Abstract
Farmers are under increasing pressure to use slurry-nutrients more efficiently in order to maximise crop utilisation and minimise losses to the environment. The objective of this field experiment was to quantify the fate of three N fractions (urine-N [U], rapid faecal-N [FR] and slow faecal-N [FS]) from cattle slurry in herbage and soil. The recovery of the three slurry-N fractions was measured in the first and second year after application on a permanent grassland in Ireland. Urine and faeces were collected from cows fed with 15N-labelled herbage, or unlabelled herbage with added 15N-labelled urea and these were recombined to produce differentially labelled experimental slurries. Slurries were applied to plots, and 15N-enrichments of the herbage and three soil layers were determined. The initial recovery of 15N (6 weeks after application) in herbage was 18%, 13%, 2%, while the residual recovery (12–63 weeks) was 4%, 6% and 7% for U, FR and FS, respectively. The total slurry-N recovery in the plant-soil system was estimated to range from 45% for urine-N to 72% for faecal-N. These results increase our mechanistic understanding of slurry-N dynamics in soil-plant systems and will inform models used to predict the fate of cattle slurry applied to grassland.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Slurry is an important source of nutrients in grassland systems and farmers are under increased pressure to improve the utilisation of slurry nitrogen (N), especially in the European Union since the implementation of the EU Nitrates Directive (91/676/EEC) (Anon 1991) and Water Framework Directive (2000/60/EC) (Stark and Richards 2008). Knowing when slurry-N is taken up by herbage is important in order to maximise crop utilisation and minimise environmental degradation. Herbage uptake of slurry-N may be immediate, i.e. prior to the first herbage cut after application, or it may be residual and taken up by the plant over a sustained period of time (Schröder 2005b; Thomsen 2005).
Approximately 50% of the N in cattle slurry is in an inorganic form (mainly ammonium-N [NH4-N] originating from urea in urine) (Anon 2010) which is directly available for plant uptake after application. However, during and after application, large losses from this fraction (up to 80%) can occur due to ammonia volatilisation (Søgaard et al. 2002; Sommer and Hutchings 2001). These losses can be minimised by using low emissions application techniques (Misselbrook et al. 2002; Smith et al. 2000; Webb et al. 2010). Some 15–35% of NH4-N is also immobilised by the soil microbial biomass in the first weeks after application, and may be gradually mobilised over time (Jensen et al. 2000; Morvan et al. 1997). A large proportion of mineral N, derived from slurry, can also be lost through nitrate leaching and denitrification (Schröder 2005a).
The remaining 50% of the slurry-N is in an organic form (faecal-N) and needs to be mineralised prior to it becoming plant-available. Mineralisation of the organic slurry-N fraction is difficult to predict as it is affected by factors such as slurry composition, soil type, temperature, pH, aeration and soil moisture conditions (Calderon et al. 2005; Chadwick et al. 2000; Fangueiro et al. 2008; Van Kessel and Reeves 2002). Labelling of animal manures with 15N stable isotope tracers has been established as an important tool to assess N release because it has a number of important advantages over conventional agronomic methods (Cusick et al. 2006; Dittert et al. 1998). The results from 15N labelling tend to be less variable, which is particularly important when attempting to quantify the relatively small residual N recoveries (Cusick et al. 2006; Muñoz et al. 2003; Thomsen 2004). Additionally, 15N can be traced in both soil and plant (as well as leachate and gaseous emissions if required), providing valuable information about the fate of N not taken up by plants (Chadwick et al. 2001; Dittert et al. 2001; Morvan et al. 1997; Sørensen and Amato 2002; Thomsen et al. 1997). Also, with 15N labelling the fate of urine and faecal-N from slurry can be monitored separately, offering a more detailed insight into the N dynamics of organic and inorganic-N fractions (Bosshard et al. 2009; Jensen et al. 1999; Sørensen et al. 1998; Thomsen et al. 1997).
Methods have been developed to label different faecal-N fractions (Powell et al. 2005; Powell et al. 2004), but there are some important pitfalls to consider. The main pitfalls for interpreting bulk 15N values include the non-homogeneous 15N labelling of faecal-N, which depends on the duration of feeding 15N labelled forage to the animal, on the type of diet, and on the metabolism of the individual animal. Non-uniform labelling may result in an over- or underestimation of the mineralised proportion of faecal-N in the soil (Powell et al. 2004; Sørensen et al. 1994). Also, the increased microbial activity upon slurry addition may result in an exchange between non-labelled biomass N with inorganic 15N, while the net amount of plant-available N may have remained constant (Dittert et al. 1998). This can lead to an over- or underestimation of the slurry-N recovery in the plant (Schröder 2005a).
Even though there have been many studies using 15N labelling to trace the fate of manure N in arable systems (Dittert et al. 1998), we have not been able to identify any studies on grassland, with the exception of studies that focussed on the fate of 15N labelled NH4-N (Chadwick et al. 2001; Dittert et al. 2001; Hoekstra et al. 2010; Morvan et al. 1997). Therefore, the objective of the present experiment was to trace the fate of three slurry-N fractions (urine-N, rapid faecal-N and slow faecal-N) in herbage and multiple soil layers in the first 62 weeks after a single slurry application on a permanent grass sward, using 15N labelling techniques. This will provide us with better insights into the immediate and residual N uptake by grass from slurry, a better understanding of soil N cycling and a quantification of N losses from such grassland systems.
Materials and methods
Experimental design
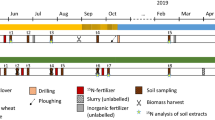
The experiment was located at the Johnstown Castle Environment Research Centre, Wexford, Ireland, in a permanent perennial ryegrass-dominated grassland on a moderately drained, fine loamy soil that was not grazed and did not receive any N fertiliser or slurry during the year 2007 prior to treatment application. The experiment used 1 m × 1 m micro-plots confined with 10 cm high metal borders, which were placed into the soil to 5 cm depth (Fig. 1).
In order to facilitate both herbage and soil sampling without affecting herbage growth for subsequent harvests, the micro-plots were divided into a herbage sampling area and soil sampling area (Fig. 1). Herbage was sampled in the central 50 cm quadrat and soil samples were taken from a grid surrounding the 50 cm quadrat, but at least 15 cm from the micro-plot boundary. There was a 50 cm discard area between main plots. The plot layout was a complete randomised block design with five blocks.
