Abstract
In many temperate ecosystems, rates of atmospheric nitrogen deposition remain high over winter despite decreased agricultural activity over this season. The extent to which this nitrogen is accessible for plant growth over the following growing season may depend strongly on uptake by plants and soil microorganisms from late fall through early spring, when the majority of aboveground plant tissue has senesced. We added Ca(15NO3)2 (5 atom %15N) at a rate of 2 g m−2 of N (corresponding to 100 mg 15N m−2) to the surface of plots in a temperate old field during either late fall, winter, spring melt or early spring. We quantified the recovery of excess 15N in the soil microbial biomass and soil extracts following spring melt and in aboveground plant tissue at the peak of the plant growing season. Nitrate additions had no significant effect on total aboveground plant biomass, relative species abundance or percent tissue nitrogen. However, mean excess 15N in aboveground plant tissue varied significantly among treatments, with values of 8.1, 2.6, 0.3 and 7.3 mg m−2 for late fall, winter, spring melt and early spring addition plots, respectively. Corresponding values of excess 15N were 3.1, 1.4 and 0.2 mg m−2 in microbial biomass, and 0.17, 0.07 and 0.03 mg m−2 in soil extracts, for late fall, winter and spring melt addition plots, respectively. Overall, these results indicate that nitrogen retention from late fall through early spring may depend highly on plant uptake in this system, and that only a small fraction of the nitrogen that accumulates in the winter snow pack may be available to plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The over-winter nitrogen dynamics of ecosystems have received increasing attention in recent years, both due to evidence that rates of soil microbial activity over this time can account for a significant proportion of annual activity (Groffman et al. 2001; Monson et al. 2006) and with speculation that climate-induced changes to soil freezing dynamics may alter N cycling (Henry 2007). Studies of soil N dynamics over winter have focused primarily on arctic and alpine systems (Campbell et al. 2005), which feature proportionally long winters, and arctic winters are projected to exhibit the greatest warming over the next century (IPCC 2007). However, N dynamics over winter can also influence annual N budgets in temperate systems (Fitzhugh et al. 2001; Campbell et al. 2005), and while these systems may not be expected to experience the most severe climate warming, they may be particularly sensitive to winter warming as a consequence of having soils that often remain close to freezing throughout winter (Henry 2008).
Unlike most arctic systems, warming in many temperate ecosystems is projected in coming decades to be coupled with large increases in atmospheric N deposition, as high as 2–5 g m−2 above pre-industrial rates, as a result of increased emissions from industrial and agricultural activities (Galloway et al. 2004). In eastern North America, nitrate is the dominant component in atmospheric nitrogen deposition, and the proportion of nitrate further increases over winter as a result of decreased ammonium emissions from agricultural sources (Sickles and Shadwick 2007). Nitrate is highly mobile in soil and thus vulnerable to leaching losses, and the extent to which it is retained in temperate systems from late fall through early spring may have important implications for plant nitrogen availability during the following growing season.
Studies of ecosystem nitrogen dynamics over winter have focused primarily on the fate of soil and plant litter nitrogen, rather than on the retention of exogenous nitrogen sources. For example, soil nitrogen release can be high over winter, particularly in late fall and early winter, as a result of fine root and microbial mortality, and the subsequent net mineralization and nitrification of organic N (Groffman et al. 2001; Kielland et al. 2006). Nitrogen uptake by plants (Andresen and Michelsen 2005) and lichens (Levia 2002) has been documented over winter, and microbial or abiotic immobilization can result in high soil nitrate retention during periods over winter when plant uptake is low (Judd et al. 2007). Nevertheless, the extent to which these potential nitrogen sinks generally offset ecosystem nitrogen losses over winter is unclear, and soil freeze-thaw cycles or cold soil temperatures over winter may alternatively promote nitrogen losses by increasing plant root mortality (Fitzhugh et al. 2001; Weih and Karlsson 2002) or the lysis of soil microorganisms (Yanai et al. 2004; Bolter et al 2005). Uptake of nutrient pulses by plants may be more important than uptake under steady state conditions over winter (Jonasson and Chapin 1991; Jonasson et al. 1999). In particular, cold-adapted species in both arctic and other systems may be highly responsive to nitrogen pulses following snow melt at the transition between late winter and early spring (Bilbrough and Caldwell 1997; Tye et al. 2005). Yet, these spring melt studies do not adequately address the potential for nitrogen uptake during periods of melt over winter, which occur frequently in many temperate systems.
