Abstract
Nitrous oxide (N2O) emission was measured in a Kobresia humilis meadow and a Potentilla fruticosa meadow in the Qinghai–Tibet Plateau from June 2003 to July 2006. Five treatments were setup in the two alpine meadows. Two bare soil treatments were setup in the K. humilis meadow (BSK) and in the P. fruticosa meadow (BSP) by removing the above- and belowground plant biomass. Three plant community treatments were setup with one in the K. humilis meadow (herbaceous community in the K. humilis meadow-HCK) and two in the P. fruticosa meadow (herbaceous community in the P. fruticosa meadow-HCP, and shrub community in the P. fruticosa meadow-SCP). Nitrous oxide emission from BSP was estimated to be 38.1 ± 3.6 μg m−2 h−1, significantly higher than from BSK (30.2 ± 2.8 μg m−2 h−1) during the whole experiment period. Rates from the two herbaceous blocks (HCK and HCP) were close to 39.5 μg m−2 h−1 during the whole experimental period whereas shrub community (SCP) showed significant high emission rates of N2O. Annual rate of N2O emission was estimated to be 356.7 ± 8.3 and 295.0 ± 11.6 mg m−2 year−1 from the alpine P. fruticosa meadow and from the alpine K. humilis meadow, respectively. These results suggest that alpine meadows in the Qinghai–Tibetan Plateau are an important source of N2O, contributing an average of 0.3 Tg N2O year−1. We concluded that N2O emission will decrease, due to a predicted vegetation shift from shrubs to grasses imposed by overgrazing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite its low abundance in the atmosphere, N2O plays an important role in the radioactive forcing (Finlayson-Pitts et al. 2000; Houghton et al. 1992). Moreover, N2O catalyzes the destruction of ozone in the stratosphere (Crutzen 1981) due to its long residence time (∼120 years) in the atmosphere (Houghton et al. 1995). Since increasing atmospheric N2O concentrations contribute to changes in the climate of the earth, there has been a growing interest in quantifying its significant sources and sinks in different ecosystems (Flessa et al. 2002).
It has been suggested that soils are a predominant source, contributing about 70% of the total N2O emitted from the biosphere to the atmosphere (Flückiger et al. 1999). In this regard agricultural lands such as fertilized fields and grasslands make a great contribution to global N2O emission (Mosier 1994). Because grasslands occupy a large proportion of terrestrial ecosystems in the globe (Adams et al. 1990), numerous studies have been conducted in grassland ecosystems for a better understanding of N2O emissions. It shows that grasslands are a source of N2O (Pei et al. 2004; Williams et al. 1999; Xu-Ri et al. 2003) and often exhibit higher emission rates than arable and forest soils (Oenema et al. 1998). Meanwhile, evidence shows that soil moisture (Bouwman 1996; Del Prado et al. 2006; Du 2006; Li et al. 2004), temperature (Bouwman 1996; Du 2006) and grazing (Velthof and Oenema 1997) as well as N fertilization (Del Prado et al. 2006; Stehfest and Bouwman 2006; Velthof and Oenema 1997) have a great impact on N2O emissions from grasslands. Recent research has identified that some plants have the capacity to emit N2O to the atmosphere (Goshima et al. 1999; Zhang and Jiang 2006; Zou et al. 2005).
As one of the most important dominant vegetation types, alpine meadows occupy 35% of the Qinghai–Tibet Plateau which extends more than 2.5 million km2 (Zheng and Zhu 2000). Previous studies showed that alpine meadows such as Kobresia and Potentilla meadows are a weak sink of atmospheric CO2 and CH4 (Cao et al. 2004, 2008; Wang et al. 2004; Zhao et al. 2007). Up to date little is known about N2O emissions from alpine meadows, although several studies have investigated N2O emissions from alpine steppes (Pei et al. 2004). This seriously hinders our understanding of N2O emissions as well as our budget of N2O fluxes in the Tibet Plateau on a regional scale. Besides, it has been suggested that future changes in N2O in response to climate change will be more strongly mediated by large-scale changes in vegetation than by direct temperature effects in wet meadows (Verville et al. 1998).
In order to clarify the effects of plant communities on N2O emissions and thus estimate the contribution to atmospheric N2O from alpine meadows a three year experiment was conducted in two alpine meadow types in the Qinghai–Tibetan Plateau.