The five treatments consisted of two control treatments (no slurry and non-labelled slurry) and three differentially labelled slurries, as described below and in Table 1:
-
Urine-labelled slurry (ULS)
-
Faeces-labelled slurry, Urea method (FLSUM)
-
Faeces-labelled slurry, Herbage method (FLSHM)
Slurry 15N labelling and application
For the 15N labelling of faeces and urine, two non-lactating cows were used, which are preferred over milking cows in order to prevent 'loss' of expensive 15N in milk, even though the manure composition may be slightly different (Powell et al. 2004). Cattle slurry was labelled using two different, modified methods based on Powell et al. (2004): a) 15N labelled herbage was fed to cows in order to label the entire faecal-N pool, including undigested feed N, or b) feed urea 15N labelling was used to label the microbial fraction and the endogenous N of the faeces.
-
a)
For the 15N-herbage labelling method, a perennial ryegrass-dominated permanent sward was fertilised with double 15N labelled (10 atom%) ammonium nitrate (both the ammonium-N and nitrate-N were enriched) at a rate of 45 kg N ha−1. The herbage was harvested after a 5-week regrowth period when the enrichment of the herbage at harvest time was approximately 3 atom%. Non-labelled herbage was produced in the same way, but using non-labelled fertiliser. This non-labelled herbage was used in the adaptation period, for the 15N-urea method (below), and to allow the continuation of similar feeding during the manure collection period after 15N herbage feeding had stopped.
For the 15N herbage-feeding method, the first cow was first adapted to the fresh grass diet for 7 days, of which the last 2 days were used to collect unlabelled faeces and urine for recombination (see below). On day 8, the 15N labelled herbage material was harvested and divided into 10 equal parts (to assure uniform 15N feeding) on a weight basis. The grass was offered to the cow five times daily (approximately 15 kg DM [dry matter] day−1) for 3 days.
-
b)
For the urea 15N labelling method, 120 g urea per day (15N at natural abundance) was sprinkled onto unlabelled grass fed to the second cow during the 7-day adaptation period. On day 8, single 24 g doses of 10 atom% 15N urea mixed with water were sprinkled over the grass and fed to the cow five times daily for 2 days (10 doses per cow).
Total faeces and urine were collected at approximately 6-hour intervals after initial 15N feeding up to at least 48 h after the 15N feeding had stopped. Urine was collected by gluing a harness to the cows with a tube connection to drain the urine into a container. Faeces were collected from a crate on the floor. The collected faeces and urine were frozen until later use.
Labelled faeces were proportionally recombined with unlabelled urine, and vice versa, in order to make four different slurries (Table 1):
-
NLS (non-labelled slurry),
-
ULS (urine-labelled slurry),
-
FLSUM (faeces-labelled slurry, urea feeding method),
-
FLSHM (faeces-labelled slurry, herbage feeding method).
Tap water was added in order to obtain a DM content of approximately 7% in each slurry type.
Slurries were applied to the field micro-plots on 29th June 2007 at a rate of 3.3 kg m−2 by watering can, mimicking broadcast application by conventional slurry tankers.
Sampling methods
The plots were harvested five times during 2007 and 2008 (Table 2); the final harvest took place 62 weeks after slurry application. The herbage from the inner square of the micro-plots (Fig. 1) was harvested with electrical grass shears at 5 cm height, weighed and a sub-sample taken for DM, total N and 15N analyses.
At each harvest, two soil cores (2 cm diameter) from each of three randomly selected grid-squares were taken to 25 cm depth (Fig. 1). The sampled grids were not revisited at later harvests. The samples were split into three depths: 0–5 cm, 5–15 cm and 15–25 cm and samples were immediately stored at 4°C.
Analytical procedures
Fresh soils were sieved through a 2 mm screen before further analyses. Slurry, grass and soil dry matter contents were determined by drying at 100°C overnight. Slurry and faecal total N were determined by Kjeldahl digestion of fresh slurries and faeces. NH4-N was extracted from fresh slurry by shaking 10 g of slurry in 200 ml 0.1 M HCl on a peripheral shaker for 1 h and filtering through a No. 2 Whatman filter paper. Concentrations of NH4-N in the filtrate were determined on an Aquakem 600 discrete analyser (Thermo Electron OY, Vantaa, Finland).
Faecal neutral detergent fibre (NDF) was extracted from a freeze dried sub-sample according to the method by Van Soest et al. (1991) (Dungait et al. 2005) and the total N in the NDF fraction (NDFN) was measured using a NA1500 automated nitrogen-carbon analyser (Carlo Erba, Milan, Italy) coupled to a 20/20 isotope ratio mass spectrometer (SerCon Ltd, Crewe, UK).
Inorganic-N, NH4-N and total oxidised-N (TON i.e. NO3 and NO2) in fresh soil were determined by extraction in 2 M KCl (40 g soil: 100 ml KCl, shaken for 1 h), with NH4-N and TON determined on an Aquakem 600 discrete analyser.
Soil microbial biomass was extracted from the soils using the fumigation-extraction (FE) method (Sparling and West 1988). Ten grams of field moist soil were extracted with 40 ml of potassium sulphate (0.5 M K2SO4), either directly or after 24 h of chloroform fumigation. The samples were shaken for 1 h and filtered. Soil microbial biomass N (SMB-N) was determined in the extract by persulphate digestion (Cabrera and Beare 1993) using a conversion factor of keN = 0.54 (Brookes et al. 1985).
A composite sub-sample of the dried grass was milled through a 2 mm screen and subsequently ground to a fine powder in a ball mill. Total N and 15N concentrations in the dried and milled grass and soil samples were measured on an ANCA 20/20 SL combustion isotope ratio mass spectrometer (IRMS) (Delta plus, Finnigan, Bremen, Germany). The 15N isotopic enrichments of total slurry-N, total faecal-N, soil inorganic-N and SMB-N were determined after diffusion of the Kjeldahl digest, KCl extract and persulphate digest, respectively (Stark and Hart 1996) and measured on an IRMS (Delta plus, Finnigan, Bremen, Germany). The 15N isotopic enrichment of the faecal-NDFN was measured on a 20/20 isotope ratio mass spectrometer (SerCon Ltd, Crewe, UK).