While still poorly understood, soil nitrogen losses over winter are receiving increased attention in temperate agricultural systems, where crop residues or nitrogen fertilizer added in late fall may result in large leaching or trace gas losses (Francis et al. 2003). When the summer crop is removed in fall, the subsequent planting of non-leguminous cover crops can increase nitrogen retention (McLenaghen et al. 1996; Weinert et al. 2002). Nevertheless, nitrogen leaching may only be significantly reduced if cover crops are established in early fall (Ritter et al. 1998). In addition, while plant uptake can reduce leaching in the fall, trace gas losses of nitrogen from leaf litter can be high over winter (Dejoux et al. 2000), and there can be poor synchronization of the mineralization of crop residues with the timing of uptake by the subsequent crop (Catt et al. 1998). Even in forage and turf grass systems, where the same crop is continuously present, little of the nitrogen fertilizer applied in late fall or winter may be taken up by plants (Miltner et al. 2004), although microbial immobilization of nitrogen from fertilizer or plant residues can be high in these systems when plant uptake is low (Blankenau et al. 2000).
Overall, despite a growing interest in winter nitrogen dynamics, the fate of atmospheric nitrogen deposition over winter has not been addressed adequately; as described above, studies of natural systems have primarily explored the release of nitrogen from plant litter, soil microorganisms or soil organic matter, whereas those of agricultural systems have focused on nitrogen from crop residues or the addition of slow release or organic fertilizers. In this study, we used 15N-labelled nitrate as a tracer to determine variation in the retention and subsequent plant uptake of nitrogen applied to a temperate old field in either late fall, mid-winter, spring melt or early spring. We hypothesized that by the peak of the following growing season, the greatest quantity of tracer would be recovered in plants from plots receiving nitrate in the early spring, based on the assumption that actively growing roots are able to respond to nutrient pulses at this time. In contrast, we hypothesized that minimal tracer would be recovered in plants, soil microorganisms and soil extracts from the late fall and winter treatments, based on the assumptions that plant nitrogen uptake is low at this time and that nitrogen losses increase as a result of multiple freeze-thaw events over winter in this system.
Materials and methods
Site description
We conducted our experiment in a temperate old field (43° 11.590′ N, 81° 18.978′ W) north of London, Ontario, Canada. The site was previously planted with rotations of cereal crops but has not been cropped for over 35 years, and has not been mowed for the last 20 years. The vegetation of this site is dominated by the grass Festuca rubra (L.), and the other graminoids Agrostis stolonifera (L.), Poa pratensis (L.), and Carex spp., and the legume Coronilla varia (L.), are also abundant. The non-leguminous forbs Aster pilosus (Willd,), Centaurea jacea (L.), Daucus carota (L.), Dipsacus fullonum (L.), Fragaria virginiana (L.), Hypericum perforatum (L.), Plantago lanceolata (L.), Portulaca oleracea (L.) and Solidago altissima (L.) are present at lower density. The soil is classified as a moderately well drained brunisolic gray brown luvisol (Hagerty and Kingston 1992). The mean annual temperature of this region is 7.5°C, with monthly averages ranging from -7 in January to 20°C in July, and the mean precipitation is 987 mm (Environment Canada, National Climate Data and Information Archive).
15N tracer addition
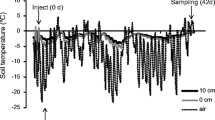
In November 2005, we selected ten replicate blocks at uniform intervals along two parallel 120 m transects separated by 50 m, and within each block delineated six 30 cm × 30 cm plots, each separated by 100 cm (Fig. 1). For four plots per block we added 900 ml of Ca(NO3)2 (5 atom %15N) using a watering can at a rate of 2 g N m−2 (100 mg 15N m−2) in either a) late fall following the senescence of most plant shoots (3 November 2005), b) during a mid-winter melt (4 January 2006), c) at spring melt (9 March 2006) or d) in early spring (6 April 2006) (Fig. 2; air temperature and snow cover data were obtained for the London International Airport, 20 km from the site, from the Environment Canada National Climate Data and Information Archive). The rate of nitrogen addition was based on projected rates of atmospheric nitrogen deposition for northeastern North America by the year 2050 (Galloway et al. 2004), and even higher rates are predicted in regions close to major urban and agricultural areas (Fenn et al. 2003). The remaining two plots per block served as controls, with one receiving an equivalent amount of Ca (as CaSO4) as the treated plots on 9 March. We applied an equal amount of deionized water to all treatments and control plots at each N addition, and treatment position within each block was randomized.
