Abstract
Two controlled microcosm experiments aimed at a critical re-assessment of the contributions of divergent arbuscular mycorrhizal (AM) fungi to plant mineral nutrition were established that specifically targeted Plantago lanceolata–Glomus intraradices (B.B/E) and –Gigaspora margarita (BEG 34) symbioses developed in a native, nutrient limited, coastal dune soil. Plant tissue nitrogen (N), phosphorus (P) and potassium (K) status as well as plant growth parameters and levels of mycorrhizal colonization were assessed at harvest. In addition to the general well-established mycorrhizal facilitation of P uptake, the study was able to demonstrate a G. intraradices-specific contribution to improved plant nitrogen and potassium nutrition. In the two respective experiments, G. intraradices-inoculated plants had 27.8% and 40.8% more total N and 55.8% and 23.3% more total K when compared to Gi. margarita inoculated counterparts. Dissimilar overall contribution of the two isolates to plant nutrition was identified in AM-genus specific differences in plant tissue N:P:K ratios. G. intraradices inoculated and non-mycorrhizal plants generally exhibited N:P:K ratios indicative of P limitation whereas for Gi. margarita mycorrhizal plants, corresponding ratios strongly implied either N or K limitation. The study provides further evidence highlighting AM functional biodiversity in respect to plant nutrient limitation experienced by mycorrhizal P. lanceolata in an ecologically relevant soil system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arbuscular mycorrhizas are symbiotic associations formed between the roots of plants and asexual arbuscular mycorrhizal (AM) fungi ascribed to the phylum Glomeromycota (Schüβler et al. 2001) that occur in an estimated 80% of terrestrial plants (Wang and Qui 2006). Maintenance of the symbiosis incurs a carbon cost for the plant host that is generally balanced by a range of benefits provided by the AM fungal mycobiont, including enhanced nutrient uptake, protection from pathogens and improved water relationships (Newsham et al. 1995; Helgason and Fitter 2009; Sikes et al. 2010).
The influence of AM symbiosis on plant mineral nutrition continues to represent a major area of research (Smith and Read 2008) as the outcome is crucial in controlling plant fitness and productivity whilst being relatively easily quantifiable through traditional plant tissue nutrient analysis techniques. Nutritional gains for the host plant from the AM symbiosis have focused mainly on immobile nutrients, namely phosphorus (P), zinc (Zn) and copper (Cu) (Smith and Read 2008), where AM fungi permit plant nutrient acquisition from a more extensive soil volume through development of a extraradical fungal mycelial network that significantly extends the nutrient mobilization zone (Jakobsen et al. 1992; Drew et al. 2003). Despite reports that demonstrate facilitation of nitrogen (N) uptake from organic (e.g., Hodge et al. 2001; Leigh et al. 2009) and mineral (e.g.; Tanaka and Yano 2006) soil resources in the presence of AM fungi, significant enhancement of N nutrition in AM plants remains in dispute (e.g., Reynolds et al. 2005; Smith and Read 2008). Similarly, the case for potassium (K) nutrition still remains unclear. Enhanced K assimilation that has been demonstrated in some studies is often linked with low pH. AM fungi appear to positively contribute to plant K nutrition only under acidic conditions (Clark and Zeto 2000) whilst in other reports the positive effect is linked with AM facilitation of P nutrition in P-deficient soils and the form and availability of N as ammonium or nitrate and even possibly soil Na+ status (Smith and Read 2008). The latter authors have stressed the need for more defined experimentation to resolve this issue.
In the case of AM-enhancement of host plant P nutrition, there is evidence to suggest different genus- and species-specific contributions to mineral P nutrition (Pearson and Jakobsen 1993; Facelli et al. 2010). However, evidence to suggest that similar AM genus- and species-specific differences operate in relation to N and K assimilation is not as abundant and, when present, has been gained in fertilized soil systems (Azcón et al. 2001). Indeed, a significant amount of our knowledge regarding the nutritional gains for the host plant from the AM symbiosis has focused on agricultural plant species (e.g., Galvez et al. 2001; Marschner et al. 2001) that are adapted to high N, P and K availability and where data has originated from experiments that have utilized fertilized soils or artificial growth substrates (e.g., Azcón et al. 2008; Hodge et al 2001; Marschner et al. 2001). As such there remains an urgent need to extend these investigations to native plant species in non-fertilized soils of un-managed ecosystems.
