Abstract
The application of inorganic nitrogen (N) fertilizers strongly influences the contribution of agriculture to the greenhouse effect, especially by potentially increasing emissions of nitrous oxide (N2O), carbon dioxide (CO2) and methane (CH4) from soils. The present microcosm-study investigates the effect of different forms of inorganic N fertilizers on greenhouse gas (GHG) emissions from two different agricultural soils. The relationship between greenhouse gas emissions and soil microbial communities, N transformation rates and plant (Hordeum vulgare L. cv. Morex) growth were investigated. Repeated N fertilization led to increased N2O emissions. In a parallel survey of functional microbial population dynamics we observed a stimulation of bacterial and archaeal ammonia oxidisers accompanied with these N2O emissions. The ratio of archaeal to bacterial ammonium monooxygenase subunit A (amoA) gene copies (data obtained from Inselsbacher et al., 2010) correlated positively with N2O fluxes, which suggests a direct or indirect involvement of archaea in N2O fluxes. Repeated N fertilization also stimulated methane oxidation, which may also be related to a stimulation of ammonia oxidizers. The fertilizer effects differed between soil types: In the more organic Niederschleinz soil N-turnover rates increased more strongly after fertilization, while in the sandy Purkersdorf soil plant growth and soil respiration were accelerated depending on fertilizer N type. Compared to addition of NH +4 and NO −3 , addition of NH4NO3 fertilizer resulted in the largest increase in global warming potential as a summary indicator of all GHG related effects. This effect resulted from the strongest increase of both N2O and CO2 emission while plant growth was not equally stimulated, compared to e.g. KNO3 fertilization. In order to decrease N losses from agricultural ecosystems and in order to minimize soil derived global warming potential, this study points to the need for interdisciplinary investigations of the highly complex interactions within plant-soil-microbe-atmosphere systems. By understanding the microbial processes underlying fertilizer effects on GHG emissions the N use efficiency of crops could be refined.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
N2O, CH4 and CO2 are the three most important greenhouse gases (GHGs), which cause radiative forcing of climate change (IPCC 2001). While the global increases in CO2 concentration are primarily due to fossil fuel use and land-use change, agricultural practice is assumed to be one of the major sources of N2O and CH4 emissions (IPCC 2007). N2O emissions from agriculture are estimated to account for more than 75% of the total global anthropogenic emissions (Duxbury et al. 1993; Isermann 1994; Abdalla et al. 2009; Jackson et al. 2009). Therefore, the contribution of agricultural soils to global warming has received increasing attention, with a major research focus on the influence of the form and quantity of N fertilizers on greenhouse gas fluxes (e.g., Bouwman 1990; Matson et al. 1998; Mosier 1998; Verma et al. 2006; Jones et al. 2007). Increased N inputs by mineral fertilizers temporarily result in a surplus of NH +4 and NO −3 in the soil, the substrates for nitrification and denitrification. As the major part of N2O emitted from soils is produced by these two processes (Hutchinson and Davidson 1993; Ambus et al. 2006), inorganic N fertilizer input potentially increases N2O emissions (Matson et al. 1998; Hall and Matson 1999; Chu et al. 2004; Cardenas et al. 2010). Besides the availability of NH +4 and NO −3 , nitrification and denitrification are regulated by a number of edaphic factors, including soil texture, water content, soil temperature, aeration, the amount of soluble organic C and pH (Bouwman 1990; Granli and Bockman 1994). In context with the prevailing weather situation there are seasonal differences in the availabilities of dissolved inorganic nitrogen (DIN), as during cold and wet spring weather NH +4 is present at high amounts after fertilization (in the forms of urea, NH +4 or NH4NO3), while NO −3 becomes the dominant N form in the soil during the warm and dry summer season (Dobbie and Smith 2003). Thus, large variability is typical for N2O emissions from agricultural soils and therefore it has been difficult to draw general conclusions on the impact of fertilizer N form on N2O emissions. Increased N2O emissions also imply losses of fertilizer N from the agricultural system and reduce the N availability for crop plants. For calculating N2O emissions from fertilized soils the IPCC report (1997) recommends a constant emission factor of 1.25% of the amount of N applied to agricultural land. However, literature emission factor values for cereal crops are extremely variable, ranging from 0.2% to 8% (Abdalla et al. 2009). Further, the response of N2O emissions to fertiliser N application rate might be non-linear, as has been shown for grazed grassland (Cardenas et al. 2010).
Fertilization not only potentially increases N2O emissions but also strongly influences CO2 emissions (Treseder 2008). Raich and Tufekcioglu (2000) estimated that plant root respiration, including microbial respiration in the rhizosphere, contributed 12%–38% to the total soil respiration in agricultural fields. Nitrogen fertilization generally increases root respiration due to increased plant growth leading to higher total CO2 emissions (Chu et al. 2007). Thereby, the form of inorganic N applied strongly affects rates of root-derived respiration, as high energy demands of NO −3 reduction in roots result in a stronger increase of respiration compared to NH +4 assimilation (Gavrichkova and Kuzyakov 2008). However, this effect differs between crop species, depending on the contribution of shoots and roots to the NO −3 reduction process. Until now identification of the mechanisms contributing to N fertilizer-induced changes in CO2 efflux from soil under agricultural crops remains challenging because of difficulties in separating root and microbial contributions to total CO2 efflux.
Not only N2O and CO2 fluxes are controlled by a multitude of factors but CH4 fluxes as well. Aerated soils act as sinks of CH4, and their sink strength has been estimated to be 3%–9% of the global annual removal of CH4 from the atmosphere (Smith et al. 2000). Application of mineral N fertilizers has been shown to reduce the natural CH4 oxidation capacity of agricultural soils and may therefore lead to increased CH4 emissions (e.g., Hütsch 2001; Hu et al. 2002). This increase has mainly been attributed to the competitive inhibition of the enzyme methane monooxygenase, and to the resulting decrease in pH when NH +4 is applied to soil (Bedard and Knowles 1989; Hütsch 1998). However, there is contradictory evidence about the effect of different inorganic N forms on CH4 emissions, and the underlying mechanisms are still poorly understood, especially in the field.
When assessing the effect of different N fertilizers on greenhouse gas fluxes in agricultural fields one is facing several difficulties. It has become more and more recognized that soil microbes cannot be treated as a uniform pool in the soil. While N fertilization may influence all soil microbes, these effects were shown to vary between different groups of microorganisms (e.g., Cavagnaro et al. 2008; Enwall et al. 2007; Szukics et al. 2009). Therefore, fertilizer-induced changes of soil processes, and consequently of greenhouse gas emissions, are dependent on the microbial community structure of a soil. Another important factor to consider is the N use efficiency of the crop plant investigated, as higher plant N uptake rates lead to decreased concentrations of N in the soil that could be lost from the system. Apart from this, changing environmental conditions in the field (soil moisture, temperature, light conditions, etc.) pose another complication to assess the effect of different N fertilizers on greenhouse gas fluxes. In field studies it is not possible to control most of these factors, and often unwanted side effects (e.g., flooding or drought stress) are masking actual influences of fertilization. To investigate the effects of different inorganic N fertilizers it is necessary to control as many other factors as possible. To achieve this, microcosm studies proved to be a helpful tool (Inselsbacher et al. 2009), when keeping in mind that results gained from such studies need to be validated in the field (Madsen 2005). This approach allows studying fertilizer effects on greenhouse gas emissions in different soil types with different microbial community structures.