Air temperature and snow cover data from the London International Airport, 20 km from the study site. The black arrows denote the timing of the late fall, winter, spring melt and early spring nitrate additions, and the open triangle denotes the soil sampling date for the microbial biomass and soil extracts
Aboveground plant biomass
In September 2006, we clipped aboveground biomass from a single 10 cm × 10 cm subplot within each plot. We separated the plants by species then dried them at 60°C for 4 days prior to weighing. For subsequent analyses, we pooled plants by functional group into legumes, graminoids and non-leguminous dicots. By analyzing leguminous and non-leguminous plants separately, we could later assess whether shifts in plant 15N/14N at the plot level resulted from incorporation of the added 15N or merely reflected shifts in the relative abundances of functional groups.
Soil extractable and microbial N
On 29 March 2006, we collected a single soil sample from each plot, with the exception of the yet untreated early spring addition plots, in order to assess the retention of N by soil and soil microorganisms over winter. We collected the samples using a 10 cm deep, 2 cm diameter corer, homogenized the soil and removed rocks and large invertebrates. For each sample, we extracted approximately 15 g of wet soil in 75 ml of K2SO4 for 1 h, filtered the extract through pre-leached Whatman no.1 filter paper, then froze the extract. We collected subsamples of each core to determine the ratio of soil fresh weight to dry weight and used the latter, along with the volume of the corer and the total soil fresh weight (minus that of small rocks) to estimate bulk density. We estimated microbial N using a modification of the chloroform fumigation method described by Witt et al. (2000). For this method, we added 1 ml of ethanol-free chloroform to approximately 20 g of wet soil in 250 ml glass Schott bottles, stirred, then incubated the soils in the dark for 24 h. We then extracted the soils in K2SO4 as described above. To measure total soluble N, we oxidized the extracts using an alkaline persulphate oxidation method (Cabrera and Beare 1993) and subsequently analyzed for nitrate. We reduced nitrate to nitrite prior to analysis (Keeney and Nelson 1982), and determined nitrate concentrations colorimetrically following reaction with sulphanilamide coupled with N-1-naphthylethylene.HCl using a Smartchem 140 discrete autoanalyzer (Westco Scientific, Brookfield, CT, U.S.A.). We estimated microbial biomass N by subtracting total soluble N in unfumigated samples from total soluble N in fumigated samples.
Stable isotope analyses
We ground plant tissue into a fine powder using a Wiley mill (20 mesh) followed by a ball mill (Spex Certiprep Geno/grinder 2000, Metuchen, NJ, U.S.A), then weighed it into tin capsules. These samples were analyzed by The Colorado Plateau Stable Isotope Laboratory at Northern Arizona University using a NC 2100 Elemental Analyzer interfaced with a Finnigan Delta Plus XL isotope ratio mass spectrometer. For analysis of soil and microbial extracts, we combined aliquots with a KNO3 carrier solution (to meet the minimum quantity of N needed by the mass spectrometer), dried the solutions, then crushed the residue into a fine powder prior to analysis. We used 15N/14N to calculate percent 15N content in plant tissue and extracts collected from treatments and control plots. We then subtracted percent 15Ncontrol from percent 15Ntreatment for plant tissue, soil and microbial extracts in each block and multiplied these differences by total N in the respective pools to estimate excess 15N.
Statistical analyses
All dependent variables were approximately normally distributed with the exception of excess 15N in aboveground biomass, soil and microbial extracts, which we log-transformed, and aboveground biomass, which we square-root transformed. We analyzed all dependent variables using ANOVA with treatment as a fixed factor and block as a random factor. Tukey’s HSD post-hoc tests were used to reveal significant pairwise differences among treatments. We included CaSO4 addition plots in all analyses but excluded them from the figures and tables based on their similarity to control plots. We conducted all statistical analyses using SPSS version 13.0 (SPSS Inc., Chicago, IL, U.S.A), and used p < 0.05 as the threshold for statistical significance.
Results
Excess 15N in aboveground plant tissues differed significantly among treatments, both analyzed over all species (p < 0.001), and for graminoids (p < 0.001) and leguminous species (p < 0.001). The highest recovery of excess 15N occurred for the late fall and early spring addition treatments, with approximately 70% and 96% less recovery than in the late fall and early spring treatments occurring for the winter and spring melt addition treatments, respectively (Fig. 3). Approximately 6–12 times higher excess 15N was recovered in graminoids than in leguminous species among treatments (Fig. 3). The average aboveground biomass of graminoids and legumes ranged from 288–367 g m−2 and 80–190 g m−2 among treatments, respectively (Table 1), and non-leguminous dicots, which averaged 38 g m−2, were only found in one third of the plots. However, there were no significant treatment effects on total aboveground plant biomass (p = 0.55) or the proportion of legumes (p = 0.21) or graminoids (p = 0.82). There were no significant treatment effects on total N in aboveground plant tissue (p = 0.33), and percent N in the aboveground plant tissues of graminoids (p = 0.19) and legumes (p = 0.07) also did not differ significantly among treatments (Table 1).
