C limited, microbes use the C to support their energy needs and they excrete plant available ammonium (NH +4 ) as a waste product into soil i.e. N is mineralised by the microbial biomass (Kaye and Hart 1997). Microbial C limitation together with the strong negative impact of defoliation on plant size and the concomitant reduction in total plant N might be the cause for the increase in N availability. As previously mentioned, it is also possible that higher rates of microbial predation in soils of defoliated plants contributed to the stimulation of soil N availability via the microbial-loop (Clarholm 1985), although this was not measured in this study.
Despite the wide spectrum in ecological traits in the plant species we used, and the subtle differences in plant growth responses to defoliation, few inter-specific differences were observed in the response of soil properties. Only for the rate of NO −3 mineralisation did we detect inter-specific differences in the responses to defoliation. Here, we found that the rate of NO −3 mineralisation was significantly lower in soils planted with the grasses which showed the highest biomass values, namely A. capillaris, A. odoratum and H. lanatus, than in soils planted with N. stricta. However, we did not detect a defoliation by species interaction, indicating that such inter-specific differences in NO −3 mineralisation were independent of defoliation. This trend of lower rates of NO −3 mineralisation in soils planted with those species which exhibited higher biomass values across defoliation treatments is difficult to interpret given that no concomitant changes in NH +4 availability were detected for the same set of species. The absence of inter-species differences in other soil properties across the species tested is in contrast to previous studies which show that plant species, and even genotypes, can have markedly different effects on soil biological properties, acting as major determinant of microbial communities in soil (Bardgett et al. 1999; Innes et al. 2004; Bezemer et al. 2006; Markham et al. 2009; Harrison and Bardgett 2010; Orwin et al. 2010). We do not know the reason for the absence of such inter-species variation in soil properties in our experiment. However, given that inter-specific differences in most soil biological properties were apparent in the undefoliated controls, but not in defoliated plants, it appears that defoliation has cancelled out any differences at the species level. Secondly, it has been shown that the effects of plant species on soil properties are dependent on soil type (Innes et al. 2004; Marschner et al. 2004; Bezemer et al. 2006). In this way, it seems that our soil might have restricted the expression of strong plant effects on soil biological properties, suggesting that other factors, such as low pH and nutrient availability, might have been primary determinants of these measures. Despite this, defoliation was found to consistently and strongly promote soil nutrient availability in soil across all species tested.
In conclusion, our results show that grassland plant species representing a broad range of life history strategies respond in a consistent way to defoliation. Across all species tested, we found that defoliation reduced plant growth, especially of root mass and length, but stimulated N availability in soil. We did not measure the consequences of this defoliation-induced stimulation of N availability, but we propose that it would, in the long term, positively feedback to the plant in terms of improved N acquisition and, potentially, improved growth. Surprisingly, we found only subtle differences in the response of different plant species to defoliation, and no inter-species variation in the response of soil properties to this treatment. This suggests that, in these soils, effects of defoliation on plant and soil properties are remarkably consistent, at least under the experimental conditions used in this study. Overall, our findings reinforce the view that aboveground and belowground components of ecosystems are strongly interrelated and that understanding the effects of grazing on ecosystems nutrient cycling requires a combined aboveground and belowground approach.