For a better comparability all measured gas emissions are commonly expressed as CO2 equivalents using the global warming potential (GWP), which is defined as the cumulative radiative forcing between the present and a selected time in the future, caused by a unit mass of gas emitted now. The GWP (with a time span of 100 years) of CO2, CH4 and N2O is 1, 25 and 298, respectively (IPCC 2007). However, despite the magnitude of studies on greenhouse gas emissions in agriculture, there is still a lack of knowledge about fertilizer-induced effects on the interactions between soil microbes and crop plants in different soil types and therefore hampering reliable predictions of soil-derived GWP. With more detailed data, it could be possible to account for these effects in greenhouse gas inventories, and thus make the achieved emission reductions visible in the official inventories as well. Management options to reduce greenhouse gas emissions in agriculture are manifold (e.g., Lou et al. 2010) but are usually neglecting the soil microbial community composition. Therefore, the aim of this study was to investigate the effect of different inorganic N fertilizers (NH4NO3, NH4Cl, KNO3) on fluxes of CO2, N2O and CH4 from soil microcosms exhibiting different soil microbial community structures before and after planting young barley (Hordeum vulgare L. cv. Morex) plants.
Material and methods
Soil sampling and experimental setup
Soil was collected in April 2006 from two sites, Purkersdorf and Niederschleinz, in the vicinity of Vienna, Austria. The soil types selected for this study are widely distributed and are frequently used for barley cultivation in this area. Both soils are well characterized and have been used in previous studies (Inselsbacher et al. 2009; Inselsbacher et al. 2010). A brief summary of site characteristics and soil properties is given in Table 1. Soil samples were collected from 0 to 20 cm depth from both sites, thoroughly mixed, homogenized and sieved (<2 mm) and immediately stored at 4°C until further analysis. General soil characteristics were analysed 2 weeks after soil sampling and thereafter the incubation experiments were conducted. Experiments were carried out using a recently developed microcosm system described by (Inselsbacher et al. 2009). Briefly, the microcosms consisted of 50 ml polypropylene centrifuge tubes complemented with two stainless steel sieves above the tube cones. Eight holes were drilled into the tube cones to allow sufficient aeration of the soils. Aliquots of sieved and homogenized field-moist soil were centrifuged (1 min, 187 g in a swing out rotor) into the test tubes to reach a final volume of 30 ml and a bulk density of 1 g DW cm−3. The microcosms were kept under controlled conditions in a climate chamber with a 15/9 h day/night cycle at 21/18°C temperature and 55% relative air moisture. Supplementary lighting was provided via eight 400 W daylight lamps. During 14 day of pre-equilibration the soil water content (WC) of both soils was adjusted gravimetrically to 62% water filled pore space (WFPS). To achieve this, 28.8 g DW of soil Purkersdorf were adjusted to 23.6% WC (% dry weight) and 25.8 g DW of soil Niederschleinz to 19.2% WC. The different amounts of soil were the result of preliminary tests determining the amount of soil needed to reach an exact volume of 30 ml within the microcosm tubes after centrifugation (see above). Seeds of barley (Hordeum vulgare L. cv. Morex) were germinated on moist filter paper for 2 day and one seedling planted per microcosm. During the experimental period the WC of the soils was adjusted gravimetrically twice daily.
Fertilizer application and sample collection
To investigate the influence of fertilizer N form on greenhouse gas emissions four experiments were conducted in parallel, modified after Inselsbacher et al. (2010). A solution of 6.25 mM K2HPO4 and either 12.5 mM NH4NO3, 25 mM NH4Cl, 25 mM KNO3 or distilled water were mixed and used for fertilization. These solutions were applied to the soils at two times: Three days before planting 1.6 ml and 5 day after planting 1.2 ml of the mixtures were applied to each microcosm, resulting in a total of 1 mg of N (except in control samples), 0.55 mg of P and 1.4 mg of K in all treatments. Homogenous distribution of fertilizer N was ensured by inserting a 7-cm long side-hole needle to the bottom of the soil cores in 4 (3) positions and slowly injecting the solution (400 μl each injection) while withdrawing the needle (Pörtl et al. 2007). Samples were taken 2 h before and 4 h, 1 day and 3 days after the first fertilization (the last sampling being equal with 2 h before the second fertilization) and 4 h, 1 day, 2 days, 3 days, 6 days and 8 days after the second fertilization. Seedlings of barley were planted into the microcosms 2 days after the first fertilizer application.
Gas fluxes measurements
For gas sampling, test tubes were supplemented with gas tight retrofit kits described by (Inselsbacher et al. 2009) to increase head space volume in order to prevent significant under-pressure during gas sampling. Test tubes were closed at both sides with butyl rubber seals and kept in the dark during the sampling period by wrapping the microcosms with aluminium foil. Gas samples were taken immediately after closing, after 30 min and after 1 h. With a gastight syringe head space air (10 ml) was transferred into evacuated headspace vials and kept at 4°C until analysis. Immediately after taking gas samples the same volume of standardized pressured air was injected into the microcosms to compensate air pressure. The linearity of measured gas emissions during this time, as well as the validation of compensating air pressure in the vials was proved in a preliminary study following a simplified experimental setup. Gas samples were analysed within 48 h as described by Kitzler et al. (2006) by automated headspace gas chromatography. Briefly, the GC was equipped with a 63Ni electron capture detector to quantify N2O concentrations and a flame ionization detector and a methanizer to quantify CO2 and CH4 concentrations. Emission rates of N2O and CO2 were then assessed by the linear increase and CH4 oxidation rates by an exponential decrease of headspace gas concentration over the closure period. The correction for dilution effects resulting from injecting standardized pressured air into microcosms was included into these calculations.
Soil and plant measurement
After gas sampling, the test tubes were opened again, plants were harvested, separated into shoots and roots and briefly rinsed with distilled water. Plant material was oven-dried (70°C for 48 h) and weighed. Dried roots and shoots were ground in a ball mill (Retsch MM2000) and subsequently total plant C and N contents were measured with an elemental analyzer (EA 1110, CE Instruments). Soil of each microcosm was quantitatively retrieved, homogenized and prepared for further analyses. Soil chemical properties were analyzed as described by (Inselsbacher et al. 2010). An aliquot (4 g) of homogenized soil was dried at 70°C for 3 days and weighed to determine soil moisture. Another aliquot (2 g) of homogenized soil was extracted in 15 ml CaSO4 (10 mM) and subsequently anions were determined by ion chromatography (DX 500, Dionex, Vienna, Austria) and conductivity detection. NO −3 was separated on an anion exchange column (AS11, 250 × 4 mm i.d., Dionex, Vienna, Austria) after chemical suppression (ASRS-Ultra, Dionex) and linear NaOH gradient elution (0.5 mM to 37.5 mM within 10 min at a flow rate of 2 ml min−1, with a column temperature of 35°C). NH +4 was extracted from aliquots (6 g) of homogenized soil with 45 ml KCl (1 M) and determined by a modified indophenol reaction method (Kandeler and Gerber 1988). Total dissolved C and N in the same KCl extracts were determined by an automated C analyzer (Shimadzu, TOC-VCPH, Japan) and a total N measuring unit (Shimadzu, TNM-1, Japan). Total C and N contents of the soils were analyzed with an elemental analyzer as given above.