Mean excess 15N recovered in the aboveground biomass of graminoids and legumes the following September for each of the nitrate addition treatments. Data are back-transformed from log-transformed data, and error bars represent standard error (n = 10). Different lower case letters above bars within a panel indicate significant pairwise differences (Tukey’s HSD post-hoc tests; p < 0.05)
Excess 15N recovered in the soil microbial biomass was highest in the late fall addition treatment, following by the winter addition, then the spring melt addition (p < 0.001; Fig. 4). Total microbial N varied from 68–86 μg per g dry soil among treatments but was not significantly affected by the timing of N addition (p = 0.06). Significantly more excess 15N was recovered from soil extracts sampled from late fall treatment plots than from winter and spring melt treatment plots (p < 0.001; Fig. 5). Total extractable N, which varied from 6–10 μg per g dry soil among treatments was not affected by the timing of N addition (p = 0.31). Overall, the maximum mean recoveries of the added 15N (e.g. from the late fall addition treatment) in the soil microbial biomass and soil extracts we sampled were approximately 3% and 0.2%, respectively, as compared with a recovery of approximately 8% of the added 15N in the aboveground plant biomass.
Mean excess 15N recovered in soil microbial biomass following spring melt for each of the nitrate addition treatments. These data are converted to mg m−2 and % of 15N added on the right hand y-axis, based on bulk density down to the sampling depth of 10 cm. Data are back-transformed from log-transformed data, and error bars represent standard error (n = 10). Different lower case letters above bars within a panel indicate significant pairwise differences (Tukey’s HSD post-hoc tests; p < 0.05)
Mean excess 15N recovered in soil extracts following spring melt for each of the nitrate addition treatments. These data are converted to mg m−2 and % of 15N added on the right hand y-axis, based on bulk density down to the sampling depth of 10 cm. Data are back-transformed from log-transformed data, and error bars represent standard error (n = 10). Different lower case letters above bars within a panel indicate significant pairwise differences (Tukey’s HSD post-hoc tests; p < 0.05)
Discussion
Retention of 15NO3 − added over winter and spring melt
Based on the combined recovery of 15N in the aboveground plant tissue, soil microbial biomass and extractable nitrogen pools (Figs. 3, 4, 5), the retention of nitrate added to the soil surface during winter, and particularly during spring melt, was substantially lower than that added in late fall or in early spring. Decreased plant nitrogen uptake over winter was not unexpected, based on decreased uptake rates of active roots at cold temperatures (Henry and Jefferies 2003; Castle et al. 2006) and root dormancy or senescence over this period. However, it was surprising that very little nitrogen retention occurred during spring melt, given that plants in many arctic and alpine systems can take up nitrogen rapidly at this time (Hiltbrunner et al. 2005; Tye et al. 2005; Edwards et al. 2006). Nevertheless, the ability of plants to exploit nitrogen pulses during spring melt can vary widely among systems (Bilbrough et al. 2000) or even among species within systems (Bowman and Bilbrough 2001). In addition, nitrogen addition in our study was designed to simulate atmospheric deposition and involved the addition of nitrate to the soil surface, whereas some studies that have demonstrated high uptake at spring melt (e.g. Andresen and Michelsen 2005) have used less mobile forms of nitrogen than nitrate and have injected the labeled substrate directly into the soil. Surface additions may be more vulnerable to runoff or denitrification under the water-saturated soil conditions in early spring (Muller et al. 2002). Although the addition of a single large pulse of 2 g N m−2 did not accurately simulate a more realistic even temporal distribution of atmospheric nitrogen deposition over the length of the winter, the winter and spring melt treatments did reasonably simulate the accumulation of atmospheric nitrogen deposition in the snow pack and subsequent release to the soil in a single pulse during a large snow melt. Our results suggest that there may be an asynchrony between spring melt and the production of active root biomass in this system (sensu Sickman et al. 2001; Lafreniere and Sharp 2005) that allows a large fraction of nitrate in melt water to be lost as leachate or as trace gases at this time. However, we did not measure these losses directly, and we cannot rule out that a portion of the 15N we could not account for was retained in the system yet sequestered in the recalcitrant and insoluble fraction of the soil organic matter. Although abiotic nitrate retention can also reduce nitrate mobility during spring melt (Davidson et al. 2003; Campbell et al. 2007), this mechanism did not appear to contribute substantially to nitrate retention in our system, given that little of the 15N label was recovered in the soil extracts.