Therefore, the aim of this study was to examine the impact of two divergent AM fungi, Glomus intraradices and Gigaspora margarita, on the growth properties and N, P and K status of Plantago lanceolata, in a N, P and K-limited, non-fertilized dune soil over two consecutive experiments. P. lanceolata was selected for investigation as it represents a very common mycotrophic perennial plant (Sagar and Harper 1964) in unmanaged-N-limited grasslands (Stevens et al. 2004) and occurred naturally at the site were the soil was obtained. Microcosm experiments utilized the widely studied AM fungal isolates Glomus intraradices (B.B/E) and Glomus margarita (BEG 34).
It was hypothesized that the two divergent AM fungal isolates, in a series of two experiments, would result in differences in the plant N, P and K status of the corresponding AM fungal inoculated plants with consequences for the type of nutrient limitation experienced by the P. lanceolata host. We tested this hypothesis under two experimental conditions: firstly in a system where mycorrhizal P. lanceolata plants could exploit soil of similar volume to non-mycorrhizal plants to absorb nutrients (Experiment 1), and further in a microcosm system where mycorrhizal hyphae could access soil unavailable to the P. lanceolata root system (Experiment 2).
Materials and methods
Soil
The soil used in both experiments was collected from coastal sand dunes at the Ainsdale Nature Reserve (Merseyside, UK; 53°34′N, 3°5′W). The reserve has Special Site of Scientific Interest (SSSI) and Special Area of Conservation (SAC) status as the nutrient-limited dune slack habitat supports a rich diversity of flora and fauna (Smith 2006; JNCC 2007). The soil was sampled at a depth of 5–15 cm from an area supporting indigenous P. lanceolata populations and transported to the laboratory in plastic bags. Soil was air dried on the bench for 2 days, sieved through a 2 mm sieve and sterilized in 5 l volumes by exposure to gamma irradiation (50 kGy for 48 h). The soil was classified as a sandy loam (70% sand, 13% silt, 17% clay) with the following properties: pH: 5.76, organic C 2.06%, Olsen P: 4.97 mg/kg, total P: 148 mg/kg, total N: 1011 mg/kg, total K: 329 mg/kg, total Ca: 105 mg/kg, total Mg: 372 mg/kg, exchangeable ammonia: 29.4 mg/kg, exchangeable nitrate: 6.5 mg/kg.
Experiment 1
Pots, (11 cm height, 8 cm diameter) that were pre-sterilized in bleach and filled with 340 g equivalent dry weight (EDW) of gamma-irradiated Ainsdale dune soil, were used in a controlled experiment. To re-establish a prokaryotic microbial population in the irradiated soil, a microbial suspension was prepared by sequential filtration [Whatman #1 (11 μm pore size) and Whatman #5 (2.5 μm pore size) under vacuum] of a soil extract obtained by orbital shaking (150 rpm) a non-sterile soil:sterile dH2O (1:9 w/v) mixture for 30 min. The microbial suspension was used to raise the soil water content (SWC) to 60% water holding capacity (WHC), and soil was incubated at this SWC (maintained gravimetrically with autoclaved distilled water) in the growth chambers under the experimental growth conditions (see below) for 2 weeks to allow equilibration of the reintroduced microbial community. Plantago lanceolata seeds (MAS Seed Specialists, Wiltshire, UK) were surface sterilized through soaking in 70% ethanol (2 min) and 5% bleach in 0.5% SDS (5 min). Following five washings in sterile H2O, seeds were left to germinate on tryptone soy agar plates for 7 days and germinated seeds showing no sign of microbial contamination were used in experiments.
Three treatments were established: non-mycorrhizal, Glomus intraradices B.B/E (Biorize France), Gigaspora margarita BEG 34 (Biorize, France). The mycorrhizal inoculum comprised 1 g chopped 3-month-old strawberry (Fragaria vesca) roots grown semi-aseptically with the respective AM species. The inoculum was positioned 1 cm below a single seedling that was transplanted to each pot. Non-mycorrhizal strawberry roots were used for the non-mycorrhizal control treatments. The experiment consisted of 60 pots (20 per treatment) that were harvested sequentially (in quadruplicate) 2, 3, 5, 7 and 10 weeks after seedling transfer and AM inoculation. The experimental growth conditions in dedicated plant growth chambers (Fitotron SGC097 equipped with CO2 monitoring and control, Weiss-Gallenkamp, Loughborough, UK) were as follows: day/night photoperiod, temperature and relative humidity of 16/8 h, 22/15°C, 53/70%, respectively and mean ambient CO2 levels of approximately 450 ppm. Throughout the whole experiment pots were watered three times a week with autoclaved distilled water on a gravimetric basis to maintain 60% WHC.