Context data and statistical analysis
Data of microbial biomass, root and shoot DW, total plant C contents, numbers of fungal and bacterial genome equivalents as well as archaeal and bacterial ammonium monooxygenase catalytic subunit A (amoA) copies were taken from a previously published set of experiments which were conducted in parallel to the present study (Inselsbacher et al. 2010). Data were analysed using one-way and multi-factorial ANOVA followed by Tukey’s HSD post-hoc test using Statistica 6.0 (StatSoft Inc., Tulsa, OK, USA). When necessary, data were either square root- or log10- transformed prior to analysis to meet the assumptions of ANOVA after testing normality using Kolmogorov-Smirnov test and homogeneity of variances using Bartlett’s test. The effects of adding different forms of N on emissions of CO2, CH4 and N2O was analysed by three-way ANOVA with soil type, N form and sampling time as independent variables. Multiple regression analyses of greenhouse gas fluxes in soils Purkersdorf and Niederschleinz were performed with Statgraphics 5.0 (Statistical Graphics Inc., Rockville, MD, USA) including microbial biomass, root and plant biomass (dry weight), total N, NH +4 , NO −3 , total dissolved C, WFPS, absolute values and ratios of bacterial and fungal gene copy numbers, absolute values and ratios of bacterial and archaeal amoA copy numbers, gross nitrification rates and gross mineralization rates.
Results
Soil properties and plant C contents
Microcosms were kept under strictly controlled conditions in a climate chamber to guarantee stable conditions throughout the test period. As intended, after equilibration no shifts in water content, and therefore also not in water filled pore space (WFPS), were observed in the soils (data not shown, P > 0.05). However, as fertilizer was applied as a solution some minor, but nevertheless significant shifts of WFPS occurred in both soils directly after fertilization (data not shown, P < 0.05). The pH values of bulk soils did not change in planted and unplanted microcosms, independent of N form and soil (one-way ANOVA, P > 0.05, n = 10). However, due to insufficient root lengths and biomass at the time of fertilization, shifts in rhizosphere pH could not be estimated.
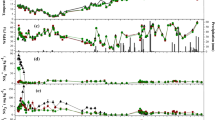
Dissolved inorganic nitrogen (DIN) pools in both soils decreased rapidly after the second fertilization event, mainly due to plant N uptake during this period. Eight days after the second fertilization event NH +4 and NO −3 pools had reached background levels in both soils (1.3 ± 0.2 and 19 ± 1.5 μg N g-1 DW, respectively). Ammonium was depleted significantly faster in Niederschleinz soil, whereas NO −3 concentrations remained elevated for a longer period in this soil than in Purkersdorf soil (Inselsbacher et al. 2010). In both soils total dissolved carbon (TDC) contents were markedly higher after the second fertilization compared to untreated soils, the latter having TDC concentrations of 40 ± 2 and 31 ± 2 μg Cg−1 DW (Purkersdorf and Niederschleinz soil, respectively; Fig. 1). Higher amounts of TDC, but also higher shifts of TDC during the test period and stronger effects of different forms of fertilizer N were found in Niederschleinz soil (three-way ANOVA, P < 0.0001). In both soils TDC contents increased directly after addition of NH4NO3, while after applying either NH4Cl or KNO3 TDC contents decreased. Compared to background levels, TDC concentrations increased least in the KNO3 treatment in both soils. After 8 days TDC contents reached a near-steady state, showing no difference in concentration between different fertilizer N forms in Purkersdorf soil (one-way ANOVA, P > 0.05), but showing still slightly lower contents after KNO3 addition in Niederschleinz soil (P < 0.05).
Concentrations of total dissolved C (TDC) in soils Purkersdorf and Niederschleinz as well as total plant C contents during 8 days after application of 3 different inorganic N fertilizers (NH4NO3, NH4Cl or KNO3). Soils were preincubated in the microcosms for 14 days before fertilization. The first samples were taken at day 3, 4 h after the second fertilization event. Symbols and bars represent means ± SE, n = 15
After 4 days total plant C contents increased significantly throughout the rest of the experimental period in both soils (P < 0.001, Fig. 1). In Purkersdorf soil highest total plant C contents were observed in the KNO3 treatment, followed by NH4NO3 and being lowest after NH4Cl fertilization (One-way ANOVA, P < 0.01). The same pattern was found in Niederschleinz soil, with the exception that no significant difference in total plant C contents was observed between the KNO3 and NH4NO3 treatments (P > 0.05). Independent of fertilizer treatment plants grown in Niederschleinz soil exhibited higher total plant C contents compared to Purkersdorf soil 8 days after the second fertilization (P < 0.05).
Nitrous oxide fluxes
Both soils were sources of N2O at all measured time points. After equilibration and before fertilization initial N2O emission rates from Purkersdorf soil were significantly (P < 0.01) lower (1 ± 0.8 μg N kg−1 DW d−1) than those from Niederschleinz soil (3.2 ± 1 μg N kg−1 DW d−1). Four hours after the first application of fertilizer, N2O emissions had increased significantly in both soils, with higher emissions from Niederschleinz soil (Fig. 2, Table 2). In both soils fertilizer N form did not affect N2O emission rates, but addition of N generally resulted in higher N2O emissions compared to water control samples (one-way ANOVA, P < 0.05). Peaks of N2O emissions rapidly decreased again to background levels during the following 3 days in both soils. Immediately (4 h) after the second fertilization, N2O emissions were markedly higher than after the first fertilization event (Fig. 2), although less N fertilizer was applied as compared to the first fertilization. In the control samples of both soils no increase in N2O emission was found (P > 0.05). Other than after the first fertilizer application, the form of N fertilizer applied had a strong influence on N2O emissions after the second fertilizer application in both soils (Table 3), with highest peaks of N2O emission after NH4NO3 application and lowest peaks after KNO3 application (one-way ANOVA, P < 0.01). In the NH4Cl and NH4NO3 treatments, N2O emissions from Niederschleinz soil were higher than those from Purkersdorf soil (P < 0.05, Table 3), whereas KNO3 application resulted in similar N2O emissions from both soils (P > 0.05). Within the following 8 days, N2O emissions decreased in both soils, showing different patterns depending on the fertilizer N form applied (Fig. 2, Table 3). N2O emissions from the NH4NO3 treatment decreased almost linearly during this time period, but did not reach background values after 8 days (one-way ANOVA, P < 0.05). On the other hand, N2O emissions from the NH4Cl treated soils decreased more rapidly and declined to background levels (P > 0.05). The slowest decrease in N2O emissions was found after KNO3 application in both soils, when they clearly remained above background levels after 8 days (P < 0.05). In Niederschleinz soil the most important factors determining the variation in N2O fluxes were soil NH +4 concentrations and the ratio of archaeal to bacterial amoA copies. Additionally, resulting from multiple regression analysis, soil NO −3 concentrations and root biomass were found to be significant factors as well (Table 4). Together these four factors explained 88% of the variability of N2O fluxes. In soil Purkersdorf soil NO −3 and TDC contents were significant factors determining N2O fluxes and explained 64% of the variation.
Cumulative CO2 emission, CH4 consumption and N2O emission from microcosms filled with soil Purkersdorf and Niederschleinz during 11 days. Soils were preincubated in the microcosms for 14 days before fertilization. Dashed arrows indicate date of planting barley seedlings. Full arrows indicate dates of inorganic N fertilization (NH4NO3, NH4Cl or KNO3). Control samples received distilled water instead of fertilizer and were measured only until day 4. Symbols represent cumulative means ± SE, n = 7
Fertilizer N lost as N2O
Taken the first fertilization event alone, average losses of applied N via N2O emission during the subsequent 3 days were as little as 0.04% in both soils (Table 5). As after 3 days N2O emissions have reached background levels again, it is unlikely that under the present laboratory conditions additional severe losses would have occurred at later time points. The proportional loss of fertilizer N as N2O from the second fertilizer dose, on the other hand, was significantly higher, with highest losses in the NH4NO3 treatments from both soils. While N2O losses after NH4Cl and KNO3 application were similar and in the same range in the two soils, N losses after NH4NO3 fertilization were higher in Niederschleinz soil. Taken together, during the monitored 11 days N losses from total applied fertilizer N via N2O emissions were below 0.3% in both soils. By extrapolating the cumulative curves of N2O emissions (shown in Fig. 2), it was possible to extrapolate N2O emissions over a time period of 1 month after fertilization (Fig. 3, Table 5). The extrapolated results indicate that the major N losses occurred during the first week after fertilization while additional potential N losses during the following 3 weeks would have accounted for a much smaller part of total N losses. Calculated relative to the amount of applied N from the second fertilizer dose alone, this resulted in average N losses of 0.62% and 0.88% in the NH4NO3 treatment one month after fertilization of Purkersdorf and Niederschleinz soils, respectively.