Retention of 15NO3 − added in the fall and early spring
In contrast to winter and spring melt, the retention of nitrogen added in late fall was relatively high, despite the aboveground plant tissues being largely senesced at this time. The quantity of excess 15N recovered in the soil microbial biomass in early spring, prior to plant green-up, was only one third of the quantity recovered in aboveground plant tissue at the end of the following growing season (comparison of Figs. 3 and 4). This comparison does not account for plants roots, which may have contained a similar quantity of excess 15N as plant shoots (the root to shoot ratio of Festuca rubra, the dominant species, is approximately one under similar field conditions; Boot and Mensink 1990; Hunt et al. 1995). Therefore, although we did not measure plant nitrogen uptake in the late fall directly, these data indicate that the quantity of excess 15N in the soil microbial biomass at the end of winter was insufficient to explain the quantity of excess 15N recovered in plants at the peak of the following growing season. Rather, a large fraction of the excess 15N recovered in plants may have been taken up by plants immediately in the fall and remobilized for aboveground growth over the following summer. In addition, uptake of the 15N by plant roots and soil microorganisms in the fall could have led to the accumulation of 15N in insoluble necromass (e.g. root litter and microbial cells walls) over winter, followed by mineralization of the necromass nitrogen and plant uptake of the 15N label over the following growing season. As predicted, the early spring addition plots contained a high quantity of excess 15N relative to the other treatments. This nitrogen would have been available to plants at the start of the growing season, when warmer soil temperatures and rapid plant growth enable high rates of nitrogen uptake (Rowarth et al. 1998; Cookson et al. 2001).
The relatively high retention of nitrate added in late fall was also contrary to our hypothesis that soil freeze-thaw cycles would subsequently lead to the loss of a large fraction of nitrogen taken up by plants or microorganisms at this time. Although soil freeze-thaw cycles can contribute to both nitrogen leaching and trace gas losses over winter (DeLuca et al. 1992; Ludwig et al. 2004), these results have primarily been obtained from studies imposing high amplitude temperature cycles on soils under laboratory conditions (Henry 2007). In contrast, other studies that have imposed more moderate cycles have demonstrated little or no effect of freezing and thawing on soil nitrogen dynamics (Herrmann and Witter 2002; Grogan et al. 2004). We observed the highest excess 15N content in the microbial biomass from late fall addition plots, which suggests that the microbial biomass may also have been tolerant of freeze-thaw cycles over winter and able to immobilize nitrogen. High freeze-thaw tolerance of the soil microbial community has been observed previously in alpine systems (Lipson and Monson 1998; Lipson et al. 2000). However, we could not distinguish between 15N taken up as nitrate directly by soil microorganisms in the late fall following fertilization and that taken up following the release of 15N from decomposing plant roots or microbial cell walls in early spring.
Fertilization effects on plant and microbial biomass and N content
Despite the strong treatment effects on excess 15N recovery in our experiment, there were no significant treatment effects of nitrogen addition on aboveground biomass, relative species abundance or tissue nitrogen content over the following growing season, nor were there significant effects on microbial or total extractable nitrogen in the early spring. These results were surprising, given that the addition rate of 2 g m−2 of N more than doubles current background deposition rates in the region of the site (Sickles and Shadwick 2007), and is consistent with projected increases in atmospheric nitrogen deposition over the next 50 years in the region (Galloway et al. 2004). This lack of a significant plant growth response could reflect a proportionately low retention of the added N across all treatments or, as suggested above, the sequestration of the 15N label in recalcitrant fractions of the soil organic matter. Alternatively, it could indicate that our site may not be nitrogen limited. Nevertheless, concerning the latter, excess 15N enrichment in the aboveground biomass of graminoids was 6–12 times higher among treatments than that of legumes (Fig. 3), whereas total graminoid nitrogen in aboveground biomass was only 1–2 times higher than in legumes (multiplication of biomass by percent N in Table 1). This relatively low proportion of excess 15N in legumes may indicate substantial N fixation by these species at the site, although it could also reflect disproportionately high nitrate uptake by graminoids from late fall through early spring, when the nitrogen was added.
Conclusions
Overall, our results reveal that low quantities of nitrogen from precipitation over winter may be retained in temperate old fields. A large contrast with results from arctic and alpine systems was the minimal nitrogen retention that occurred during spring melt, at a time when accumulated nitrogen may be released from the melting snow pack. Although these nitrogen losses may be of little consequence for primary productivity in terrestrial systems that have become nitrogen saturated, they represent potentially important nitrate inputs to aquatic systems.