At each harvest, plant material was separated into root and shoot fractions. A small proportion of the root (in the first two harvests approximately 40%; in the other three harvests less than 10%) was sampled for mycorrhizal staining while the rest of the root was dried for 3 days at 80°C. Root dry weight was corrected to account for the mass removed for mycorrhizal staining on the basis of the wet weight-to-dry weight ratio (obtained from the proportion of the root that was dried). The shoot fraction was also dried at 80°C. Percentage of mycorrhizal colonization was assessed on stained roots according to the grid-line intersect method (Giovannetti and Mosse 1980). Oven dried plant material from the 10th week harvest was ball-milled and subjected to an acid digestion as described below.
Experiment 2
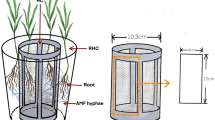
Small cylindrical microcosms (12 cm height, 9 cm diameter) were each constructed to include a centrally located longitudinal 40 μm stainless steel mesh divider (Fig. 1). Both equivolume compartments, after sterilization in bleach, were filled with gamma irradiated Ainsdale soil totalling 490 g EDW. The mesh barrier was designed to inhibit the growth of roots, but not AM extraradical hyphae, into the non-planted (root-free) compartment. The bacterial suspension was prepared as in Experiment 1 but instead of H2O, saline phosphate buffer pH 7.2 with 0.1% (v/v) Tween 80 was used as a more efficient microbial extractant and dispersant (Khanmar et al. 2004; Macdonald 1986). Microbial equilibration in soils lasted 3 weeks and the experiment included the following treatments: non-mycorrhizal plants and mycorrhizal plants inoculated with G. intraradices or Gi. margarita (eight replicate microcosms per treatment). Mycorrhizal inoculum consisted of 20–25 spores, so that the amount of nutrients added in each microcosm would be minimized, that were added to a planting hole approximately 1 cm below the seedling. Spores were isolated from 1-year-old strawberry cultures through the sucrose flotation method (York Mycorrhiza Research Group 2000), surface sterilized for 20 mins in 2% chloramine T supplemented with streptomycin (400 μg/l) according to Giovannetti et al. (1999), and cold shocked overnight at 4°C. The 1-week-old surface sterilized P. lanceolata seedlings (prepared as in Experiment 1) were sown so that in each microcosm only one compartment contained a single centrally placed seedling. Plant growth conditions maintained in the Fitotron growth chamber were as in Experiment 1 but in this case CO2 was actively regulated and maintained in the range 350–380 ppm through computer software control supplemented by a soda lime-based scrubber, which consisted of a tray containing soda lime (∼100 g) placed inside the growth chamber that was replaced on a daily basis. Plants were grown for 13 weeks in the growth chamber and, at harvest, treated as in the previous experiment.
Photograph of the bottom part of a typical microcosm at harvest in Experiment 2. The 40 μm mesh separates the microcosm into two halves: the rooted compartment were the plant is located, and the root-free compartment. Note the high density of roots in the rooted compartment. The drainage mesh has been partially removed to reveal the texture of the soil
Nutrient analysis
Acid digestion was carried out through addition of 2 ml H2SO4 and ∼100 μg of 1:100 (w / w) selenium: lithium catalyst to approximately 0.75 g ball-milled plant material. The mix was digested at 360°C for 5 h (Gupta 1987). N (as NH +4 ) and K+ were determined through ion chromatography (Dionex DX 100 – Dionex Pac CS16 analytical column – IonPacCG16 guard column) while P was assessed through ICP-AES (Varian Vista AX with a CCD detector) analysis at 177.433 nm. Shoots and roots were digested separately and total plant tissue nutrient content was calculated according to the formula:
Nutrient limitation assessment
To indirectly assess plant growth nutrient limitation type, the approach developed by Hoosbeek et al. (2002) for bog vegetation was generalized. This approach assumes that vegetation N:P and N:K ratios can be indicative of the type of plant growth nutrient limitation experienced. In this respect, a plot is created based on plant sample N:P and N:K ratios and critical N:P, N:K and P:K ratios as established in the literature for the type of vegetation studied.
Data analysis
Statistical analyses were conducted using ANOVA in Minitab version 15 (Minitab, State College, PA). All data were examined for normality using Kolmogorov-Smirnov tests. Multiple comparisons were made according to Fisher’s least significant difference (LSD) test (Student 1908), provided that a significant F test at the same significance level was previously obtained. Significance of LSD comparisons of P ≤ 0.05 was adopted.