Calculations and estimations of other pathways
15N recovery of labelled fractions
For each of the three slurry labelling treatments (ULS, FLSUM and FLSHM), the percentage of slurry 15N recovered in herbage (15 NRH) was calculated as:
where H=total herbage N uptake, S=slurry-N applied, a=atom% 15N of applied slurry, b=atom% 15N of unlabelled slurry, c=atom% 15N of herbage from labelled plots, d=atom% 15N of herbage from control plots.
Similar equations were applied to calculate the 15N recovery in the total soil (15 NRS T ), in the inorganic soil N fraction (15 NRS I ) and soil microbial biomass N (15 NRS SMB ).
While the recovery of ULS is a direct reflection of the slurry urine fraction, the two faecal labelling methods are more confounded. For FLSHM, the total faecal-N is enriched, and therefore both the slow and rapid faecal fractions are labelled. In the FLSUM labelled faeces, only the microbial and endogenous N fractions are enriched. In Fig. 2, we present two different theories, on the basis of which the contribution of both the rapid and slow faecal fractions to total 15N recovery can be determined, as explained below.
Conceptual presentation of 15N labelling of the slowly (FS) and rapidly (FR) degradable faecal-N fractions with the urea and herbage methods. Theory 1 is based on work by Powell et al. (2004) and Theory 2 is based on Sørensen et al. (1994). Abbreviations: TFN=total faecal-N, NDF=neutral detergent fibre, NDFN=N in the NDF fraction
Theory 1 is derived from the work by Powell et al. (2004), and is based on two main assumptions:
-
The urea method labels the rapid faecal-N (FR) fraction only, which is equivalent to the non-NDFN fraction of the faeces
-
The herbage method labels both the slow (FS, which is equivalent to NDFN) and rapid fraction of faeces, and the labelling is homogenous i.e. the enrichment of NDFN is similar to the enrichment of non-NDFN (or total faecal-N).
Therefore the recovery from the rapid faecal fraction (15NR_FR) is equal to the recovery from the urea-labelled slurry:
The 15N recovery from FLSHM is the sum of the recovery from the fast and slow faecal fractions multiplied by the proportion of the slow and rapid fractions in the total faecal-N, and can be represented by the following equation:
which can be rewritten as:
in which 15NR_FLSHM, 15NR_FLSUM and 15NR_FS are the 15N recovery of the herbage-labelled faecal-N (both slow and rapid fractions), urea-labelled faecal-N (rapid fraction only) and slow faecal-N fraction, respectively. NDFN is the N in the NDF fraction (equivalent to slow faecal-N) and TFN is total faecal-N.
However, both assumptions in Theory 1 can cause considerable problems. Firstly, it is notoriously difficult to achieve homogenous labelling of the whole faecal fraction (Powell et al. 2004; Sørensen et al. 1994). Secondly, work by Sørensen et al. (1994) suggests that the divide between the slow and rapid fractions is not between undigested feed N and the remaining faecal-N, but that some of the microbial faecal fraction (microbial N from the rumen) can also be considered to be slowly available. This would suggest that the urea feeding method not only labels the rapidly available pool, but also part of the slowly available pool (Fig. 2, Theory 2). Therefore, we propose Eq. 4 to describe the enrichment of the UM and HM labelled faeces as a function of the enrichments of the slow and rapid faecal fractions (NDFN and non-NDFN) and their proportion in total faecal-N:
in which δ15NTF, δ15NFS and δ15NFR are the 15N atom% excess of the total faecal-N, the slow faecal-N (NDFN) and the rapid faecal-N (non-NDFN) (excess 15N refers to the absolute 15N enrichment minus the natural abundance, which was 0.369%) and TFN, FS and FR refer to the N content of the total faeces, and the slow and rapid faecal-N fractions, respectively. Therefore the proportion (P) of the total 15N excess in faeces that can be attributed to the slow fraction (P15NFS) and fast fraction (P15NFR) is calculated as:
This proportion of the total faecal 15N excess in the slow fraction is then used to attribute the total 15NR of FLSUM and FLSHM (as calculated from Eq. 1) to the 15N recovery from the slow and rapid faecal fractions (15NR_FS and 15NR_FR) by solving Eq. 6a and 6b simultaneously.
By using this method, the results from non-homogenously labelled faecal slurry can still be analysed and the recovery of the slow and rapid faecal fractions can be calculated individually.
Apparent N recovery
The apparent N recovery (ANR) from slurry in herbage was calculated as:
where N=total N taken up by herbage or applied in slurry, respectively.
15N recovery in stubble and roots
Due to repeated harvests in the experimental design, the recovery of 15N in the stubble (<5 cm) and roots was not measured. The 15N recovery in the stubble was estimated based on the recovery in stubble (as a % of the recovery in the harvested herbage) as measured in another experiment applying the same labelled slurries to soil cores which were harvested at similar dates (Hoekstra et al., unpublished data). Similarly, data from Whitehead and Bristow (1990) on the 15N recovery of urine applied to a permanent sward were used to estimate the 15N recovery in roots as a function of the 15N recovery in the above-ground herbage and time after application.
15N lost through ammonia volatilisation
The proportion of total ammoniacal N volatilisation was estimated using the ALFAM model (Søgaard et al. 2002). The variables included in the model were soil moisture content, air temperature, wind speed, manure type, dry matter content and ammoniacal nitrogen content of manure, application method and application rate (Søgaard et al. 2002). The model was run with the slurry composition data in Table 1, the weather conditions during 24 h after application (mean temperature 15.7°C, average wind speed 3.4 m s−1); the measuring technique was set to micro-meteorological mass balance, the soil moisture content to dry soil, and application method to broadcast application.
Statistical analysis
Statistical analysis was carried out using PROC MIXED in SAS. The fixed factors were slurry labelling method and harvest number. The experiment was corrected for repeated measures (harvests) by including a heterogeneous first-order autoregressive variance covariance structure in the model.