References
Andresen LC, Michelsen A (2005) Off-season uptake of nitrogen in temperate heath vegetation. Oecologia 144:585–597 doi:10.1007/s00442-005-0044-1
Bilbrough CJ, Caldwell MM (1997) Exploitation of springtime ephemeral N pulses by six Great Basin plant species. Ecology 78:231–243
Bilbrough CJ, Welker JM, Bowman WD (2000) Early spring nitrogen uptake by snow-covered plants: a comparison of arctic and alpine plant function under the snowpack. Arct Antarct Alp Res 32:404–411 doi:10.2307/1552389
Blankenau K, Olfs HW, Kuhlmann H (2000) Effect of microbial nitrogen immobilization during the growth period on the availability of nitrogen fertilizer for winter cereals. Biol Fertil Soils 32:157–165 doi:10.1007/s003740000230
Bolter M, Soethe N, Horn R, Uhlig C (2005) Seasonal development of microbial activity in soils of northern Norway. Pedosphere 15:716–727
Boot RGA, Mensink M (1990) Size and morphology of root systems of perennial grasses from contrasting habitats as affected by nitrogen supply. Plant Soil 129:291–299
Bowman WD, Bilbrough CJ (2001) Influence of a pulsed nitrogen supply on growth and nitrogen uptake in alpine graminoids. Plant Soil 233:283–290 doi:10.1023/A:1010571920890
Cabrera ML, Beare MH (1993) Alkaline persulfate oxidation for determining total nitrogen in microbial biomass extracts. Soil Sci Soc Am J 57:1007–1012
Campbell JL, Mitchell MJ, Groffman PM, Christenson LM, Hardy JP (2005) Winter in northeastern North America: a critical period for ecological processes. Front Ecol Environ 3:314–322
Campbell JL, Mitchell MJ, Mayer B, Groffman PM, Christenson LM (2007) Mobility of nitrogen-15-labeled nitrate and sulfur-34-labeled sulfate during snowmelt. Soil Sci Soc Am J 71:1934–1944 doi:10.2136/sssaj2006.0283
Castle ML, Crush JR, Rowarth JS (2006) The effect of root and shoot temperature of 8°C or 24°C on the uptake and distribution of nitrogen in white clover (Trifolium repens L.). Aust J Agric Res 57:577–581 doi:10.1071/AR05209
Catt JA, Howse KR, Christian DG, Lane PW, Harris GL, Goss MJ (1998) Strategies to decrease nitrate leaching in the Brimstone Farm Experiment, Oxfordshire, UK, 1988-93: the effect of straw incorporation. Plant Soil 203:57–69 doi:10.1023/A:1004389426718
Cookson WR, Rowarth JS, Cameron KC (2001) The fate of autumn-, late winter- and spring-applied nitrogen fertilizer in a perennial ryegrass (Lolium perenne L.) seed crop on a silt loam soil in Canterbury, New Zealand. Agric Ecosyst Environ 84:67–77 doi:10.1016/S0167-8809(00)00196-1
Davidson EA, Chorover J, Dail DB (2003) A mechanism of abiotic immobilization of nitrate in forest ecosystems: the ferrous wheel hypothesis. Glob Change Biol 9:228–236 doi:10.1046/j.1365-2486.2003.00592.x
Dejoux JF, Recous S, Meynard JM, Trinsoutrot I, Leterme P (2000) The fate of nitrogen from winter-frozen rapeseed leaves: mineralization, fluxes to the environment and uptake by rapeseed crop in spring. Plant Soil 218:257–272 doi:10.1023/A:1014934924819
DeLuca TH, Keeney DR, McCarty GW (1992) Effect of freeze-thaw events on mineralization of soil nitrogen. Biol Fertil Soils 14:116–120 doi:10.1007/BF00336260
Edwards KA, McCulloch J, Kershaw GP, Jefferies RL (2006) Soil microbial and nutrient dynamics in a wet Arctic sedge meadow in late winter and early spring. Soil Biol Biochem 38:2843–2851 doi:10.1016/j.soilbio.2006.04.042
Fenn ME, Haeuber R, Tonnesen GS, Baron JS, Grossman-Clarke S, Hope D, Jaffe DA, Copeland S, Geiser L, Rueth HM, Sickman JO (2003) Nitrogen emissions, deposition, and monitoring in the western United States. Bioscience 53:391–403 doi:10.1641/0006-3568(2003)053[0391:NEDAMI]2.0.CO;2
Fitzhugh RD, Driscoll CT, Groffman PM, Tierney GL, Fahey TJ, Hardy JP (2001) Effects of soil freezing, disturbance on soil solution nitrogen, phosphorus, and carbon chemistry in a northern hardwood ecosystem. Biogeochemistry 56:215–238 doi:10.1023/A:1013076609950