Cumulative N2O emission from microcosms filled with soils Purkersdorf and Niederschleinz during 1 month. Soils were preincubated in the microcosms for 14 days before fertilization. Full arrows indicate dates of inorganic N fertilization (NH4NO3, NH4Cl or KNO3). The dotted line at day 11 indicates the end of direct measurements. Values from day 11 onwards were calculated with best fitting exponential growth equations
Carbon dioxide fluxes
Initial emissions of CO2 from Purkersdorf soil were low (4.0 ± 2.1 mg C kg−1 DW d−1) and similar to CO2 emissions from Niederschleinz soil (3.2 ± 0.8 mg C kg−1 DW d−1). The first fertilization event did not affect CO2 emissions but after seedlings were transferred to the microcosms, CO2 emissions increased rapidly in both microcosms (soil and plants), with higher emissions from Purkersdorf microcosms (Fig. 2, Table 2). No direct influence of N application was observed, as CO2 emissions did not differ between fertilizer N forms and the water control (P > 0.05). After the second fertilization CO2 emissions from Purkersdorf microcosms were markedly higher than from Niederschleinz microcosms and were highest in the NH4NO3 treatment and lowest in the KNO3 treatment. Interestingly, in Niederschleinz microcosms the opposite effects of fertilizer N forms were found, although much less pronounced (Fig. 2, Table 3). Integrated over the whole test period (11 days) this resulted in 2.6, 2.1 and 1.6-fold higher cumulative CO2 emissions (calculated by linear interpolation between sampling occasions) from Purkersdorf microcosms than from Niederschleinz microcosms in the NH4NO3, NH4Cl and KNO3 treatments, respectively (Fig. 2). Multiple regression analysis was performed to determine the most significant determinant factors on CO2 fluxes (Table 6). Accepting a threshold probability of 95%, in Purkersdorf microcosms concentrations of soil NH +4 and NO −3 and the bacterial to fungal genome equivalent ratio were found to be significant factors determining CO2 fluxes, explaining 77% of the variability in CO2 emissions. In Niederschleinz microcosms the bacterial to fungal genome equivalent ratio was likewise significant, but instead of DIN concentrations root biomass was the second main factor, together explaining 39% of CO2 emission variability.
Methane fluxes
Before fertilization and planting, both soils were net sources of CH4. Initial emission rates did not differ between the two soils and were 2.4 ± 3.2 and 0.6 ± 2.2 μg C kg−1 DW d−1 from soils Purkersdorf and Niederschleinz, respectively (one-way ANOVA, P > 0.05, Fig. 2). After the first fertilizer application both soils became sinks of CH4, with soil Niederschleinz exhibiting higher consumption rates than soil Purkersdorf (Table 2). No effect of fertilizer N form was observed as net CH4 consumption rates were equal in the fertilized and in the control samples (Fig. 2). Three days after the first fertilizer dose net CH4 fluxes reached background levels again (P > 0.05) and therefore, on average, both soils became net sources of CH4 again. The second fertilizer application was followed by a stronger increase of CH4 consumption compared to the first fertilization event, and again soil Niederschleinz showed higher CH4 consumption rates than soil Purkersdorf (Fig. 2). As revealed by three-way ANOVA fertilizer N form did not affect CH4 consumption rates directly, but a significant interaction effect between fertilizer N form and sampling time was observed (Table 3). While CH4 consumption rates decreased in both soils in the NH4NO3 and NH4Cl treatments to near-background levels 8 days after fertilization, CH4 consumption rates of soils treated with KNO3 declined to steady state rates already 3 d after fertilization, though at significantly higher levels of CH4 consumption (−1.9 ± 0.2 and −3.7 ± 0.2 μg C kg−1 DW d−1 in soils Purkersdorf and Niederschleinz, respectively). Total amounts of CH4 consumed over the whole time period of 11 days were higher in soil Niederschleinz than in soil Purkersdorf only after KNO3 application (44 and 24 μg C kg−1 DW, respectively, P < 0.05), but were similar in the other N treatments (between 17 and 33 μg C kg−1 DW, P > 0.05, Fig. 2). Multiple regression analysis revealed that, accepting a threshold probability of 95%, in soil Purkersdorf concentrations of soil NO −3 and TDC were the only significant factors determining CH4 fluxes and together explained 57% of the variability in CH4 fluxes (Table 7). Unexpectedly, no correlations between CH4 fluxes and WFPS or NH +4 concentrations were found (P > 0.05). On the contrary, in soil Niederschleinz WFPS, soil NH +4 concentrations and the ratio of archaeal to bacterial ammonium monooxygenase catalytic subunit A (amoA) copies were the determining factors, together explaining 58% of CH4 fluxes.
Greenhouse gas balance
N2O and CH4 have 298 and 25 times higher global warming potentials (GWP) than CO2, respectively, in a time horizon of 100 years (IPCC 2007). In our study the GWP calculated as total CO2 equivalents over an 11-day period after fertilization was markedly higher in Purkersdorf microcosms than in Niederschleinz microcosms. In detail, total CO2 equivalents were 2.45, 2.09 and 1.53 times higher in the NH4NO3, NH4Cl and the KNO3 treatment, respectively (Table 8). While the GWP of Purkersdorf microcosms was highest after NH4NO3 and lowest after KNO3 fertilization, the opposite was the case in Niederschleinz microcosms. This was clearly related to the opposite pattern of CO2 emissions in the two soils (Fig. 2). In Purkersdorf microcosms CO2 contributed 98 ± 6% to GWP in all treatments, while N2O contributed only approximately 2 ± 0.5%. Even if the contribution of N2O was highest in the NH4NO3 treatment (2.4 ± 0.4%), this difference was not significant. On the contrary, the contribution of N2O to total GWP in Niederschleinz microcosms was clearly higher, ranging between 3.7 and 8.6%. Additionally this soil showed a stronger influence of fertilizer N form, as the contribution of N2O after NH4NO3 application was approximately 2-fold higher than in the other treatments. Independent of fertilizer N form, soil CH4 consumption had only little influence on total GWP decreasing it by 0.1 ± 0.03% and 0.2 ± 0.1% in Purkersdorf and Niederschleinz microcosms, respectively.