Materials and methods
Site description
The study site was located at Haibei Alpine Meadow Research Station (37°32′ N, 101°15′ E, 3280 m a.s.l.), the Chinese Academy of Sciences. Annual precipitation averaged 560 mm in the past 20 years, of which 85% was concentrated in growing seasons from May to September (Li et al. 2004). Annual precipitation was 546, 536, 450 and 540 mm, while annual air temperature was −0.9, −1.1, −0.5 and −0.7°C for the year of 2003, 2004, 2005 and 2006, respectively.
A Kobresia humilis meadow and a Potentilla fruticosa meadow were selected for N2O emission measurements. Both alpine meadows have been fenced and used as winter grazing pasture from late September to the end of April since 1982. The Kobresia meadow only contains a herbaceous layer, and is dominated by K. humilis, Saussurea superba, Potentilla saundersiana, Leontopodium nanum, Lancea tibetica, Festuca ovina, Festuca rubra, Stipa aliena, Elymus nutans, Helictotrichon tibetica, Koeleria cristata, and Poa crymophila. Vegetation coverage ranges from 75% to 80%, of which 98% by grasses and 2% by bare soil. The P. fruticosa meadow consists of two layers: a shrub layer dominated by P. fruticosa and an herbaceous layer dominated by F. rubra, Stipa alpine, K. humilis and E. nutans with Polygonum viviparum, Poa pratensis and P. saundersiana. Vegetation coverage ranges from 60% to 75%, of which 50% by shrubs, and 48% by grasses and 2% by bare soil (Zhou and Wu 2006). Alpine grasses are about 25–30 cm high, while alpine shrubs are 50–70 cm high.
The soils developed in alpine meadows and in alpine shrub meadows were Mat-Gryic Cambisols and Mol-Gryic Cambisols (Chinese Soil Taxonomy Research Group 1995). They are rich in organic carbon content and have an Udic soil moisture regime (Bao et al. 1995; Cao et al. 1998). The soil basic properties for both sites are presented in Table 1.
Experimental design
In May 2003, three areas, one in the K. humilis meadow (herbaceous community in the K. humilis meadow—HCK) and two in the P. fruticosa meadow (herbaceous community in the P. fruticosa meadow—HCP, and shrub community in the P. fruticosa meadow—SCP) were selected for this study. The net primary production (NPP) of the three communities was estimated by harvesting three squares (0.5 m2) close to the plots which were used for N2O measurements in 2005. It was 386.3 ± 12.3 g m−2 for HCK, 341.4 ± 17.0 g m−2 for HCP and 386.6 ± 40.4 g m−2 for SCP. In each meadow bare soil plots were prepared by digging three pits, 1 m2 and 1 m deep. Soils were refilled according to soil layers after roots were removed by sieving. After about 6 weeks they were used for N2O measurements. The measurement sites are summarized in Table 2.
Samplings and measurements
Nitrous oxide emission was measured by a static chamber method (Cao et al. 2008; Wang et al. 2001). For each vegetation community three chamber bases were installed and one chamber base was placed on each bare plot. In total, 15 chamber bases were used. The stainless steel bases (0.5 × 0.5 × 0.1 m3) were permanently placed into the ground. Sticky clay soil was used between base and soil, in order to avoid gas leakage between soil and air. Twelve plexiglas chambers (0.5 × 0.5 × 0.5 m3) were used to collect N2O from bare soil and grass communities. Three larger chambers (0.5 × 0.5 × 1 m3) were used for the taller shrub communities. The chambers were equipped with two electric fans to mix the air and a thermo-probe to monitor temperature within the chamber during measurements. In order to prevent an increase of temperature inside the chambers caused by solar radiation, chambers were covered with foam and white waterproof cloth.
For flux measurement chambers were sealed onto the bases for 30 min periods between 9:00 to 10:00 o'clock in the morning. Gas samples were collected from the chambers every 10 min using 100 ml plastic syringes. Flux measurements were made every 4–5 days during growing seasons and twice per month during winter. The sampling time of 9:00 to 10:00 o'clock local time was chosen, because diurnal measurements (every 2 h) showed that these fluxes were close to the diurnal average. Throughout this study the diurnal cycle was monitored once per month, sampling every 2 h during daytime and every 3 h during nighttime. All gas samples were analyzed within 2 days of collection. There was snow cover when flux measurements were made in winter. Snow was removed outside the chamber area, but remained undisturbed inside the chambers.