References
Ayres E, Dromph KM, Cook R, Ostle N, Bardgett RD (2007) The influence of below-ground herbivory and defoliation of a legume on nitrogen transfer to neighbouring plants. Funct Ecol 21:256–263
Bardgett RD, Wardle DA (2003) Herbivore-mediated linkages between aboveground and belowground communities. Ecology 84:2258–2268
Bardgett RD, Wardle DA (2010) Aboveground-belowground linkages: biotic interactions, ecosystem processes, and global change. Oxford University Press Inc, New York
Bardgett RD, Wardle DA, Yeates GW (1998) Linking above-ground and below-ground interactions: how plant responses to foliar herbivory influence soil organisms. Soil Biol Biochem 30:1867–1878
Bardgett RD, Mawdsley JL, Edwards S, Hobbs PJ, Rodwell JS, Davies WJ (1999) Plant species and nitrogen effects on soil biological properties of temperate upland grasslands. Funct Ecol 13:650–660
Bardgett RD, Streeter T, Bol R (2003) Soil microbes compete effectively with plants for organic nitrogen inputs to temperate grasslands. Ecology 84:1277–1287
Bazot S, Mikola J, Nguyen C, Robin C (2005) Defoliation-induced changes in carbon allocation and root soluble carbon concentration in field-grown Lolium perenne plants: do they affect carbon availability, microbes and animal trophic groups in soil? Funct Ecol 19:886–896
Berg G, Esselink P, Groeneweg M, Kiehl K (1997) Micropatterns in Festuca rubra-dominated salt-marsh vegetation induced by sheep grazing. Plant Ecol 132:1–14
Bezemer TM, Lawson CS, Hedlund K, Edwards AR, Brook AJ, Igual JM, Mortimer SR, Van der Putten WH (2006) Plant species and functional group effects on abiotic and microbial soil properties and plant-soil feedback responses in two grasslands. J Ecol 94:893–904
Briske DD (1996) Strategies of plant survival in grazed systems: a functional interpretation. In: Hodgson J, Illius AW (eds) The ecology and management of grazing systems. CAB International, Wallingford, pp 37–67
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Burke IC, Lauenroth WK, Vinton MA, Hook PB, Kelly RH, Epstein HE, Aguiar MR, Robles MD, Aguilera MO, Murphy KL, Gill RA (1998) Plant-soil interactions in temperate grasslands. Biogeochemistry 42:121–143
Cabrera ML, Beare MH (1993) Alkaline persulfate oxidation for determining total nitrogen in microbial biomass extracts. Soil Sci Soc Am J 57:1007–1012
Campbell CA, Ellert BH, Jame Y (1993) Nitrogen mineralization potential in soils. In: Carter MR (ed) Soil sampling and methods of analysis. Lewis, Boca Raton, pp 341–349
Chapin SF, Slack M (1979) Effect of defoliation upon root growth, phosphate absorption and respiration in nutrient-limited tundra graminoids. Oecologia 42:67–79
Clapham AR, Tutin TG, Moore DM (1987) Flora of the British Isles, 3rd edn. Cambridge University Press, New York
Clarholm M (1985) Interactions of bacteria, protozoa and plants leading to mineralization of soil nitrogen. Soil Biol Biochem 17:181–187
Crawley MJ (1990) Rabbit grazing, plant competition and seedling recruitment in acid grassland. J Appl Ecol 27:803–820
Cumming DHM, Cumming GS (2003) Ungulate community structure and ecological processes: body size, hoof area and trampling in African savannas. Oecologia 134:560–568
Day TA, Detling JK (1990) Grassland patch dynamics and herbivore grazing preference following urine deposition. Ecology 71:180–188
de Mazancourt C, Loreau M, Abbadie L (1998) Grazing optimization and nutrient cycling: when do herbivores enhance plant production? Ecology 79:2242–2252
Elberse WT, Berendse F (1993) A comparative study of the growth and morphology of eight grass species from habitats with different nutrient availabilities. Funct Ecol 7:223–229
Engqvist L (2005) The mistreatment of covariate interaction terms in linear model analyses of behavioural and evolutionary ecology studies. Anim Behav 70:967–971
Ferraro DO, Oesterheld M (2002) Effect of defoliation on grass growth. A quantitative review. Oikos 98:125–133
Gastal F, Dawson LA, Thornton B (2010) Responses of plant traits of four grasses from contrasting habitats to defoliation and N supply. Nutr Cycl Agroecosyst 88:245–258