Discussion
Nitrous oxide emissions
One major aim of this study was to evaluate the influence of different inorganic N fertilizer forms on N2O emissions and especially on relative amounts of fertilizer N lost from agricultural soils as N2O. In both soils, N2O emissions were highest after applying a mixture of NH4NO3 compared to application of NH +4 or NO −3 alone. These findings indicate that both nitrification and denitrification might have contributed simultaneously to N2O losses in the mixed fertilizer treatment, which provided N sources for both processes. Principally, the experimental setup allowed both nitrification and denitrification to occur, as soil moisture, an important controlling factor for N2O emissions was steadily kept at 62% WFPS (e.g., Davidson 1993; Dobbie and Smith 2001). When only NO −3 was applied, concentrations of NH +4 in both soils were very low (0.5 to 1.5 μg N g−1 DW) and might have been limiting for nitrification (Garrido et al. 2002). On the other hand, when only NH +4 was applied during 1 week after fertilization NO −3 concentrations were nevertheless higher than 20 μg N g−1 DW, which was found to be the threshold concentration above which denitrification is not limited (Myrold and Tiedje 1985a, b). Together with the fact that other controlling factors (soil moisture, temperature) were kept constant, these findings indicate that in our study the availabilities of NH +4 and NO −3 clearly were the main driving factors for nitrification and denitrification and consequently directly influenced N2O emissions as reported previously (Mosier et al. 1986; Baggs and Blum 2004). Additionally, gross nitrification rates, which were estimated within parallel 15N labelling experiments (Inselsbacher et al. 2010), were significantly higher in Niederschleinz than in Purkersdorf soil. This fits well with the fact that N2O emissions from Niederschleinz soil were significantly higher directly after applying either NH4NO3 or NH +4 , and was also reflected in a strong positive correlation between N2O emissions rates and soil NH +4 concentrations.
While the importance of denitrification and nitrification for N2O emissions is well recognized (e.g., Ambus et al. 2006), there is still controversy about the contribution of different groups of microbes to these processes. For example, although it is known that ammonia-oxidizing archaea might numerically be more abundant than ammonia-oxidizing bacteria in soils (Leininger et al. 2006), until now their ecological role remains uncertain (Francis et al. 2007; Hayatsu et al. 2008). There is strong evidence that mainly bacteria, rather than archaea, functionally dominate ammonia oxidation in agricultural (Jia and Conrad 2009) and grassland soils (Di et al. 2009). However, in a recent study Martens-Habbena et al. (2009) found that ammonia-oxidizing archaea successfully competed with heterotrophic phytobacteria and phytoplankton for reduced N indicating a strong contribution of archaea to nitrification in marine environments. Also in agricultural soil the potential importance of ammonia-oxidizing archaea was already highlighted (Offre et al. 2009). Similarly, in the soils used in the present study archaeal amoA gene copy numbers were increasing stronger than bacterial amoA gene copy numbers during the first week after NH +4 application, indicating that archaea were contributing significantly to nitrification (e.g., Inselsbacher et al. 2010). The strong correlation between N2O emissions and archaeal amoA abundance in soil Niederschleinz in the present study indicates that archaea may have directly or indirectly contributed to N2O emissions and therefore to soil derived GWP as well. In Purkersdorf soil, on the other hand, gross nitrification rates were found to be 2 to 3 times lower and archaeal amoA abundance up to 10 times lower than in Niederschleinz soil (Inselsbacher et al. 2010). Further, no correlation between N2O emissions and archaeal amoA abundance was found in this soil. These results stress the importance of assessing the different functional groups within the soil microbial community in different soils in greater detail. Thereby analyses of bulk soil alone are not sufficient without taking small-scale variations into account as may be the case in the rhizosphere. Ongoing studies in our group indicate, that in the rhizosphere bacterial amoA abundance is affected much stronger by ammonium application than archaeal amoA abundance (Glaser et al. 2010). Besides the importance of soil processes and the microbial community composition, also plant growth strongly influenced N2O emissions. It has been shown that barley plants grown on both soils were the strongest sink of fertilizer N (45% to 80% of N applied) after 8 days (Inselsbacher et al. 2010). Plant N uptake became obviously stronger with continuing plant growth but was rather low during the first 5 days. Therefore, during this time there was more N available for N2O production. This was reflected in the decrease in N2O emissions after an initial pulse directly after the second fertilization, due to N depletion during continuous plant N uptake and by a strong negative correlation of N2O emissions and root biomass in soil Niederschleinz. Additionally to reducing the substrate availability for nitrification and denitrification, plants may control N2O emissions by providing an additional C source via root exudation allowing increased denitrifier activity (Philippot et al. 2008), or by a combination of both. Root penetration into the soil may decrease soil compaction and create channels for gas transfer, therefore causing faster diffusion of N2O from the soil to the atmosphere (Philippot et al. 2008). Both soils in the present study exhibited higher N2O emissions after fertilizer N application in the presence of growing plants than from soils without plants, which supports these suggestions and is in agreement with several other studies (e.g., Klemedtsson et al. 1987; Kilian and Werner 1996). However, we cannot reconcile which of the aforementioned effects was most important for the increased N2O emissions.
Our study underlines previous findings that increases in N2O emissions following N-fertilization are short-term responses only (8 to 14 days) and typically decline to base line levels thereafter (Mosier 1994; Bouwman 1996; Mosier 1998; Dobbie and Smith 2003; Ambus 2005; Jones et al. 2007). The highest peaks of N2O emissions were observed directly after the second application, despite the fact that the first application of fertilizer represented more than half of the total N applied. A similar result was found by Abdalla et al. (2009) who proposed that changing water conditions were responsible for this effect. In our study soil moisture and temperature were kept stable and therefore could not have been responsible for this increase. More likely, increased denitrification rates induced by root exudation and increased enzyme activities induced by the first fertilization event were responsible for this effect. Based on the results of a previous 15N tracer study it was found that on average 15% of applied fertilizer N was lost to the atmosphere (Inselsbacher et al. 2010). While it has been recently shown that N2 emissions can account for a significantly larger part of total gaseous N losses compared to N2O or NO emissions (e.g., Spott et al. 2006; Scheer et al. 2009), this study focused on N2O alone. In terms of relative amount of fertilizer N emitted as N2O, the influence of plants and of repeated fertilization resulted in a marked increase of N losses. Independent of fertilizer N form only 0.03% of applied N was lost as N2O after the first fertilization. Contrary, the amount of N lost from the second fertilization was markedly higher and depended on the fertilizer N form. In both soils cumulative N2O losses over 11 days were more or less equal when only one N source (either NH +4 or NO −3 ) was supplied (0.11–0.14%), but significantly higher in the NH4NO3 treatment (0.22 and 0.29% from soils Purkersdorf and Niederschleinz, respectively). These findings indicate that providing sources for nitrification and denitrification at the same time led to highest N2O emissions from both soils and therefore should be seen critically in terms of N2O-derived GWP. Estimation of proportional N2O losses from total fertilizer N added integrated over a one-month period showed that N2O emission factors in both soils (0.14–0.37%) were in the range of previous studies (Abdalla et al. 2009) though below the default value (1.25% per year) reported by the IPCC (1997). Our results also stress that N2O emissions during the first 11 days after fertilization contributed 67–85% of total N2O emissions extrapolated to 1 month. Therefore, the correct timing of fertilizer application in regard to weather conditions and plant N demands is of uttermost importance. While the emission factors calculated in the present microcosm study are valuable for short-term laboratory studies, annual site-specific N2O emission factors still need to be validated directly in the field.
Methane fluxes
Methane fluxes were not influenced by the amount or form of N fertilizer in either of the two soils. This was unexpected, as previous studies showed that CH4 oxidation is potentially inhibited by NH +4 (e.g., Steudler et al. 1989; Mosier et al. 1991; Hütsch 2001; Hu et al. 2002) or NO −3 (Kightley et al. 1995; Wang and Ineson 2003; Reay and Nedwell 2004). Still, our results are supported by several other studies that found a lack of inhibition or even stimulation of CH4 oxidation following N addition (e.g., Bodelier et al. 2000; Hilger et al. 2000; Sitaula et al. 2000; De Visscher and Van Cleemput 2003). In our study, CH4 oxidation was enhanced after fertilizer injection. It is difficult to clarify this observed pattern as the net CH4 flux in terrestrial ecosystems is the result of simultaneous gross CH4 production and gross CH4 consumption rates (Chu et al. 2007; Kammann et al. 2009; von Fischer and Hedin 2007). In our study it was not possible to distinguish whether the observed increase in CH4 consumption resulted from a stimulation of CH4 oxidizers, an inhibition of CH4 producers or by interaction effects between these communities. Nevertheless, amoA gene copy numbers increased after fertilization which may have contributed to CH4 oxidation as the enzyme ammonium monooxygenase can also oxidize CH4. Archaeal amoA gene copy numbers were increasing stronger than bacterial ones, and there was a significant relationship between arch/bact amoA, NH +4 concentration and CH4 flux in the more organic Niederschleinz soil.