Francis GS, Trimmer LA, Tregurtha CS, Williams PH, Butler RC (2003) Winter nitrate leaching losses from three land uses in the Pukekohe area of New Zealand. N Zeal J Agr Res 46:215–224
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM, Michaels AF, Porter JH, Townsend AR, Vorosmarty CJ (2004) Nitrogen Cycles: past, present and future. Biogeochemistry 70:153–226 doi:10.1007/s10533-004-0370-0
Groffman PM, Driscoll CT, Fahey TJ, Hardy JP, Fitzhugh RD, Tierney GL (2001) Colder soils in a warmer world: a snow manipulation study in a northern hardwood forest ecosystem. Biogeochemistry 56:135–150 doi:10.1023/A:1013039830323
Grogan P, Michelsen A, Ambus P, Jonasson S (2004) Freeze-thaw regime effects on carbon and nitrogen dynamics in sub-arctic heath tundra mesocoms. Soil Biol Biochem 36:641–654 doi:10.1016/j.soilbio.2003.12.007
Hagerty TP, Kingston MS (1992) The soils of Middlesex County. Ontario Ministry of Agriculture and Food
Henry HAL (2007) Soil freeze-thaw cycle experiments: trends, methodological weaknesses and suggested improvements. Soil Biol Biochem 39:977–986 doi:10.1016/j.soilbio.2006.11.017
Henry HAL (2008) Climate change and soil freezing dynamics: historical trends and projected changes. Clim Change
Henry HAL, Jefferies RL (2003) Interactions in the uptake of amino acids, ammonium and nitrate ions in the Arctic salt-marsh grass, Puccinellia phryganodes. Plant Cell Environ 26:419–428 doi:10.1046/j.1365-3040.2003.00973.x
Herrmann A, Witter E (2002) Sources of C and N contributing to the flush in mineralization upon freeze-thaw cycles in soil. Soil Biol Biochem 34:1495–1505 doi:10.1016/S0038-0717(02)00121-9
Hiltbrunner E, Schwikowski M, Korner C (2005) Inorganic nitrogen storage in alpine snow pack in the Central Alps (Switzerland). Atmos Environ 39:2249–2259 doi:10.1016/j.atmosenv.2004.12.037
Hunt R, Hand DW, Hannah MA, Neal AM (1995) Temporal and nutritional influences on the response to elevated CO2 in selected British grasses. Ann Bot (Lond) 75:207–216 doi:10.1006/anbo.1995.1014
IPCC (2007) Summary for Policymakers. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA
Jonasson S, Chapin FS (1991) Seasonal uptake and allocation of phosphorus in Eriophorum vaginatum L. measured by labeling with 32 P. New Phytol 118:349–357 doi:10.1111/j.1469-8137.1991.tb00987.x
Jonasson S, Michelsen A, Schmidt IK (1999) Coupling of nutrient cycling and carbon dynamics in the Arctic, integration of soil microbial and plant processes. Appl Soil Ecol 11:135–146 doi:10.1016/S0929-1393(98)00145-0
Judd KE, Likens GE, Groffman PM (2007) High nitrate retention during winter in soils of the hubbard brook experimental forest. Ecosystems (N Y, Print) 10:217–225 doi:10.1007/s10021-007-9027-x
Keeney DR, Nelson DW (1982) Nitrogen-inorganic forms. In: Page AL (ed) Methods of soil analysis. Part 2, 2nd edition, Agronomy Monograph 9. American Society of Agronomy and Soil Science Society of America, Madison
Kielland K, Olson K, Ruess RW, Boone RD (2006) Contribution of winter processes to soil nitrogen flux in taiga forest ecosystems. Biogeochemistry 81:349–360 doi:10.1007/s10533-006-9045-3
Lafreniere MJ, Sharp MJ (2005) A comparison of solute fluxes and sources from glacial and non-glacial catchments over contrasting melt seasons. Hydrol Process 19:2991–3012 doi:10.1002/hyp.5812
Levia DF (2002) Nitrate sequestration by corticolous macrolichens during winter precipitation events. Int J Biometeorol 46:60–65 doi:10.1007/s00484-001-0118-7
Lipson DA, Monson RK (1998) Plant-microbe competition for soil amino acids in the alpine tundra: effects of freeze-thaw and dry-rewet events. Oecologia 113:406–414 doi:10.1007/s004420050393
Lipson DA, Schmidt SK, Monson RK (2000) Carbon availability and temperature control the post-snowmelt decline in alpine soil microbial biomass. Soil Biol Biochem 32:441–448 doi:10.1016/S0038-0717(99)00068-1