Grayston SJ, Griffith GS, Mawdsley JL, Campbell CD, Bardgett RD (2001) Accounting for variability in soil microbial communities of temperate upland grassland ecosystems. Soil Biol Biochem 33:533–551
Grime JP (2007) Comparative plant ecology: a functional approach to common British species. Castlepoint, Dalbeattie
Grime JP, Hunt R (1975) Relative growth-rate: its range and adaptive significance in a local flora. J Ecol 63:393–422
Guitian R, Bardgett RD (2000) Plant and soil microbial responses to defoliation in temperate semi-natural grassland. Plant Soil 220:271–277
Hamilton EW III, Frank DA (2001) Can plants stimulate soil microbes and their own nutrient supply? Evidence from a grazing tolerant grass. Ecology 82:2397–2402
Hamilton EW III, Frank DA, Hinchey PM, Murray TR (2008) Defoliation induces root exudation and triggers positive rhizospheric feedbacks in a temperate grassland. Soil Biol Biochem 40:2865–2873
Harrison KA, Bardgett RD (2010) Influence of plant species and soil conditions on plant-soil feedback in mixed grassland communities. J Ecol 98:384–395
Hartley SE, Amos L (1999) Competitive interactions between Nardus stricta L. and Calluna vulgaris (L.) Hull: the effect of fertilizer and defoliation on above- and below-ground performance. J Ecol 87:330–340
Hill MO, Carey PD (1997) Prediction of yield in the Rothamsted park grass experiment by Ellenberg indicator values. J Veg Sci 8:579–586
Hokka V, Mikola J, Vestberg M, Setälä H (2004) Interactive effects of defoliation and an AM fungus on plants and soil organisms in experimental legume-grass communities. Oikos 106:73–84
Holland J (1995) Effects of above-ground herbivory on soil microbial biomass in conventional and no-tillage agroecosystems. Appl Soil Ecol 2:275–279
Holland JN, Cheng W, Crossley DA (1996) Herbivore-induced changes in plant carbon allocation: assessment of below-ground C fluxes using carbon-14. Oecologia 107:87–94
Ilmarinen K, Mikola J, Nieminen M, Vestberg M (2005) Does plant growth phase determine the response of plants and soil organisms to defoliation? Soil Biol Biochem 37:433–443
Innes L, Hobbs PJ, Bardgett RD (2004) The impacts of individual plant species on rhizosphere microbial communities in soils of different fertility. Biol Fertil Soils 40:7–13
Kaye JP, Hart SC (1997) Competition for nitrogen between plants and soil microorganisms. Trends Ecol Evol 12:139–143
Kembel SW, Cahill JF Jr (2005) Plant phenotypic plasticity belowground: a phylogenetic perspective on root foraging trade–offs. Am Nat 166:216–230
Kimura K, Kikuchi S, Yamasaki S (1999) Accurate root length measurement by image analysis. Plant Soil 216:117–127
Klironomos JN, McCune J, Moutoglis P (2004) Species of arbuscular mycorrhizal fungi affect mycorrhizal responses to simulated herbivory. Appl Soil Ecol 26:133–141
Markham JH, Grime JP, Buckland S (2009) Reciprocal interactions between plants and soil in an upland grassland. Ecol Res 24:93–98
Marschner P, Crowley D, Yang CH (2004) Development of specific rhizosphere bacterial communities in relation to plant species, nutrition and soil type. Plant Soil 261:199–208
Massey FP, Ennos AR, Hartley SE (2007) Grasses and the resource availability hypothesis: the importance of silica-based defences. J Ecol 95:414–424
Mawdsley JL, Bardgett RD (1997) Continuous defoliation of perennial ryegrass (Lolium perenne) and white clover (Trifolium repens) and associated changes in the composition and activity of the microbial population of an upland grassland soil. Biol Fert Soils 24:52–58
McNaughton SJ, Banyikwa FF, McNaughton MM (1998) Root biomass and productivity in a grazing ecosystem: the serengeti. Ecology 79:587–592
Medina-Roldán E, Arredondo Moreno JT, García-Moya E, Huerta-Martínez FM (2007) Soil water content dynamics along a range condition gradient in a shortgrass steppe. Rangel Ecol Manag 60:79–87
Mikola J, Kytöviita M (2002) Defoliation and the availability of currently assimilated carbon in the Phleum pratense rhizosphere. Soil Biol Biochem 34:1869–1874