The samples were analyzed by an improved gas chromatograph (HP4890D, Agilent Co. Produced) system with electron capture detector (ECD). Injection/ detection and column oven (SS-3 m × 2 mm × porapak Q) temperature were 55°C and 330°C, respectively. Ultra pure N2 was used as carrier gas with a flow rate of 30 ml min−1 (Wang et al. 2003; Wang and Wang 2003). A certified N2O standard with a concentration of 355 ppbv (China National Research Center for Certified Reference Materials, Beijing) was used for calibration. Analysis accuracy of samples is ±5 ppbv for N2O measurements. The analytical range of measured concentrations is between 163 and 9,412 ppbv. Three replicates were measured for each treatment.
Soil temperature at 5 cm depth was measured using JM624 thermometer and volumetric soil moisture at 10 cm soil depth was measured by a moisture meter (Time-domain reflectometer, Campbell Scientific, Inc., North Logan, UT, USA).
Calculation, uncertainties and statistics
Hourly N2O emissions (μg N2O m−2 h−1) were calculated based on the slope of the linear increase in N2O concentration over the sampling period, as follows:
where \({\text{Flux}}_{{\text{N}}_2 {\text{O}}} \) is the hourly N2O emission (μg N2O m−2 h−1) gas flux, ρ is gas density inside the chamber, dC t /dt is the slope of linear increase in N2O concentration during sampling period, V is the volume of the chamber, A is the surface area of the chamber, P 0 is the air pressure at sea level, P is the air pressure at sampling site, T 0 is the temperature (Kelvin) at sea level and T is chamber temperature (Kelvin).
Our estimation of N2O emissions are suffering from three uncertainties as follows: Firstly, the major uncertainty arises from low resolution of measurements of N2O. In order to polish this uncertainty, more diurnal measurements of N2O in these meadows are needed in future studies. Secondly, the removal of above- and below ground plant biomass might affect microbial composition as well as soil environments and thus change the production of N2O. Thirdly, analytic accuracy of the equipment also has an impact on the estimation of N2O emission.
The difference among treatments and correlation analysis were tested by multivariate analysis based on independent-samples T-test, whilst the correlation between N2O emission and environment factors such as temperature and moisture by bivariate process correlations (SPSS11.5).
Results
Nitrous oxide emissions from bare soils
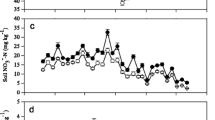
Bare soil patches in the two meadows (BSK and BSP) showed obvious seasonal dynamics of N2O emissions (Fig. 1a). The maximal emission rates appeared in growing seasons from May to September, while they reached the lowest in dormancy seasons from October to the next April. Nitrous oxide emission from the BSP treatment to the atmosphere averaged 38.1 ± 3.6 μg m−2 h−1, significantly higher than from the BSK treatment with a averaged rate of 30.2 ± 2.8 μg m−2 h−1 during the entire experimental period (Table 3). Nitrous oxide emissions from BSK and BSP were also significantly different in growing seasons (p < 0.05, T-test), but there was no significant difference between the two treatments during dormant seasons. No significant difference was found for either BSK or BSP between growing seasons/dormant periods.
Seasonal dynamic of N2O efflux (a), soil temperature at 5 cm depth (b) and soil moisture at 10 cm depth (c) from bare soil plots in the K. humilis meadow (BSK) and P. fruticosa meadow (BSP). Means ± standard errors (SE) of three replicate measurements were presented. No soil moisture data (c) were collected in winter, when soils were frozen
Nitrous oxide emissions from different plant communities
Nitrous oxide emissions from each of the three plant communities were significantly larger in growing seasons than in dormancy periods (p < 0.01, Table 3). It is interesting that N2O emissions from the two herbaceous communities (HCK and HCP) were very similar during the entire experimental period (Table 3, Fig. 2a), in spite of different soil moisture contents (p < 0.01, 40.3 ± 5.9% for HCK and 35.9 ± 7.6% for HCP). Nitrous oxide emissions from HCK were significantly larger than from BSK, whereas there was no difference in N2O emission between BSP and HCP (Table 3, Fig. 1a and 2a). For the whole experimental period average N2O emission from the shrub community (SCP) was estimated at 51.6 μg N2O m−2 h−1. This was significantly larger than from either HCK (39.4 ± 2.8 μg N2O m−2 h−1) or HCP (39.8 ± 2.7 μg N2O m−2 h−1; p < 0.01, Table 3).