Carbon dioxide emissions
CO2 emissions were strongly dependent on plant growth and on soil microbial community composition in both soils. Further, in both soils CO2 emissions were positively correlated with root biomass, indicating that root respiration, including microbial respiration in the rhizosphere, contributed significantly to soil total CO2 emissions. While in Niederschleinz soil this correlation was highly significant, a similar strong, but less significant correlation was found in Purkersdorf soil (correlation coefficient 0.45, P = 0.011). However, the strong negative correlation between plant available inorganic N pools and CO2 emissions in soil Purkersdorf also rather reflected the increasing depletion of these N pools due to root uptake together with a simultaneous increase in root respiration then a direct influence of DIN concentrations on CO2 emissions per se. These findings are in agreement with previous studies who found that root respiration contributed 12–38% to the total soil respiration in crop lands (Raich and Tufekcioglu 2000), and might be even enhanced at the onset of plant growth due to enhanced root activity (Chu et al. 2005). Fertilization increased CO2 emissions significantly in the planted microcosms, but not in bare soil. These results indicate that soil microorganisms were C limited in unplanted soil, but were stimulated by root exudation as soon as plants were grown in the microcosms, as has been suggested previously (Inselsbacher et al. 2010). The initial C limitation might have been caused by C exhaustion during sample handling which could explain the low initial CO2 emissions from both soils. In a preliminary study testing 5 contrasting soils (packed into the same microcosms used in this study) no changes in soil TDC were found during a time period of 1 month after packing proving that the microcosms were stable during the experiments (Inselsbacher et al. 2009). Still, we cannot exclude the possibility and consequences of reduced soil TDC contents in the homogenized soils compared to field conditions. Further, it is likely that also shoot respiration contributed to total CO2 emissions in planted microcosms, as gas samples were taken in the presence of intact plants, even if no significant correlation between CO2 emissions and shoot biomass could be found. However, CO2 emissions were also influenced by the form of fertilizer N applied, surprisingly with opposing effects in the two soils. One reason for this finding was that the two soils exhibited different soil microbial communities which were affected differently depending on fertilizer N form applied. In a parallel study using the same soils and the same setup, it has been shown that bacterial genome equivalents did not change over time in both soils, but that fungal genome equivalents increased in all fertilizer treatments in soil Niederschleinz, while they decreased in soil Purkersdorf (Inselsbacher et al. 2010). Nevertheless, compared with plant respiration, it is likely that microbial respiration in the bulk soil contributed less to total CO2 production, although the contribution of microbial respiration in the rhizosphere remains hard to distinguish (Gavrichkova and Kuzyakov 2008).
Soil derived global warming potential
In order to estimate the soil-derived global warming potential (GWP), which determines the relative contribution of a gas to the greenhouse effect, all gas emissions were converted to CO2 equivalents (IPCC 2007). Soil derived GWP was much higher in Purkersdorf than in Niederschleinz soil, due to higher CO2 emissions from Purkersdorf microcosms. CO2 emissions clearly were the strongest contributors to GWP in both microcosms, while CH4 and N2O emissions influenced GWP much less. However, while CO2 was emitted from the system, C was simultaneously fixed in plants. In this study the C fixation was estimated by calculating the increase in total C content of the plants during the experimental period. In our microcosm setup C fixation by plants (25.5 and 29.6 mg CO2-C plant−1 in Purkersdorf and Niederschleinz soil, respectively) occurred at much higher rates than CO2 emissions (8.7 and 3.8 mg CO2-C microcosm−1 in Purkersdorf and Niederschleinz soil, respectively), resulting in a net sink of C in the test systems. Due to slower plant growth in the NH +4 treatment (Inselsbacher et al. 2010) less C was fixed in plants during the estimated 11 days in this treatment, consequently resulting in higher net GWP in both soils compared to NH4NO3 or KNO3 fertilization. CH4 uptake during the experimental period additionally, but only negligibly, decreased total GWP, similar to previous findings from non-flooded temperate soils (Flessa et al. 2002; Chu et al. 2007; Regina et al. 2007; Soussana et al. 2007). Compared to C alone, the relative contribution of N2O to the total soil-derived GWP became more important and even more significant after N fertilization. This fertilization effect was much stronger in Niederschleinz soil, due to higher nitrification rates in this soil as explained above. This suggests that the contribution of N2O to total GWP was strongly influenced by the soil microbial community. The strong correlation of archaeal amoA abundance and soil N2O emissions together with the previously found correlation with nitrification rates (Inselsbacher et al. 2010) indicates that archaea potentially contributed to total N2O emissions. Although it still needs to be validated in the field, we here for the first time report that archaea may potentially influence total soil derived GWP. Taken together, our results indicate that during the initial growth period of barley, both soils were net sinks of CO2 equivalents, as CO2, N2O and CH4 emissions could not compensate the C fixation by plants. Nevertheless, due to weight-based calculations within this study and the small scale of the microcosms used, these findings have to be validated in the field together with area-based calculations.
Conclusion
Overall, the results of our study indicate that assessing the soil microbial community structure is of prime importance when studying the effect of N fertilizers on soil-derived GWP in agricultural soils. The proper choice of inorganic N fertilizer in order to potentially reduce the soil-derived GWP may not only depend on the physico-chemical properties of the soil, but also on the composition, abundance and spatial distribution of different functional groups of soil microorganisms. Here we show that the contribution of N2O to the soil-derived GWP was on the one hand strongly dependent on the form of mineral N fertilizer and on the other hand on the soil microbial community composition. Especially ammonia-oxidizing archaea may contribute stronger to N2O emissions than has been recognized previously. Generally, nitrification can potentially increase N2O emissions, and therefore determining the optimal ratio of NH +4 to NO −3 contained in fertilizers, as well as the use of nitrification inhibitors and a better match of N supply with crop demands, have to be considered in order to decrease N2O emissions from agricultural soils. Fertilization also strongly increased plant respiration, leading to higher CO2 emissions and to higher microbial activities induced by enhanced root exudation. Our study provides evidence that interdisciplinary research covering multiple aspects of plant-soil-microbe interactions will promote a better understanding of the factors influencing GHG emissions.