Mikola J, Yeates GW, Barker GM, Wardle DA, Bonner KI (2001a) Effects of defoliation intensity on soil food-web properties in an experimental grassland community. Oikos 92:333–343
Mikola J, Yeates GW, Wardle DA, Barker GM, Bonner KI (2001b) Response of soil food-web structure to defoliation of different plant species combinations in an experimental grassland community. Soil Biol Biochem 33:205–214
Mikola J, Setälä H, Virkajärvi P, Saarijärvi K, Ilmarinen K, Voigt W, Vestberg M (2009) Defoliation and patchy nutrient return drive grazing effects on plant and soil properties in a dairy cow pasture. Ecol Monogr 79:221–244
Milchunas DG, Lauenroth WK (1993) Quantitative Effects of grazing on vegetation and soils over a global range of environments. Ecol Monogr 63:328–366
Oesterheld M (1992) Effect of defoliation intensity on aboveground and belowground relative growth rates. Oecologia 92:313–316
Orwin KH, Buckland SM, Johnson D, Turner BL, Smart S, Oakley S, Bardgett RD (2010) Linkages of plant traits to soil properties and the functioning of temperate grassland. J Ecol 98:1074–1083
Owen DF (1980) How plants may benefit from the animals that eat them. Oikos 35:230–235
Paterson E, Sim A (1999) Rhizodeposition and C-partitioning of Lolium perenne in axenic culture affected by nitrogen supply and defoliation. Plant Soil 216:155–164
Paterson E, Sim A (2000) Effect of nitrogen supply and defoliation on loss of organic compounds from roots of Festuca rubra. J Exp Bot 51:1449–1457
Pinheiro J, Bates D, DebRoy S, Sarkar D, The R core team (2008) nlme: linear and nonlinear mixed effects models
R Development Core Team (2010) R: a language and environment for statistical computing. Vienna, Austria
Rasband WS (2007) ImageJ. US National Institutes of Health, Bethesda, MD (http://rsb.info.nih.gov/ij/)
Ritchie ME, Tilman D, Knops JMH (1998) Herbivore effects on plant and nitrogen dynamics in oak savanna. Ecology 79:165–177
Roduit N (2007) JMicroVision: un logiciel d’analyse d’images pétrographiques polyvalent. Terre et Environnement, University of Geneva
Rodwell JS (1992) Grasslands and montane communities, British plant communities, 3rd edn. Cambridge University Press, New York
Sankaran M, Augustine DJ (2004) Large herbivores suppress decomposer abundance in a semiarid grazing ecosystem. Ecology 85:1052–1061
Seagle SW, McNaughton SJ, Ruess RW (1992) Simulated effects of grazing on soil nitrogen and mineralization in contrasting Serengeti grasslands. Ecology 73:1105–1123
Sparling GP, Feltham CW, Reynolds J, West AW, Singleton P (1990) Estimation of soil microbial C by a fumigation-extraction method: use on soils of high organic matter content, and a reassessment of the k sub (EC)-factor. Soil Biol Biochem 22:301–307
Van der Graaf AJ, Stahl J, Bakker JP (2005) Compensatory growth of Festuca rubra after grazing: can migratory herbivores increase their own harvest during staging? Funct Ecol 19:961–969
Vance E, Brookes P, Jenkinson D (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Wardle DA, Barker GM, Yeates GW, Bonner KI, Ghani A (2001) Introduced browsing mammals in New Zealand natural forests: aboveground and belowground consequences. Ecol Monogr 71:587–614
Welch D (1986) Studies in the grazing of heather moorland in North-East Scotland. V. trends in Nardus stricta and other unpalatable graminoids. J Appl Ecol 23:1047–1058
Zar JH (1998) Biostatistical analysis, 4th edn. Prentice Hall, Upper Saddle River
Acknowledgements
We are grateful to the Mexican National Council for Science and Technology (CONACYT) for the doctoral scholarship granted to EMR, to Helen Quirk for technical advice in the laboratory and to Jorge Paz-Ferreiro and two anonymous reviewers for their valuable comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Rights and permissions
About this article
Cite this article
Medina-Roldán, E., Bardgett, R.D. Plant and soil responses to defoliation: a comparative study of grass species with contrasting life history strategies. Plant Soil 344, 377–388 (2011). https://doi.org/10.1007/s11104-011-0756-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-0756-4