Results
Experiment 1
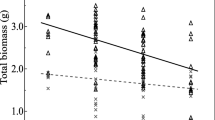
The absence of colonization was verified for all plants assigned to the non-mycorrhizal treatment that did not receive AM inocula, whereas, with the exception of the first harvest, AM structures were detected in roots of all plants inoculated with G. intraradices and Gi. margarita (arbuscules + vesicles and arbuscules, respectively). Percent AMF colonization, plant biomass and S:R ratios throughout the experiment are presented in Table 1, and the dry weight status and S:R ratios of the plants in the final harvest are presented in Fig. 2a, b, respectively. There was moderate evidence for a mycorrhizal treatment effect on S:R ratio (F2,27 = 2.54, P = 0.097) and plant size (F2,27 = 3.64 P = 0.04) across the last three harvests. Data from the first two harvests was ignored as it was not informative due to the small size of the plants.
Means (± SE) of total dry weight, shoot:root ratio and total tissue N, P and K content in final harvests of Experiments 1 and 2. Total dry weight was calculated as the aggregate of shoot and root dry weight nutrient tissue contents as the aggregate of the shoot and root products of the respective nutrient tissue concentrations with the respective shoot or root dry weights. P values above each graph represent the level of significance of the analysis of variance. Treatment means with a different letter are significantly different according to LSD comparisons at P = 0.05. NM Non mycorrhizal, Gi Glomus intraradices, Gm Gigaspora margarita. a–e Experiment 1, f–j Experiment 2
There was moderate evidence for a treatment effect on P. lanceolata N nutrition (P = 0.098; Fig. 2c). Shoot tissue P concentrations varied from an average of 0.41 mg/kg (non-mycorrhizal treatment) to 1.22 mg/kg (Gi. margarita treatment) (data not shown); values below or close to the minimum shoot tissue P concentrations that have been recorded historically for P. lanceolata (0.9 mg/kg) (Güsewell and Koerselman 2002). Mycorrhizal plants were able to forage more efficiently for soil P (Fig. 2d; P < 0.05) whilst G. intraradices plants had significantly higher K content than those of the two other treatments (P = 0.05) (Fig. 2e). N:P ratio values of total tissue ranged from 17.2 to 94 and N:K ratios ranged from 1.6 to 4 (Fig. 3a). With the exception of Gi. margarita-inoculated samples that appeared to be K limited P. lanceolata plants were, according to Hoosberg approach, mainly P limited, (Fig. 3a).
N:P vs N:K ratio plots of individual samples. The nutrient concentrations that were used for the plot of ratios were calculated as total shoot and root nutrient content divided by total plant dry weight. Each plot is comprised by five zones that correspond to N:P:K ratios characteristic of N, P, K limited, N and P co-limited and N and K co-limited plant growth . The critical values approach was adopted from Hoosbeek et al. (2002). Note that the N-P and N-K co-limitation zones are assumed to be narrow. a Experiment 1, b Experiment 2
Experiment 2
Both G. intraradices and Gi. margarita treatments were mycorrhizal at the 13-week harvest, and most plants had flowered or were flowering. Mycorrhizal colonization, assessed according to the grid-line intersect method (mean ± standard error), in these two treatments was 27% (±5.3%) and 26% (±4.4%), respectively. No mycorrhizal structures were detected in non-mycorrhizal plants. Arbuscules in both mycorrhizal treatments were in a state of collapse or had already collapsed, and in the G. intraradices treatment mycorrhizal colonization was mainly in the form of vesicles. Colonization of the non-planted root-free compartment was verified through the recovery of spores from this compartment for all the microcosms with mycorrhizal plants. Plant roots had, however, penetrated through the drainage mesh and into the root-free compartment in four, three and six microcosms in non-mycorrhizal, G. intraradices and Gi. margarita treatments, respectively. Mean dry weights of plants in intact:penetrated microcosms were as follows: 3.3 g:2.3 g for non-mycorrhizal plants, 6.1 g:5.5 g for G. intraradices inoculated plants and 4.0 g:5.2 g for Gi. margarita inoculated plants. Two sample t-tests assuming unequal variances on the impact of root penetration in the root-free compartment on dry weights, N, P and K acquisition revealed that the effect of the rupture on each of the growth parameters was not significant (P > 0.2). Thus, all samples were included in the analysis as it was speculated that root ingress occurred at a late stage of plant growth and did not greatly affect plant nutrition.