References
Abdalla M, Jones M, Ambus P, Williams M (2009) Emissions of nitrous oxide from Irish arable soils: effects of tillage and reduced N input. Nutr Cycl Agroecosyst. doi:10.1007/s10705-009-9273-8
Ambus P (2005) Relationship between gross nitrogen cycling and nitrous oxide emission in grass-clover pasture. Nutr Cycl Agroecosyst 72:189–199
Ambus P, Zechmeister-Boltenstern S, Butterbach-Bahl K (2006) Sources of nitrous oxide emitted from European forest soils. Biogeosciences 3:135–145
Baggs EM, Blum H (2004) CH4 oxidation and emissions of CH4 and N2O from Lolium perenne swards under elevated atmospheric CO2. Soil Biol Biochem 36:713–723
Bedard C, Knowles R (1989) Physiology, biochemistry, and specific inhibitors of CH4, NH +4 , and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev 53:68–84
Bodelier PLE, Roslev P, Hencke T, Frenzel P (2000) Stimulation by ammonium-based fertilizers of methane oxidation in soil around rice roots. Nature 403:421–424
Bouwman AF (1990) Exchange of greenhouse gases between terrestrial ecosystems and the atmosphere. In: Bouwman AF (ed) Soils and the greenhouse effect. Wiley, England, pp 61–127
Bouwman AF (1996) Direct emission of nitrous oxide from agricultural soils. Nutr Cycl Agroecosyst 45:53–70
Cardenas LM, Thorman R, Ashlee N, Butler M, Chadwick D, Chambers B, Cuttle S, Donovan N, Kingston H, Dhanoa MS, Scholefield D (2010) Quantifying annual N2O emission fluxes from grazed grassland under a range of inorganic fertiliser nitrogen inputs. Agr Ecosyst Environ 136:218–226
Cavagnaro TR, Jackson LE, Hristova K, Scow KW (2008) Short-term population dynamics of ammonia oxidizing bacteria in an agricultural soil. Appl Soil Ecol 40:13–18
Chu H, Hosen Y, Yagi K (2004) Nitrogen oxide emissions and soil microbial properties as affected by N-fertilizer management in a Japanese Andisol. Soil Sci Plant Nutr 50:287–292
Chu HY, Hosen Y, Yagi K (2007) NO, N2O, CH4 and fluxes in winter barley field of Japanese Andisol as affected by N fertilizer management. Soil Biol Biochem 39:330–339
Chu HY, Hosen Y, Yagi K, Okada K, Ito O (2005) Soil microbial biomass and activities in a Japanese Andisol as affected by controlled release and application depth of urea. Biol Fertil Soils 42:89–96
Davidson EA (1993) Soil water content and the ratio of nitrous oxide to nitric oxide emitted from soil. In: Oremland RS (ed) Biogeochemistry of global change. Radiatively active trace gases. Chapman & Hall, New York, pp 369–384
De Visscher A, Van Cleemput O (2003) Induction of enhanced CH4 oxidation in soils: NH +4 inhibition patterns. Soil Biol Biochem 35:907–913
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ (2009) Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat Geosci 2:621–624
Dobbie KE, Smith KA (2001) The effects of temperature, water-filled pore space and land use on N2O emissions from an imperfectly drained gleysol. Eur J Soil Sci 52:667–673
Dobbie KE, Smith KA (2003) Nitrous oxide emission factors for agricultural soil in Great Britain: the impact of soil water-filled pore space and other controlling variables. Glob Change Biol 9:204–218
Duxbury JM, Harper LA, Mosier AR (1993) Contribution of agroecosystems to global climate change. In: Harper LA, Mosier AR, Duxbury JM, Rolston DE (eds) Agricultural ecosystem effects on trace gases and global climate change. ASA Special Publication No. 55. American Society of Agronomy, Madison, pp 1–18
Enwall K, Nyberg K, Bertilsson S, Cederlund H, Stenström J, Hallin S (2007) Long-term impact of fertilization on activity and composition of bacterial communities and metabolic guilds in agricultural soil. Soil Biol Biochem 39:106–115
Flessa H, Ruser R, Dörsch P, Kamp T, Jimenez MA, Munch JC, Beese F (2002) Integrated evaluation of greenhouse gas emissions (CO2, CH4, N2O) from two farming systems in southern Germany. Agr Ecosyst Environ 91:175–189
Francis CA, Beman JM, Kuypers MMM (2007) New processes and players in the nitrogen cycle: the microbial ecology of anaerobic and archaeal ammonia oxidation. ISME J 1:19–27
Garrido D, Henault C, Gaillard H, Perez S, Germon JC (2002) N2O and NO emissions by agricultural soils with low hydraulic potentials. Soil Biol Biochem 34:559–575
Gavrichkova O, Kuzyakov Y (2008) Ammonium versus nitrate nutrition of Zea mays and Lupinus albus: effect on root-derived CO2 efflux. Soil Biol Biochem 40:2835–2842
Glaser K, Hackl E, Inselsbacher E, Strauss J, Wanek W, Zechmeister-Boltenstern S, Sessitsch A (2010) Dynamics of ammonia oxidizing communities in barley planted bulk soil and rhizosphere following nitrate and ammonium fertilizer amendment. FEMS Microbiol Ecol. doi:10.1111/j.1574-6941.2010.00970.x
Granli T, Bockman OC (1994) Nitrous oxide from agriculture. Norw J Agr Sci 12(Suppl):1–128
Hall SJ, Matson PA (1999) Nitrogen oxide emissions after nitrogen additions in tropical forests. Nature 400:152–155
Hayatsu M, Tago K, Saito M (2008) Various players in the nitrogen cycle: diversity and functions of the microorganisms involved in nitrification and denitrification. Soil Sci Plant Nutr 54:33–45
Hilger HA, Barlaz MA, Wollum AG (2000) Landfill methane oxidation response to vegetation, fertilization, and liming. J Environ Qual 29:324–334
Hu R, Hatano R, Kusa K, Sawamoto T (2002) Effect of nitrogen fertilization on methane flux in a structured clay soil cultivated with onion in central Hokkaido, Japan. Soil Sci Plant Nutr 48:797–804
Hutchinson GL, Davidson EA (1993) Processes for production and consumption of gaseous nitrogen oxides in soil. In: Harper LA, Mosier AR, Duxbury JM, Rolston DE (eds) Agricultural ecosystem effects on trace gases and global climate change. ASA Special Publication No. 55. American Society of Agronomy, Madison, pp 79–94
Hütsch BW (1998) Tillage and land use effects on methane oxidation rates and their vertical profiles in soil. Biol Fertil Soils 27:284–292
Hütsch BW (2001) Methane oxidation in non-flooded soils as affected by crop production—invited paper. Eur J Agron 14:237–260
Inselsbacher E, Hinko-Najera Umana N, Stange FC, Gorfer M, Schüller E, Ripka K, Zechmeister-Boltenstern S, Hood-Novotny R, Strauss J, Wanek W (2010) Short-term competition between crop plants and soil microbes for inorganic N fertilizer. Soil Biol Biochem 42:360–372
Inselsbacher E, Ripka K, Klaubauf S, Fedosoyenko D, Hackl E, Gorfer M, Hood-Novotny R, Von Wiren N, Sessitsch A, Zechmeister-Boltenstern S, Wanek W, Strauss J (2009) A cost-effective high-throughput microcosm system for studying nitrogen dynamics at the plant-microbe-soil interface. Plant Soil 317:293–307
IPCC (1997) In: Watson RT, Zinyowera MC, Moss RH (eds) Special report on regional impacts of climate change: an assessment of vulnerability. A special report of IPCC working group II. Cambridge University Press, UK
IPCC (2001) Climate change 2001, The scientific basis—contribution of working group I to the third assessment report of IPCC. Cambridge University Press, Cambridge
IPCC (2007) Climate change 2007: Synthesis report. Contribution of working groups I, II and III to the fourth assessment report of the intergovernmental panel on climate change [Core Writing Team, Pachauri RK, Reisinger A (eds.)]. IPCC, Geneva