Results
Slurry composition and weather conditions
On average, the DM content of the slurry was 65 g kg−1 and the N content was 84 g kg−1 DM, with NH4-N making up 55% of the total slurry-N (Table 1), which is within the range reported for cattle slurry. The slurry 15N enrichment was 0.369, 0.937, 0.563 and 1.140 atom% for NLS, ULS, FLSUM and FLSHM, respectively. For FLSUM and FLSHM, both the slow and the rapid faecal fraction were enriched, but the slow fraction was less enriched than the rapid fraction (Table 1). Therefore, only method 2 (Eqs. 4, 5a and 5b) was suitable for calculating the 15NR of the slow and rapid faecal fractions.
The weather conditions during the growing season in 2008 were extremely wet, and the mean daily rainfall during the last harvest interval was over twice the 30-year average for the period. As a result, the calculated excess drainage was 3 mm day−1 during the last harvest interval in 2008, which was four times higher than in the same period in 2007 (Table 2).
Herbage dry matter yield and N uptake
The herbage dry matter yields (DMY) were on average 4.5, 1.4, 0.8, 7.3 and 2.9 t ha−1 for the harvests at weeks 6, 12, 38, 53 and 62, respectively (data not shown). At week 6, the DMY for the control plots were significantly (p < 0.001) lower compared to the plots with slurry applied (2.4 versus 5.0 t ha−1), but there were no significant differences in DMY in the remaining harvests. There was no significant effect of slurry application on herbage-N concentration, and therefore the herbage-N uptake followed the same temporal patterns as DMY (data not shown). The ANR was on average 21% after 6 weeks and the residual ANR (weeks 12–62) was 4.8%, but the variation was very large, particularly for the residual cuts (data not shown).
Recovery of slurry 15N in herbage (15NRH)
There was a significant interaction (p < 0.0001) between harvest and slurry fraction on 15NRH (Fig. 3a). At week 6, the 15NRH of urine (U), the rapid faecal fraction (FR) and the slow faecal fraction (FS) was 18.3, 12.3 and 2.7%, respectively. The 15NRH of U decreased to 2.2% at week 12 and remained on average 0.8% for the later harvests. The 15NRH of FR decreased to on average 1.8%, and there were no significant differences between subsequent harvests. For FS, the 15NRH was similar across harvests and was on average 1.7%.
a The 15N recovery (%) of urine-N (U, white bars), rapidly degradable faecal-N (FR, grey bars) and slowly degradable faecal-N (FS, black bars) in herbage at 6, 12, 38, 53 and 62 weeks after application, and b the total slurry 15N recovery in herbage and the contribution of U, FR and FS. Error bars = 2 × SE (n = 5)
In order to calculate the contribution of the three fractions to the total slurry 15NRH, the 15NRH of the three fractions were multiplied with their relative proportions in the slurry total N (62, 25 and 13% for U, FR and FS, respectively) (Table 1 and Fig. 3b). There was a significant (p < 0.0001) effect of harvest on total slurry 15NRH which was highest at 6 weeks (14.7%), and declined to 2.1%, 0.9%, 1.6% and 0.8% at 12, 38, 42 and 53 weeks, respectively. There was a significant interaction (p < 0.0001) between harvest and slurry fraction on the contribution of the different fractions to total slurry 15NRH. At 6 weeks, approximately 78% of the total slurry 15NRH was from U, 20% from FR and 2% from FS, whereas from 38 to 62 weeks, on average 40% of total slurry 15NRH was from U and the remainder from FR and FS.
Recovery of slurry 15N in soil
15N recovery in soil total N (15NRST)
The total soil N content varied from 3.0 g kg−1 dry soil in layer a (0–5 cm depth) to 1.5 g kg−1 dry soil in layer c (15–25 cm depth) (data not shown).
There was a significant (p < 0.001) slurry fraction × depth × time interaction for 15NRST (Fig. 4).
The 15N recovery (%) of urine-N (U), rapidly degradable faecal-N (FR), slowly degradable faecal-N (FS) and total slurry-N (calculated from weighted fractions) in the total soil N for three soil layers (a = 0–5 cm, b = 5–15 cm and c = 15–25 cm) at 6, 12, 38, 53 and 62 weeks after application. Error bars = 2 × SE (n = 5)
The 15NRST from U summed over the three layers was 11.5% after 6 weeks, increased up to 20.7% after 53 weeks, and declined to 17.3% after 62 weeks, but these changes were not significant. During the first three harvests, most of the 15N was recovered in layer a, but the recovery in layer b increased significantly (p < 0.0001) with time, and after 62 weeks layer b contained about 50% of the 15N recovered in soil. The recovery in layer c did not change significantly over time and was on average 8% of the 15NRST.
For FR, the 15NRST increased from 33% after 6 weeks to 55% after 53 weeks, after which it declined to 34% at 62 weeks. Similar to the 15NRST recovery from U, the relative recovery in layer a was largest at 6 weeks, and decreased with time, whereas the relative recovery in layer b increased and the recovery in layer c remained fairly stable at, on average, 12% of 15NRST.
The 15NRST from FS was 15% at 6 weeks, increased to 65% after 53 weeks and decreased (non-significantly) to 50% after 62 weeks. During the first two harvests, no 15N was recovered in layer b and layer c, but for the last 3 harvests the proportion of 15NRST recovered was 34%, 56% and 11% for layer a, b and c, respectively.
There was a significant effect of harvest on the calculated recovery of total slurry-N in soil (15NRST of fractions multiplied by the relative proportion of fraction in total slurry-N), which increased from 15% at 6 weeks to 35% after 53 weeks and declined to 26% after 62 weeks. There was a significant (p < 0.001) interaction between layer and harvest, and the relative recovery in layer a decreased from 69% at 6 weeks to 53% at 62 weeks, whereas the relative recovery in layer c was stable at on average 9% of 15NRST.
15N recovery in soil inorganic-N (15NRSI)
Soil mineral N content varied over the different harvests but showed no significant treatment effect. 15NRSI was very small, and represented on average <1% of 15NRST. There were no significant treatment effects (data not shown).