A significant treatment effect was detected with respect to plant biomass as non-mycorrhizal plants were significantly (P < 0.05) smaller than mycorrhizal plants (Fig. 2f). S:R ratio for G. intraradices, was significantly lower than the non-mycorrhizal control (P < 0.05) (Fig. 2g). N and K nutrition in G. intraradices inoculated plants was significantly (P < 0.05) enhanced compared to the Gi. margarita and non-mycorrhizal plant treatments (Fig. 2h, j) while Gi. margarita plants appeared to contain more K than non-mycorrhizal plants (P < 0.05) (Fig. 2j). There was strong evidence (P < 0.05) that the P content of mycorrhizal plants was higher (Fig. 2i). N:P ratio of total tissue ranged from 9.88 to 39 and the N:K ratio ranged from 0.6 to 2.3 (Fig. 3b). With the exception of Gi. margarita-inoculated samples that experienced all, N-, P- and K- plant-growth nutrient limitation types, the other P. lanceolata plants were, according to Hoosberg approach, P limited (Fig. 3b).
Discussion
The study presented here examined the effect of AM fungi on the N, P and K status of P. lanceolata. In Experiment 1, mycorrhizal and non-mycorrhizal plants had access to nutrients from comparable volumes of soil, whereas in Experiment 2 the microcosm system enabled, probably more realistically, mycorrhizal plants to have access to additional soil volume. This effect combined with the increased duration of the experiment could explain the more pronounced contrasts between non-mycorrhizal controls and mycorrhizal treatments with respect to accumulation of nutrients in mycorrhizal plants in Experiment 2. Elevated CO2 (ca. 450 ppm) levels in Experiment 1, in addition to the impact on mycorrhizal colonization (Treseder 2004), could have been expected to have permitted enhanced dry weight accumulation per unit of limiting N (Zak et al. 1993), though no such an effect has been detected in the cases when plant growth is limited by P (Edwards et al. 2005). There is no record in the literature, however, to indicate that elevated CO2 might have any effect on the balance and the proportional plant uptake of nutrients other than N.
Both experiments clearly illustrate the well-known rule that, under P limiting conditions, plant growth is critically dependent on the AM symbiosis in facilitating more efficient P assimilation (Pearson and Jakobsen 1993). More importantly, the data suggest that plants symbiotically associated with G. intraradices exhibited more efficient N and K uptake compared to Gi. margarita and non-mycorrhizal counterparts. Thus, in agreement with our proposed hypothesis, an AM genus-specific effect was demonstrated for these two nutrients. In fact, comparison between the Gi. margarita and non-mycorrhizal treatments in Experiment 1 revealed that, despite the larger size of the mycorrhizal plants, no additional N or K was assimilated in the Gi. margarita treatment suggesting not only more efficient K uptake in G. intraradices compared to Gi. margarita but a Gi. margarita-induced repression of N and K uptake when expressed on a plant biomass basis.
The recorded improvement in N and K nutrition could be attributed to either a direct AM fungi effect in enhancing uptake, or, to the better access to soil P that allowed enhanced growth in AM plants, thus increasing N and K uptake as an indirect consequence of the AM symbiosis. G. intraradices plants acquired significantly more N (Experiment 2) and K (Experiment 1 and 2). However, in both experiments, G. intraradices- and Gi. margarita-inoculated plants did not differ significantly in biomass or P uptake, which may suggest that the enhancement in N and K nutrition in the G. intraradices treatments, when compared to Gi. margarita, was due to a direct influence of G. intraradices in nutrient uptake.
Gi. margarita belongs to the family of Gigasporaceae that is distantly related to the Glomeraceae family, which includes G. intraradices. In reviewing studies that have demonstrated facilitation of N uptake by AM fungi, it is worth noting that the majority of these investigated isolates belonged to the family Glomeaceae. For example, Glomus fasciculatum (Azcón-Aguilar et al. 1993), Glomus hoi (Hodge et al. 2001; Leigh et al. 2009), G. intraradices (Hawkins et al. 2000; Govindarajulu et al. 2005; Tanaka and Yano 2006; Azcón et al. 2008; Leigh et al. 2009) and Glomus mosseae (Ames et al. 1983; Hawkins et al. 2000). Porras-Soriano et al. (2009) investigated inter-species responses within Glomeaceae in respect to N, P and K assimilation. In the single study where non-Glomeaceae AM fungi were also included, i.e., Gigaspora gigantea, Gigaspora decipiens, Archaeospora trappei (Reynolds et al. 2005), no facilitation in N assimilation was detected although it should be noted that a Glomus species that was included in the same experiment, despite having a significant (P = 0.05) ameliorating effect on total N content compared to the other AM isolates in three out of the five cases examined, did not promote plant N acquisition under the experimental conditions adopted. The results of our study demonstrate differential AM-species-specific N and K assimilation in P. lanceolata. Assuming the mechanism to be a result of direct, as opposed to indirect, AM fungal-facilitated uptake, this observation may rest in a distinct physiology of the two families that have so far been demonstrated to differ in morphological and life history traits such as their colonization strategy in terms of respective intra-radical and extra-radical allocation of AM fungal biomass (Gigasporaceae colonize soil more extensively) and the ability to propagate from root fragments (a low capacity for Gigasporaceae isolates) (Hart and Reader 2002; Klironomos and Hart 2002). It is clear that more research is needed to reveal whether, indeed, Glomus isolates may possess increased efficiency in facilitating plant N and K nutrition under N and P limiting soil conditions.