Isermann K (1994) Agriculture’s share in the emission of trace gases affecting the climate and some cause-oriented proposals for sufficiently reducing this share. Environ Pollut 83:95–111
Jackson J, Choudrie S, Thistlethwaite G, Passant N, Murrells T, Watterson J, Mobbs D, Cardenas L, Thomson A, Leech A (2009) UK greenhouse gas inventory, 1990 to 2007 annual report for submission under the framework Convention on Climate Change, AEA Technology, p.71
Jia Z, Conrad R (2009) Bacteria rather than archaea dominate microbial ammonia oxidation in an agricultural soil. Environ Microbiol 11:1658–1671
Jones SK, Rees RM, Skiba UM, Ball BC (2007) Influence of organic and mineral N fertiliser on N2O fluxes from a temperate grassland. Agr Ecosyst Environ 121:74–83
Kammann C, Hepp S, Lenhart K, Müller C (2009) Stimulation of methane consumption by endogenous CH4 production in aerobic grassland soil. Soil Biol Biochem 41:622–629
Kandeler E, Gerber H (1988) Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol Fertil Soils 6:68–72
Kightley D, Nedwell DB, Cooper M (1995) Capacity for methane oxidation in landfill cover soils measured in laboratory-scale soil microcosms. Appl Environ Microbiol 61:592–601
Kilian S, Werner D (1996) Enhanced denitrification in plots of N2-fixing faba beans compared to plots of a non-fixing legume and non-legumes. Biol Fertil Soils 21:77–83
Kitzler B, Zechmeister-Boltenstern S, Holtermann C, Skiba U, Butterbach-Bahl K (2006) Nitrogen oxides emission from two beech forests subjected to different nitrogen loads. Biogeosciences 3:293–310
Klemedtsson L, Svensson BH, Rosswall T (1987) Dinitrogen and nitrous oxide produced by denitrification and nitrification in soil with and without barley plants. Plant Soil 99:303–310
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C (2006) Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809
Lou J, De Klein CAM, Ledgard SF, Saggar S (2010) Estimation of nitrous oxide emission from ecosystems and its mitigation technologies. Agr Ecosyst Environ 136:282–291
Madsen EL (2005) Identifying microorganisms responsible for ecologically significant biogeochemical processes. Nat Rev Microbiol 3:439–446
Martens-Habbena W, Berube PM, Urakawa H, DeLaTorre JR, Stahl DA (2009) Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 461:976–979
Matson PA, Naylor R, Ortiz-Monasterio I (1998) Integration of environmental, agronomic, and economic aspects of fertilizer management. Science 280:112–115
Mosier AR (1994) Nitrous oxide emissions from agricultural soils. Fertil Res 37:191–200
Mosier AR (1998) Soil processes and global change. Biol Fertil Soils 27:221–229
Mosier AR, Guenzi WD, Schweiger EE (1986) Soil losses of dinitrogen and nitrous oxide from irrigated crops in northeastern Colorado. Soil Sci Soc Am J 50:344–348
Mosier AR, Schimel D, Valentine D, Bronson K, Parton W (1991) Methane and nitrous oxide flux in native, fertilized and cultivated grasslands. Nature 350:330–332
Myrold DD, Tiedje JM (1985a) Diffusional constraints on denitrification in soil. Soil Sci Soc Am J 49:651–657
Myrold DD, Tiedje JM (1985b) Establishment of denitrification potential in soils: effects of carbon, nitrate and moisture. Soil Biol Biochem 17:819–822
Offre P, Prosser JI, Nicol GW (2009) Growth of ammonia-oxidizing archaea in soil microcosms is inhibited by acetylene. FEMS Microbiol Ecol 70:99–108
Philippot L, Hallin S, Börjesson G, Baggs EM (2008) Biochemical cycling in the rhizosphere having an impact on global change. Plant Soil 321:61–81
Pörtl K, Zechmeister-Boltenstern S, Wanek W, Ambus P, Berger TW (2007) Natural 15 N abundance of soil N pools and N2O reflect the nitrogen dynamics of forest soils. Plant Soil 295:79–94
Raich JW, Tufekcioglu A (2000) Vegetation and soil respiration: correlations and controls. Biogeochemistry 48:71–90
Reay DS, Nedwell DB (2004) Methane oxidation in temperate soils: effects of inorganic N. Soil Biol Biochem 36:2059–2065
Regina K, Pihlatie M, Esala M, Alakukku L (2007) Methane fluxes on boreal arable soils. Agric Ecosyst Environ 119:346–352
Scheer C, Wassmann R, Butterbach-Bahl K, Lamers JPA, Martius C (2009) The relationship between N2O, NO and N2 fluxes from fertilized and irrigated dryland soils of the Aral Sea Basin, Uzbekistan. Plant Soil 314:273–282
Sitaula BK, Hansen S, Sitaula JIB, Bakken LR (2000) Methane oxidation potentials and fluxes in agricultural soil: effects of fertilisation and soil compaction. Biogeochemistry 48:323–339
Smith C, Dobbie KE, Ball BC, Bakken LR, Sitaula BK, Hansen S, Brumme R, Borken W, Christensen S, Prieme A, Fowler D, Macdonald AJ, Skiba U, Klemedtsson L, Kasimir-Klemedtsson A, Degorska A, Orlanski P (2000) Oxidation of atmospheric methane in northern European soils, comparison with other ecosystems, and uncertainties in the global terrestrial sink. Glob Change Biol 6:791–803
Soussana JF, Allard V, Pilegaard K, Ambus P, Amman C, Campbell C, Ceschia E, Clifton-Brown J, Czobel S, Domingues R, Flechard C, Fuhrer J, Hensen A, Horvath L, Jones M, Kasper G, Martin C, Nagy Z, Neftel A, Raschi A, Baronti S, Rees RM, Skiba U, Stefani P, Manca G, Sutton M, Tuba Z, Valentini R (2007) Full accounting of the greenhouse gas (CO2, N2O, CH4) budget of nine European grassland sites. Agric Ecosyst Environ 121:121–134
Spott O, Russow RWB, Apelt B, Stange F (2006) A N-15-aided artificial atmosphere gas flow technique for online determination of soil N2 release using the zeolite Köstrolith SX6. Rapid Commun Mass Spectrom 20:3267–3274
Steudler PA, Bowden RB, Melillo JM, Aber JD (1989) Influence of nitrogen fertilization on methane uptake in temperate forest soils. Nature 341:314–315
Szukics U, Hackl E, Zechmeister-Boltenstern S, Sessitsch A (2009) Contrasting response of two forest soils to nitrogen input: rapidly altered NO and N2O emissions and nirK abundance. Biol Fertil Soils 45:855–863
Treseder KK (2008) Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11:1111–1120
Verma A, Tyagi L, Yadav S, Singh SN (2006) Temporal changes in N2O efflux from cropped and fallow agricultural fields. Agric Ecosyst Environ 116:209–215
von Fischer JC, Hedin LO (2007) Controls on soil methane fluxes: Tests of biophysical mechanisms using stable isotope tracers. Global Biogeochem Cy 2:GB2007
Wang Z, Ineson P (2003) Methane oxidation in a temperate coniferous forest soil: effects of inorganic N. Soil Biol Biochem 35:427–433
Yavitt JB, Downey DM, Lang GE, Sextone AJ (1990) Methane consumption in two temperate forest soils. Biogeochemistry 9:39–52
Acknowledgements
This work was supported by Project Nitrogenome (Enhancing nitrogen use efficiency by opening up the microbial black-box) LS-05-36 granted by the Vienna Science and Technology Fund WWTF. It was also partly funded by the European Commission through the NitroEurope Integrated Project 017841.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Per Ambus.
Rights and permissions
About this article
Cite this article
Inselsbacher, E., Wanek, W., Ripka, K. et al. Greenhouse gas fluxes respond to different N fertilizer types due to altered plant-soil-microbe interactions. Plant Soil 343, 17–35 (2011). https://doi.org/10.1007/s11104-010-0597-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-010-0597-6