Seasonal N2O emission (a), soil temperature at 5 cm depth (b) and soil moisture at 10 cm depth (c) from herbaceous communities in the K. humilis (HCK) and the P. fruticosa (HCP) meadows and shrub community in the P. fruticosa (SCP) meadow. Means ± standard errors (SE) of three replicate measurements were presented. No soil moisture data (c) were collected in winter, when soils were frozen
On the whole ecosystem basis K. humilis meadows emitted 295.0 ± 11.6 mg N2O m−2 year−1 while P. fruticosa meadows showed larger N2O emissions, 356.7 ± 8.3 mg N2O m−2 year−1. Nitrous oxide emissions were larger in growing seasons than in dormancy periods in both meadow types. It was 167.0 ± 24.9 vs. 128.0 ± 36.4 mg N2O m−2 in the K. humilis meadow and 227.6 ± 23.2 vs. 129.1 ± 31.5 mg N2O m−2 in the P. fruticosa meadow.
Discussion
As observed by previous studies (Pei et al. 2004), alpine soils are a source of N2O (Table 3). Rates of N2O emission from constructed bare soil plots on both meadow types (BSK, 30 μg N2O m−2 h−1 and BSP, 38 μg N2O m−2 h−1) were five to 47 times more than those from bare patches on alpine steppe soils (0.8 μg N2O m−2 h−1, Pei et al. 2004) and on temperate steppe soils (5.9 μg N2O m−2 h−1, Du et al. 2001). The great difference in N2O emission between these grasslands may be caused by different soil moisture contents, e.g. higher moisture in alpine meadow soils than in alpine steppe soils or temperate steppe soils (Du et al. 2001; Pei et al. 2004). The N2O fluxes in both BSK and BSP were however smaller than in N fertilized agriculture soils of the Northern China Plain (57 μg N2O m−2 h−1, Zhang et al. 2000), indicating that in the latter high N fertilizer inputs may have enhanced emissions of N2O from soils (Del Prado et al. 2006; Stehfest and Bouwman 2006; Velthof and Oenema 1997).
On an ecosystem level grasslands are often regarded as a significant source of N2O (Williams et al. 1999), especially fertilized and grazed grasslands. For these very high rates of N2O emission are often reported (e.g. 179 to 358 μg N2O m−2 h−1, Velthof and Oenema 1997). However, few studies have been conducted to compare the difference in N2O emission between plant communities and bare soils. Our experimental setup allowed us to do this analysis. It showed that plant communities, except HCP in the P. fruticosa meadow, significantly enhanced N2O emission from alpine meadows (Table 3). There are three possible explanations for this. Firstly, root exudates derived from plant photosynthesis may stimulate nitrifying bacteria and denitrifying bacteria responsible for N2O production, because root exudates are rapidly utilized by rhizosphere microorganisms and regulate rhizomicrobial activity (Anderson and Domsch 1985; Kuzyakov and Cheng 2001). Secondly, a growing number of studies have shown that terrestrial plants can emit N2O to the atmosphere (Goshima et al. 1999; Zhang and Jiang 2006; Zou et al. 2005), this may also be true for alpine plants. In this study we made a crude calculation of N2O emissions by plants based on the difference between N2O emission from plant communities and N2O emission from bare soils. Grasses in K. humilis meadows (9 μg N2O m−2 h−1) and shrubs in P. fruticosa meadows (14 μg N2O m−2 h−1) showed strong N2O emissions, while grasses in the P. fruticosa meadows emitted only very little N2O (2 μg N2O m−2 h−1). These values are significantly lower than those observed from the bare soils. Thirdly, soil moisture differences may contribute to the difference in N2O emissions between the plant communities. Soil moisture is an important factor controlling N2O emissions (Du 2006). It was reported for temperate grasslands that nitrification is dominant in N2O emissions because soil moisture was often kept at low water holding capacity (WHC; Du 2006). Denitrification to N2 is dominant when soil moisture reached more than 70% of the WHC (Yu et al. 1995). In this study the average soil moisture content in both HCK and SCP was 40.3 ± 5.9% and 35.9 ± 6.0% and ranged from 24.1 to 50.0% and 16.4 to 49.3% in HCK and SCP, respectively. This was equivalent to about 80% of the WHC in HCK and 35% of the WHC in SCP. High soil moisture content (80% of the WHC) and the optimal soil pH (Table 1) in HCK in theory provide good conditions for denitrification to N2. The significantly lower rate of N2O emission from HCK compared to SCP (Table 3) may be due to denitrification to N2. This suggests that different processes control N2O emissions in both grass and shrub communities. It is possible that nitrification is dominant in SCP while denitrification to N2 is dominant in HCK. However, HCK and HCP showed similar flux rates despite a significant difference in soil moisture at 10 cm depth (p < 0.01, T-test), implying that N2O emissions are more complicated and possibly controlled by multifactor in alpine meadows.