To examine if differences in plant nutrient assimilation patterns amongst the treatments may affect the type of plant growth nutrient limitation experienced by P. lanceolata, the approach of Hoosbeek et al. (2002) where N:P ratios of samples were plotted against respective N:K ratios was adopted for data derived from Experiment 1 (Fig. 3a) and Experiment 2 (Fig. 3b). Though this approach has been applied for bog vegetation, which has been more extensively studied in terms of growth limitation in the absence of other limiting factors, an N:P ratio of 15 has consistently been found, irrespective of type of vegetation, to delineate a cut off between P limitation (N:P > 15) and N limitation (N:P < 15) (Güsewell 2004) with a symmetrical zone of N and P co-limitation for N:P ratios around 15, the extent of which is determined from the characteristics of the ecosystem (Craine et al. 2008). Similarly, there appears to be consistency in the interpretation of N:K ratios (N:K ratios below 1.2 suggest N-limited plant growth and above 1.4, K-limited plant growth) irrespective of type of vegetation (Lawniczak et al. 2009; Venterink et al. 2003). According to Fig. 3a, plant growth in Experiment 1 was limited by either P or K availability, while in Experiment 2 (Fig. 3b), N limitation was also recorded in some samples. However, it was only in the Gi. margarita inoculated plants where the N:P:K status shifted from being predominantly P-limited to N- or K-limited. N, P and K limitation are the dominant forms of nutrient limitation in unmanaged terrestrial ecosystems, and are the main controllers of plant growth in the absence of the other growth limiting factors, e.g., light, temperature, diseases and toxicity (Güsewell and Koerselman 2002). That some plant individuals appeared to be N-limited in Experiment 2 may be a result of the longer duration (13 weeks compared to 10 weeks for Experiment 1) of this experiment. Both N and P are mobile nutrients within plants (Taiz and Zeiger 2006). Mature leaves while still photosynthetically active (N demanding process) are no longer growing (P-demanding process) and, therefore, critical N:P values for mature plants appear to be higher (Usuda 1995).
What was interesting in the analytical approach adopted was that the identity of the AM symbiont appeared to affect the type of nutrient limitation that the plant experienced, i.e., P limitation when P. lanceolata was inoculated with G. intraradices or no AM fungus, and K, P or N when inoculated with Gi. margarita. Thus, in agreement with our hypothesis, AM species-mediated differences in P. lanceolata N, P and K status manifested in the type of nutrient limitation experienced by the plant host. In the literature, there is evidence that the AM fungi that belong to the family of Gigasporaceae (when compared to corresponding AM fungi that belong to Glomeaceae) occupy a distinct ecological niche as they are more sensitive to N fertilization (Johnson et al. 2003; Egerton-Warburton et al. 2007), prefer mesic ecosystems and are unable to cope well with aridity (Egerton-Warburton et al. 2007). As a result, the relative proportion of isolates that belong to the family of Gigasporaceae in the AM fungal population may vary considerably across different ecosystems. Working under the assumption that the differences noted in terms of plant nutrition are dependent on the identity of the AM-fungal symbionts, the distinct ecology of the two taxa of AM fungi could become a potentially decisive determinant of the type of growth limitation that the vegetation encounters in natural ecosystems. It should be stressed that the critical N:P:K ratios that were used in this experiment should be treated with caution as they represent indirect indicators of potential nutrient limitations and more targeted experimentation, through addition of nutrients to establish the type of plant growth limitation, is needed to reinforce the inferences made here. However, the possibility that AM fungi can potentially alter the limiting nutrient experienced by their host plant in an AM genus-specific way has implications for the role of AM diversity in influencing the nature of plant–plant interactions in natural ecosystems.