15N recovery in soil microbial biomass (15NRSSMB)
On average 4% of the total soil N was in the form of SMB (data not shown). After 6 weeks, on average 4.6% of 15N was recovered in the SMB (summed over 3 depths), and this declined to 2.3% after 62 weeks (Fig. 5). After 6 weeks the 15NRSSMB tended to be higher for U and FR compared to FS, but this difference between slurry fractions was not significant. There was a significant (p < 0.05) interaction between harvest week and depth. After 6 weeks 15NRSSMB was significantly higher in layer a compared to b and c, whereas after 62 weeks it was higher in layers a and b than in c.
After 6 weeks 15NRSSMB represented 45%, 22% and 11% of the total 15N recovered in soil for U, FR and FS, respectively, and after 62 weeks this had decreased to 10%, 11% and 2%, respectively.
Estimated recovery in stubble and roots
The estimated 15N recovery in the stubble decreased over time from 10.4% at week 6 (U) to <0.4% at week 62 (FR and FS) (Fig. 6). A similar trend was found for the calculated 15N recovery in the roots, which ranged from 5.2% at week 6 (U) to 1.2% at week 62 (FS) (Fig. 6).
The fate of 15N (%) of urine-N (U), rapidly degradable faecal-N (FR), slowly degradable faecal-N (FS) and total slurry-N (calculated from weighted fractions), in herbage (cumulative), soil (0–25 cm), roots (calculated), stubble (calculated), ammonia volatilisation (predicted by ALFAM model), and on the soil surface (estimated) at 6, 12, 38, 53 and 62 weeks after application. For measured data, error bars = 2 × SE (n = 5)
The fate of slurry 15N
There was a significant (p < 0.0001) harvest × fraction interaction for the sum of the 15NR in herbage (cumulated over 5 harvests), soil, roots and stubble (15NRSUM). For U, 15NRSUM was on average 46% and did not change significantly between harvests (Fig. 6). The predicted ammonia volatilisation accounted for another 46%, thus total urine-15N recovery was >90%. For FR, the total 15NR increased from 58% at 6 weeks to 75% at 53 weeks, after which it declined to 57% at 62 weeks. For FS, the initial recovery at 6 weeks was only 21%, the recovery increased sharply to 75% at 53 weeks and declined to 59% at 62 weeks.
On average, 51% of slurry-N could be accounted for in 15NRSUM, whereas another 28% was predicted to be lost through ammonia volatilisation. Therefore, on average, 21% of slurry-N remained unaccounted for.
Discussion
Labelling and calculation method
In this study, the 15N herbage feeding method and the 15N urea feeding method (Powell et al. 2004) were used to produce 15N enriched faeces. The method was thought to produce homogeneous labelling by recombining equal amounts of faeces around the peak 15N enrichment, but this was not observed. The lower 15N enrichment of the NDFN fraction in the FLSHM may have been partly due to the enrichment of the herbage NDFN fraction, which tends to be lower than the enrichment of the grass cell contents (Bosshard 2007). Additionally, the NDFN fraction of the FLSUM was also enriched contrary to the findings of Powell et al. (2004), who suggested that only the rapidly available fraction would be enriched.
Therefore, we developed a new calculation method applying the theory by Sørensen et al. (1994) that divides faecal-N components into a slowly-decomposable pool consisting of undigested feed N and microbial N from the rumen, and a more rapidly decomposable pool consisting of N compounds in living microbes, partly-decomposed microbial tissues, digestive secretions and dead cells from tissues in the digestive tract (Fig. 2). As we had data for two differentially labelled slurries, both containing a labelled slow and rapid faecal-N pool, we could calculate the recovery of the two faecal fractions separately by comparison (see 15N recovery of labelled fractions and Eqs. 4, 5a, 5b, 6a, 6b). There are two major advantages to this method: Firstly, we could accurately calculate the recovery of slurry that is not labelled homogenously, and secondly, for the first time we were able to calculate the recoveries of three slurry-N pools (U, FR and FS) and, based on that, the total slurry 15N recovery. The main downside of this method is a potential increase in the error, because two measurements are compared, as opposed to a direct calculation of the 15NR.
Fate of slurry-N in herbage
The total slurry 15NR in herbage was 15% after 6 weeks, which is lower than the ANR of 21%, indicating a substitution of tracer 15N, resulting in a potential underestimation of the slurry-N recovery in the herbage with the 15N method (Dittert et al. 1998; Schröder 2005a). However, direct comparison of these results requires caution, because the ANR is based on the mean of the three different slurries, whereas the 15NR is based on the weighted recovery of the labelled fraction in each slurry.
As expected, the 15NR in herbage 6 weeks after application was highest for the urine fraction (18.3%) followed by the FR (12.3%) and FS (2.7%), reflecting the plant availability of the three slurry fractions. The 18% recovery from the urine fraction is similar to recoveries reported for broadcast summer applied slurry by Hoekstra et al. (2010) using slurry of which the NH4-N pool was labelled with 15N. There was a large difference between the recovery of the slow and the rapid faecal fractions, confirming that the two N pools had distinctly different N availabilities.
The total slurry 15N recovery in herbage of 15% after 6 weeks was in close agreement with the apparent N recovery for broadcast slurry applied in June as reported by Lalor et al. (in press), but was higher than the agronomic advice in Ireland (Nitrogen Fertiliser Replacement Value [NFRV]=5%) (Coulter 2004). It should be noted that the NFRV is a ratio of recoveries, i.e. the recovery of slurry-N over the recovery of mineral fertiliser N, as opposed to N recovery. Therefore, the discrepancy between our results and those of Coulter is likely to be even larger (assuming a mineral fertiliser recovery of <100%).
The residual recovery in herbage (cumulated over the remaining 4 harvests) was 4.7%, 6.8% and 5.7% for U, FR and FS, respectively, indicating that the residual recovery of the faecal fractions is slightly higher than for urine-N, which is in agreement with other studies (Bosshard et al. 2009; Sørensen et al. 1998; Thomsen et al. 1997). The main difference between the residual effect from urine and faecal-N is that for the faecal fractions, the residual effect is mainly the result of gradual mineralisation of organic N, whereas for U it is caused by the gradual mobilisation of inorganic-N immobilised shortly after application (Jensen et al. 2000; Sørensen and Amato 2002).