Ludwig B, Wolf I, Teepe R (2004) Contribution of nitrification and denitrification to the emission of N2O in a freeze-thaw event in an agricultural soil. J Plant Nutr Soil Sci 167:678–684 doi:10.1002/jpln.200421462
McLenaghen RD, Cameron KC, Lampkin NH, Daly ML, Deo B (1996) Nitrate leaching from ploughed pasture and the effectiveness of winter catch crops in reducing leaching losses. N Zeal J Agr Res 39:413–420
Miltner ED, Stahnke GK, Johnston WJ, Golob CT (2004) Late fall and winter nitrogen fertilization of turfgrass in two pacific northwest climates. HortScience 39:1745–1749
Monson RK, Lipson DA, Burns SP, Turnipseed AA, Delany AC, Williams MW, Schmidt SK (2006) Winter forest soil respiration controlled by climate and microbial community composition. Nature 439:711–714 doi:10.1038/nature04555
Muller C, Martin M, Stevens RJ, Laughlin RJ, Kammann C, Ottow JCG, Jager HJ (2002) Processes leading to N2O emissions in grassland soil during freezing and thawing. Soil Biol Biochem 34:1325–1331 doi:10.1016/S0038-0717(02)00076-7
Ritter WF, Scarborough RW, Chirnside AEM (1998) Winter cover crops as a best management practice for reducing nitrogen leaching. J Contam Hydrol 34:1–15 doi:10.1016/S0169-7722(98)00087-4
Rowarth JS, Boelt B, Hampton JG, Marshall AH, Rolston MP, Sicard G, Silberstein T, Sedcole JR, Young WC III (1998) The relationship between applied nitrogen, seed yield and nitrogen concentration in herbage in perennial ryegrass (Lolium perenne L.). I. Cv. Grasslands Nui at five sites around the globe. J Appl Seed Prod 16:105–114
Sickles JE III, Shadwick DS (2007) Changes in air quality and atmospheric deposition in the eastern United States: 1990–2004. J Geophys Res 112:D17301 doi:10.1029/2006JD007843
Sickman JO, Leydecker A, Melack JM (2001) Nitrogen mass balances and abiotic controls on N retention and yield in high-elevation catchments of the Sierra Nevada, California, United States. Water Resour Res 37:1445–1461 doi:10.1029/2000WR900371
Tye AM, Young SD, Crout NMJ, West HM, Stapleton LM, Poulton PR, Laybourn-Parry J (2005) The fate of N-15 added to high Arctic tundra to mimic increased inputs of atmospheric nitrogen released from melting snowpack. Glob Change Biol 11:1640–1654 doi:10.1111/j.1365-2486.2005.01044.x
Weih M, Karlsson PS (2002) Low winter soil temperature affects summertime nutrient uptake capacity and growth rate of mountain birch seedlings in the subarctic, Swedish lapland. Arct Antarct Alp Res 34:434–439 doi:10.2307/1552201
Weinert TL, Pan WL, Moneymaker MR, Santo GS, Stevens RG (2002) Nirogen recycling by nonleguminous winter cover crops to reduce leaching in potato rotations. Agron J 94:365–372
Witt C, Gaunt JL, Galicia CC, Ottow JCG, Neue H (2000) A rapid chloroform-fumigation extraction method for measuring soil microbial biomass carbon and nitrogen in flooded rice soils. Biol Fertil Soils 30:510–519 doi:10.1007/s003740050030
Yanai Y, Toyota K, Okazaki M (2004) Effects of successive soil freeze-thaw cycles on soil microbial biomass and organic matter decomposition potential of soils. Soil Sci Plant Nutr 50:821–829
Acknowledgements
This work was supported by a Natural Sciences and Engineering Research Council of Canada Discovery Grant to HALH. We thank Dr. Jane Bowles for assistance in plant species identification, and two anonymous reviewers for their comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Lars S. Jensen.
Rights and permissions
About this article
Cite this article
Joseph, G., Henry, H.A.L. Retention of surface nitrate additions in a temperate old field: implications for atmospheric nitrogen deposition over winter and plant nitrogen availability. Plant Soil 319, 209–218 (2009). https://doi.org/10.1007/s11104-008-9862-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9862-3