References
Ames RN, Reid CPP, Porter L, Cambardella C (1983) Hyphal uptake and transport of nitrogen from two 15N-labelled sources by Glomus mosseae, a vesicular-arbuscular mycorrhizal fungus. New Phytol 95:381–396
Azcón R, Ruiz-Lozano JM, Rodriguez R (2001) Differential contribution of arbuscular mycorrhizal fungi to plant nitrate uptake (15N) under increasing N supply to the soil. Can J Bot 79:1175–1180
Azcón R, Rodriguez R, Amora-Lazcano E, Ambrosano E (2008) Uptake and metabolism of nitrate in mycorrhizal plants as affected by water availability and N concentration in soil. Eur J Soil Sci 59:131–138
Azcón-Aguilar C, Alba C, Montilla M, Barea JM (1993) Isotopic (15N) evidence of the use of less available N forms by VA mycorrhizas. Symbiosis 15:39–48
Galvez L, Douds DD Jr, Drinkwater LE, Wagoner P (2001) Effect of tillage and faming system upon VAM fungus populations and mycorrhizas and nutrient uptake of maize. Plant Soil 228:299–308
Clark RB, Zeto SK (2000) Mineral acquisition by arbuscular mycorrhizal plants. J Plant Nutr Soil Sci 23:867–902
Craine JM, Morrow C, Stock WD (2008) Nutrient concentration ratios and co-limitation in South African grasslands. New Phytol 179:829–836
Drew EA, Murray RS, Smith SE, Jakobsen I (2003) Beyond the rhizosphere: growth and function of arbuscular mycorrhizal external hyphae in sands of varying pore sizes. Plant Soil 251:105–114
Edwards EJ, McCaffery S, Evans JR (2005) Phosphorus availability and elevated CO2 affect biological nitrogen fixation and nutrient fluxes in a clover-dominated sward. New Phytol 169:157–169
Egerton-Warburton LM, Johnson NC, Allen EB (2007) Mycorrhizal community dynamics following nitrogen fertilization: a cross-site test in five grasslands. Ecol Monogr 77:527–544
Facelli E, Smith SE, Facelli JM, Christophersen HM, Smith FA (2010) Underground friends or enemies: model plants help to unravel direct and indirect effects of arbuscular mycorrhizal fungi on plant competition. New Phytol 185:1050–1061
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Giovannetti M, Azzolini D, Citernesi AS (1999) Anastomosis formation and nuclear and protoplasmic exchange in arbuscular mycorrhizal fungi. Appl Environ Microbiol 65:5571–5575
Govindarajulu M, Pfeffer PE, Jin HR, Abubaker J, Douds DD, Allen JW, Bucking H, Lammers PJ, Shachar-Hill Y (2005) Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 435:819–823
Gupta PL (1987) A comparative study of ashing procedures for the analysis of N,P and K in small amounts of plant materials. In: Rowland AP (ed) Chemical analysis in environmental research. Institute of Terrestrial Ecology, Huntingdon
Güsewell S (2004) N:P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266
Güsewell S, Koerselman W (2002) Variation in nitrogen and phosphorus concentrations of wetland plants. Perspect Plant Ecol Evol Syst 5:37–61
Hart MM, Reader RJ (2002) Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol 153:335–344
Hawkins H-J, Johansen A, George E (2000) Uptake and transport of organic and inorganic nitrogen by arbuscular mycorrhizal fungi. Plant Soil 226:275–285
Helgason T, Fitter AH (2009) Natural selection and the evolutionary ecology of the arbuscular mycorrhizal fungi (Phylum Glomeromycota). J Exp Bot 60:2465–2480
Hodge A, Campbell CD, Fitter AH (2001) An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 413:297–299
Hoosbeek MR, Van Breemen N, Vasander H, Buttler A, Berendse F (2002) Potassium limits potential growth of bog vegetation under elevated atmospheric CO2 and N deposition. Glob Chang Biol 8:1130–1138
Jakobsen I, Abbot LK, Robson AD (1992) External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. 1. Spread of hyphae and phosphorus inflow into roots. New Phytol 120:371–380
Johnson NC, Rowland DL, Gorkidi L, Egerton-Warburton LM, Allen EB (2003) Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology 84:1895–1908
JNCC (2007) Second report by the UK under article 17 on the implementation of the Habitats Directive from January 1001 to December 2006. Joint Nature Conservation Committee, Peterborough. Available from: http://www.jncc.gov.uk/article17
Khanmar N, Malhautier L, Degrange V, Lensi R, Fanio JL (2004) Evaluation of dispersion methods for enumeration of microorganisms from peat and activated carbon biofilters treating volatile organic compounds. Chemosphere 54:243–254