Fate of slurry-N in soil
The recovery of urine-N and faecal-N in soil was comparable with results from other non-grassland based studies in which 15N labelled manures were applied to soils (Bosshard et al. 2009; Sørensen et al. 1998; Thomsen et al. 1997).
The recovery of the faecal fractions was variable, with a strong increase in recovery (particularly for FS) up to 38 weeks, followed by a decline after 53 weeks. This strong increase may be related to the incorporation of faecal matter that had previously remained in the stubble and on top of the soil after application. Our soil sampling method would not have accounted for this fraction, as cores were taken from the bare soil surface between grass tillers, and stubble residues were removed from the samples before sieving. It is estimated that (Fig. 6, on soil surface) 6 weeks after application, over 70% and 20% of the slow and rapid faecal fractions, respectively, had not been incorporated into the soil, equating to just over 10% of total slurry-N. The fact that it took 38 weeks (the winter period) before FS was recovered in the deeper soil layers, would confirm the slow incorporation of the more fibrous slurry component into the soil.
The decrease in slurry-N recovery in soil after 53 weeks may be related to the excessive rainfall and resulting net drainage during this period (Table 2), which may have resulted in increased leaching (of both nitrate and dissolved organic N) (Van Kessel et al. 2009; Wachendorf et al. 2005) or enhanced loss of N as N2 and N2O due to higher denitrification rates (Jahangir et al. 2010).
After 6 and 62 weeks, <1% of slurry 15N was recovered in the inorganic soil N pool (KCl-extract), which is in line with previous work (Hoekstra et al. 2010; Morvan et al. 1997) and is due to the rapid uptake into herbage and immobilisation of mineral N in soil.
Immobilisation of NH4-N in soil after fertiliser application can vary considerably, depending on slurry composition, placement, soil and crop type and temperature (Jackson et al. 1989; Ledgard et al. 1989; Morvan et al. 1997; Petersen 2006). The proportion of slurry NH4-N (urine fraction) immobilised after 6 weeks in this study was relatively low at <5% (Fig. 5), compared to reported slurry NH4-N recoveries in SMB in grassland of 15% (Morvan et al. 1997) up to 60% (Jackson et al. 1989). This could be related to the relatively large losses of NH4-N through volatilisation. When the urine-N recovery in SMB was expressed as a percentage of the 15N recovered in total soil, the recovery was 45%, which is more in line with these other studies. Even though there was no significant difference in 15NRSSMB as a percentage of total 15N applied, the proportion of soil 15N recovered in the SMB was lower for the faecal fractions compared to urine (on average 20% and 45%, respectively). This is to be expected because faecal (organic) N is more resistant to microbial degradation. We have been unable to identify any other studies that have investigated the recovery of the faecal slurry fraction in SMB-N.
Cumulative recovery and losses
The 15N recovery in the roots and stubble were not measured in this experiment, however, calculations based on recoveries reported in the literature (Whitehead and Bristow 1990) and from related experiments (Hoekstra et al., unpublished), suggested that even though roots and stubble were significant sinks for 15N during the initial phase of the experiment (up to 13% of total slurry-N, Fig. 6), the contribution after 62 weeks would have been very small (3.3% of total slurry-N).
When adding up the total recovery of U, FR and FS over all the measured (herbage and soil) and estimated (stubble, roots, on top of soil) sinks, the recovery was on average 45% for U and 72% for FR and FS. The U not accounted for can largely be attributed to losses through ammonia volatilisation after application (Fig. 6). The remaining 10% could have been lost through leaching and denitrification (Schulte et al. 2006). The faecal-N not accounted for is harder to explain and may be attributed to losses through mineralisation followed by leaching or denitrification. Additionally, some faecal-N was likely incorporated into the soil faunal biomass. For example, earthworm populations, which had a biomass of up to 189 g m−2 in a pasture at the same research station (Curry et al. 2008), have been shown elsewhere under field conditions to assimilate slurry-derived N (Schmidt and Ostle 1999). Transport of faecal-N below the sampling depth (25 cm) by deep-burrowing earthworms such as Lumbricus terrestris or through their vertical channels, could also have occurred.
Implications and future work
Results indicated that the bulk of the slurry-N recovery in herbage (approximately 17% of slurry-N applied) after a June application occurs in the year of application, with only 3% recovered in the subsequent year. This 3% (equivalent to 5 kg N ha−1) may seem negligible from an agronomic point of view. However, it is crucial to consider that, with the practice of yearly repeated slurry application, as is customary in winter housing grassland systems, this small effect may add up to significant cumulative effects (e.g. Schröder 2005b).
However, a 20% recovery of slurry N in herbage over 2 years is still quite low compared to countries such as The Netherlands and Denmark, where NFRV’s for cattle slurry on grassland are set at 50 and 70%, respectively and there is pressure on Ireland to reach the same targets.
It should be noted that the conditions and methods of slurry application during this experiment were not aimed at optimising the slurry N recovery. Research has shown that slurry N utilisation is higher for spring applied slurry (as opposed to summer applied) and band application or injection (as opposed to broadcast) (Hoekstra et al., 2010; Lalor et al. 2009, in press) due to a combination of better crop utilisation and lower losses through ammonia volatilisation. Currently, best practice in Ireland is to apply slurry with a trailing shoe in spring time, and this could increase the N recovery in the first year up to at least 25% (Lalor et al. in press). Also, the weather conditions during 2007, but particularly late summer 2008 were exceptionally wet, which may have resulted in an N recovery which is lower than could be expected in other years. Additionally, the isotope substitution effect may have resulted in a slight underestimation of the slurry N recovery in herbage. However, based on current Irish studies, it is unlikely that NFRV’s of over 40% are achievable in practice under current Irish conditions.
Slurry is notorious for its variable composition, which may be the result of differences in animal type, animal feeding, slurry storage, etc. In this study, we have distinguished between three separate slurry fractions for the first time. Further work is needed to investigate whether we can establish a link between the recovery of these different slurry fractions and slurry composition (e.g. NIRS, structural and chemical analysis), in order to improve the prediction of the recovery of slurry-N from various sources.
Conclusions
-
A new calculation method was developed that can be used to quantify the recovery of both the slow and rapidly available faecal-N fractions based on the combined results from the herbage and urea 15N labelling methods. This method has the important benefit that results from non-homogenously labelled faecal-N can be correctly interpreted. By applying this method, the N recovery from three different slurry-N fractions (urine, rapid faecal and slow faecal) could be distinguished for the first time.
-
The initial recovery (6 weeks after application) in herbage was 18%, 13%, 2% for U, FR and FS, respectively, and the residual recovery (12–63 weeks after application) was 4%, 6% and 7% for U, FR and FS, respectively.
-
The total recovery in the plant-soil system ranged from 45% for urine-N to 72% for faecal-N.
-
The 15N results in combination with results of agronomic studies will form the basis for models predicting both the initial and residual nitrogen recoveries from cattle slurry on grassland.
References
Anon (1991) Directive of the Council of December 12 concerning the protection of waters against pollution caused by nitrates from agricultural sources (91/676/EEC). European Commission, Brussels, 8 pp
Anon (2010) Fertiliser recommendations for agricultural and horticultural crops (RB 209). DEFRA, The Stationery Office, Norwich, UK
Bosshard C (2007) Nitrogen dynamics in conventional and organic cropping systems. PhD thesis, ETH Zurich
Bosshard C, Sørensen P, Frossard E, Dubois D, Mäder P, Nanzer S, Oberson A (2009) Nitrogen use efficiency of 15N-labelled sheep manure and mineral fertiliser applied to microplots in long-term organic and conventional cropping systems. Nutr Cycl Agroecosyst 83:271–287
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Cabrera ML, Beare MH (1993) Alkaline persulfate oxidation for determining total nitrogen in microbial biomass extracts. Soil Sci Soc Amer J 57:1007–1012
Calderon FJ, McCarty GW, Reeves JB III (2005) Analysis of manure and soil nitrogen mineralization during incubation. Biol Fert Soils 41:328–336
Chadwick DR, Pain BF, Brookman SKE (2000) Nitrous oxide and methane emissions following application of animal manures to grassland. J Env Qual 29:277–287
Chadwick DR, Martinez J, Marol C, Beline F (2001) Nitrogen transformations and ammonia loss following injection and surface application of pig slurry: a laboratory experiment using slurry labelled with 15N-ammonium. J Agric Sci (Cambr) 136:231–240
Coulter BS (2004) Nutrient and trace element advice for grassland, tillage, vegetable and fruit crops. Teagasc, Ireland, 96 p
Curry JP, Doherty P, Purvis G, Schmidt O (2008) Relationships between earthworm populations and management intensity in cattle-grazed pastures in Ireland. Appl Soil Ecol 39:58–64
Cusick P, Kelling K, Powell JM, Muñoz G (2006) Estimates of residual dairy manure nitrogen availability using various techniques. J Env Qual 35:2170–2177
Dittert K, Goerges T, Sattelmacher B (1998) Nitrogen turnover in soil after application of animal manure and slurry as studied by the stable isotope 15N: A review. Z Pflanzenern Bodenk 161:453–463
Dittert K, Bol R, King R, Chadwick D, Hatch D (2001) Use of a novel nitrification inhibitor to reduce nitrous oxide emission from N-15-labelled dairy slurry injected into soil. Rapid Commun Mass Spectr 15:1291–1296
Dungait JAJ, Bol R, Evershed RP (2005) Quantification of dung carbon incorporation in a temperate grassland soil following spring application using bulk stable carbon isotope determination. Isot Environ Health Stud 41:3–11
Fangueiro D, Chadwick D, Bol R (2008) Assessment of the potential N mineralization of different particle-size fractions in two dairy cattle slurries. J Plant Nutr Soil Sci-Z Pflanzenern Bodenk 171:313–315
Hoekstra NJ, Lalor STJ, Richards KG, O’Hea N, Lanigan GJ, Dyckmans J, Schulte RPO, Schmidt O (2010) Slurry 15NH4-N recovery in herbage and soil: effects of application method and timing. Plant Soil 330:357–368
Jackson LE, Schimel JP, Fireston MK (1989) Short-term partitioning of ammonium and nitrate between plants and microbes in an annual grassland. Soil Biol Biochem 21:409–415
Jahangir MMR, Khalil MI, Johnston P, Cardenas L, Hatch D, Richards KG (2010) Denitrification and the N2/(N20+N2) ratios at various soil depths under grazed grassland in Ireland. In: Proceedings of the Joint Scottish Agricultural College (SAC) and Scottish Environment Protection Agency (SEPA) Biennial Conference, University of Edinburgh, Scotland, UK, pp 282–287
Jensen B, Sørensen P, Thomsen IK, Jensen ES, Christensen BT (1999) Availability of nitrogen in 15N-labeled ruminant manure components to successively grown crops. Soil Sci Soc Amer J 63:416–423
Jensen LS, Perdersen IS, Hansen TB, Nielsen NE (2000) Turnover and fate of 15N-labelled cattle slurry ammonium-N applied in the autumn to winter wheat. Eur J Agron 12:23–35
Lalor STJ, Kirwan L, Schulte RPO (2009) Maximising the utilisation of slurry nitrogen in grassland. Efficient and reliable utilisation of manure nutrients—Manure application management conference, Teagasc, Johnstown Castle, Wexford, Ireland, pp 21–32
Lalor STJ, Schröder JJ, Lantinga EA, Oenema O, Kirwan L, Schulte RPO (In press) Nitrogen fertilizer replacement value of cattle slurry in grassland as affected by method and timing of application. J Env Qual
Ledgard SF, Brier GJ, Sarathchandra SU (1989) Plant uptake and microbial immobilization of 15N-labelled ammonium applied to grass-clover pasture - influence of simulated winter temperature and time of application. Soil Biol Biochem 21:667–670
Misselbrook TH, Smith KA, Johnson RA, Pain BF (2002) Slurry application techniques to reduce ammonia emissions: results of some UK field-scale experiments. Biosyst Engin 81:313–321