Klironomos JN, Hart MM (2002) Colonization of roots by arbuscular mycorrhizal fungi using different sources of inoculum. Mycorrhiza 12:181–184
Lawniczak AE, Güsewell S, Verhoeven JTA (2009) Effect of N:K supply ratios on the performance of three grass species from herbaceous wetlands. Basic Appl Ecol 10:715–725
Leigh J, Hodge A, Fitter AH (2009) Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytol 181:199–207
Macdonald RM (1986) Sampling soil microfloras: dispersion of soil by ion exchange and extraction of specific microorganisms from suspension by elutriation. Soil Biol Biochem 18:399–406
Marschner P, Crowley DE, Lieberei R (2001) Arbuscular mycorrhizal infection changes the bacterial 16S rDNA community composition in the rhizosphere of maize. Mycorrhiza 11:297–302
Newsham KK, Fitter AH, Watkinson AR (1995) Multi-functionality and biodiversity in arbuscular mycorrhizas. Trends Ecol Evol 10:407–411
Pearson JN, Jakobsen I (1993) Symbiotic exchange of carbon and phosphorus between cucumber and three arbuscular mycorrhizal fungi. New Phytol 124:481–488
Porras-Soriano A, Soriano-Martín ML, Porras-Piedra A, Azcón R (2009) Arbuscular mycorrhizal fungi increased growth, nutrient uptake and tolerance to salinity in olive trees under nursery conditions. J Plant Physiol 166:1350–1359
Reynolds HL, Hartley AE, Vogelsang KM, Bever JD, Schultz PA (2005) Arbuscular mycorrhizal fungi do not enhance nitrogen acquisition and growth of old-field perennials under low nitrogen supply in glasshouse culture. New Phytol 167:869–880
Sagar GR, Harper JL (1964) Plantago major L., P. media L. and P. lanceolata L. J Ecol 52:189–221
Schüβler A, Schwarzott D, Walker C (2001) A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol Res 105:1413–1421
Sikes BA, Powell JR, Rillig MC (2010) Deciphering the relative contributions of multiple functions within plant–microbe symbiosis. Ecology 91:1591–1597
Smith PH (2006) Changes in the floristic composition of sand-dune slacks over a twenty-year period. Watsonia 26:41–49
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Elsevier, London, p 605
Stevens CJ, Dice NB, Mountford JO, Cowing DJ (2004) Impact of nitrogen deposition on the species richness of grasslands. Science 303:1876–1879
Student (1908) The probable error of a mean. Biometrika 19:151
Taiz L, Zeiger E (2006) Plant physiology. Sinauer, Sunderland, p 700
Tanaka Y, Yano K (2006) Nitrogen delivery to maize via mycorrhizal hyphae depends on the form of N supplied. Plant Cell Environ 28:1247–1254
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355
Usuda H (1995) Phosphate deficiency in maize. V. Mobilization of nitrogen and phosphorus within shoots of young plants and its relationship to senescence. Plant Cell Physiol 36:1041–1049
Venterink HO, Wassen MJ, Verkroost WM, de Ruiter PC (2003) Species richness-productivity patterns differ between N-, P-, and K- limited wetlands. Ecology 84:2191–2199
Wang B, Qui YL (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16:299–363
York Mycorrhiza Research Group (2000) Techniques in Arbuscular Mycorrhiza research laboratory manual. Below-ground research team. The University of York, York
Zak DR, Pregitzer KS, Curtis PS, Teeri JA, Fogel R, Randlett DL (1993) Elevated atmospheric CO2 and feedback between carbon and nitrogen cycles. Plant Soil 151:105–117
Acknowledgments
We are very grateful to Mr. Mike Downey (Natural England) for providing soil sampling permission at the Ainsdale Nature Reserve. The study would not have been feasible without the technical support of Mr. David McKendry. The authors would like to thank Professor Demetrios Veresoglou and four anonymous reviewers for their comments on earlier drafts of the manuscript. We also thank Dr Tony Scallan for statistical advice and Mr. Paul Warren for ICP analyses. The study was partially funded by The Chloros Trust.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Katharina Pawlowski.
Rights and permissions
About this article
Cite this article
Veresoglou, S.D., Shaw, L.J. & Sen, R. Glomus intraradices and Gigaspora margarita arbuscular mycorrhizal associations differentially affect nitrogen and potassium nutrition of Plantago lanceolata in a low fertility dune soil. Plant Soil 340, 481–490 (2011). https://doi.org/10.1007/s11104-010-0619-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-010-0619-4