Morvan T, Leterme P, Arsene GG, Mary B (1997) Nitrogen transformations after the spreading of pig slurry on bare soil and ryegrass using 15N-labelled ammonium. Eur J Agron 7:181–188
Muñoz GR, Powell JM, Kelling KA (2003) Nitrogen budget and soil N dynamics after multiple applications of unlabeled or 15Nitrogen-enriched dairy manure. Soil Sci Soc Amer J 67:817–825
Petersen J (2006) Crop 15N recovery course affected by spatial distribution of animal slurries in soils. Nutr Cycl Agroecosyst 75:15–27
Powell JM, Wu Z, Kelling K, Cusick P, Muñoz G (2004) Differential nitrogen-15 labeling of dairy manure components for nitrogen cycling studies. Agron J 96:433–441
Powell JM, Kelling K, Muñoz G, Cusick P (2005) Evaluation of dairy manure nitrogen-15 enrichment methods on short-term crop and soil nitrogen budgets. Agron J 97:333–337
Schmidt O, Ostle NJ (1999) Tracing nitrogen derived from slurry in earthworms using 15N/14N stable isotope ratios at natural abundances. Appl Soil Ecol 12:7–13
Schröder J (2005a) Revisiting the agronomic benefits of manure: a correct assessment and exploitation of its fertilizer values spares the environment. Bioresour Technol 96:253–261
Schröder JJ (2005b) Manure as a suitable component of precise nitrogen nutrition. In: Proceedings No. 574, International Fertiliser Society, York, UK, pp 1–30
Schulte RPO, Diamond J, Finkele K, Holden NM, Brereton AJ (2005) Predicting the soil moisture conditions of Irish grasslands. Irish J Agric Food Res 44:95–110
Schulte RPO, Richards K, Daly K, Kurz I, McDonald EJ, Holden NM (2006) Agriculture, meteorology and water quality in Ireland: A regional evaluation of pressures and pathways of nutrient loss to water. Biology and Environment: Proceedings of the Royal Irish Academy 106B:117–133
Smith KA, Jackson DR, Misselbrook TH, Pain BF, Johnson RA (2000) Reduction of ammonia emission by slurry application techniques. J Agric Engin Res 77:277–287
Søgaard HT, Sommer SG, Hutchings NJ, Huijsmans JFM, Bussink DW, Nicholson F (2002) Ammonia volatilization from field-applied animal slurry - the ALFAM model. Atm Env 36:3309–3319
Sommer SG, Hutchings NJ (2001) Ammonia emission from field applied manure and its reduction - invited paper. Eur J Agron 15:1–15
Sørensen P, Amato M (2002) Remineralisation and residual effects of N after application of pig slurry to soil. Eur J Agron 16:81–95
Sørensen P, Jensen ES, Nielsen NE (1994) Labelling of animal manure nitrogen with 15N. Plant Soil 162:31–37
Sørensen P, Jensen B, Thomsen IK, Jensen ES, Christensen BT (1998) The fate of 15N-labelled animal manure components in soil. In: Proceedings of the 16th world congress of soil science, Montpellier, France
Sparling GP, West AW (1988) Modifications to the fumigation extraction technique to permit simultaneous extraction and estimation of soil microbial C and N. Comm Soil Sci Plant Anal 19:327–344
Stark JM, Hart SC (1996) Diffusion technique for preparing salt solutions, Kjeldahl digests, and persulfate digests for nitrogen-15 analysis. Soil Sci Soc Amer J 60:1846–1855
Stark CH, Richards KG (2008) The continuing challenge of nitrogen loss to the environment: Environmental consequences and mitigation strategies. Dynamic Soil, Dynamic Plant 2:41–55
Thomsen IK (2004) Nitrogen use efficiency of 15N-labeled poultry manure. Soil Sci Soc Amer J 68:538–544
Thomsen IK (2005) Crop N utilization and leaching losses as affected by time and method of application of farmyard manure. Eur J Agron 22:1–9
Thomsen IK, Kjellerup V, Jensen B (1997) Crop uptake and leaching of 15N applied in ruminant slurry with selectively labelled faeces and urine fractions. Plant Soil 197:233–239
Van Kessel JS, Reeves JBI (2002) Nitrogen mineralization potential of dairy manures and its relationship to composition. Biol Fert Soils 36:118–123
Van Kessel C, Clough T, Van Groenigen JW (2009) Dissolved organic nitrogen: An overlooked pathway of nitrogen loss from agricultural systems? J Env Qual 38:393–401
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fibre, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74:3583–3597
Wachendorf C, Taube F, Wachendorf M (2005) Nitrogen leaching from 15N labelled cow urine and dung applied to grassland on a sandy soil. Nutr Cycl Agroecosyst 73:89–100
Webb J, Pain B, Bittman S, Morgan J (2010) The impacts of manure application methods on emissions of ammonia, nitrous oxide and on crop response—A review. Agric Ecosyst Env 137:39–46
Whitehead DC, Bristow AW (1990) Transformations of nitrogen following the application of 15N-labelled cattle urine to an established grass sward. J Appl Ecol 27:667–678
Acknowledgements
This research was financially supported by the Research Stimulus Fund 2005, administered by the Department of Agriculture and Food as part of the National Development Plan, Ireland. The authors would like to express their thanks to Ciara Coughlan, Nicola Rochford and David Ryan for their technical assistance, and to Dr Laura Kirwan for her advice on statistical analyses.
We thank Dr Jens Dyckmans and the KOSI team (Göttingen, Germany) for professional isotopic analysis of seemingly endless numbers of samples and Liz Dixon at North Wyke Research for 15N analysis of slurry fractions.
The advice and input received during this research project from Dr John Murphy (Teagasc, Moorepark, Ireland), Dr Ibrahim Khalil (EPA, Ireland), Dr Ken Smith (ADAS, UK), Dr Jaap Schröder (Plant Research International, The Netherlands), Dr Ruth Grant (NERI, Denmark) and Dr Dave Chadwick (North Wyke Research, UK) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Dan Murphy.
Rights and permissions
About this article
Cite this article
Hoekstra, N.J., Lalor, S.T.J., Richards, K.G. et al. The fate of slurry-N fractions in herbage and soil during two growing seasons following application. Plant Soil 342, 83–96 (2011). https://doi.org/10.1007/s11104-010-0669-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-010-0669-7