Over the entire experimental period all the treatments showed a significant N2O emission peak in summer 2005 (Fig. 1a, 2a). Low annual precipitation and high annual air temperature may account for a part of variations. However, there was a poor correlation between N2O emission and soil temperature at 5 cm depth in the two bare soil treatments in spite of a strong correlation between them in the three plant communities (Fig. 3). The discrepancy is possibly ascribed to the variation in the nutrient and oxygen concentration profiles of the soils imposed by living roots. Nitrous oxide emission did not show a significant correlation with soil moisture at 10 cm depth as other studies observed (Du 2006), although soil moisture generally plays an important role in N2O emissions.
Kobresia and Potentilla shrub meadows have been suffering from overgrazing in the Tibetan plateau. This leads to degradation of Kobresia meadows to bare areas and of some Potentilla shrub meadows to Kobresia meadows (Klein et al. 2007). Therefore, N2O emissions from alpine meadows may be strongly altered by this process. We have used our data to estimate if alpine meadows and a shift in vegetation type due to grazing pressure would have an impact on N2O emissions at the global scale. For this purpose we assumed that alpine meadows are completely occupied by bare soils or by shrubs. Total N2O emission from alpine meadows in the Tibetan Plateau was estimated to range from 0.2 to 0.4 Tg N2O year−1, with an average of 0.3 Tg N2O year−1. This value is 11% of the estimated global emission from fertilized grasslands (2.8 Tg N2O year−1, Stehfest and Bouwman 2006). This indicates that alpine meadows are not a negligible source of N2O in the Tibet Plateau. Vegetation shift caused by heavy grazing gradually reduces the carbon storage potential of grasslands (Allard et al. 2007) and strongly enhances CH4 and CO2 emissions (Cao et al. 2004), but as this study has shown can decrease N2O emissions from alpine meadows.
Although many studies have shown that alpine meadows are a weak sink of atmospheric CO2 and CH4 (Cao et al. 2004; Wang et al. 2004; Zhao et al. 2007), the contribution of greenhouse gases from terrestrial ecosystems to climate warming should be assessed based on integrated research. The reason is that there may be a trade-off between mitigation of CH4 and N2O emissions and maintenance of carbon sink activity (Soussana et al. 2007). We here made an estimation based on previous studies together with this study. An average amount of 0.2 Tg CH4 year−1 (Cao et al. 2008) and of 46.5 Tg CO2-C year−1 (Kato et al. 2004; Yan et al. 2006; Zhao et al. 2007) have been estimated to be absorbed by alpine meadows in the Tibetan Plateau. Radiative forcing of N2O and CH4 is 298 and 25 times more than of CO2 (IPCC 2007). This means that the whole alpine meadows (more than 8,750 ha) in the Tibet Plateau emit 60.0 Tg CO2-eq year−1 and take up 4.8 Tg CO2-eq year−1. Consequently, Tibetan alpine meadows contribute 94 Tg CO2-eq year−1 to the atmosphere. This clearly implies that an accurate assessment of the contribution of terrestrial ecosystems to climate warming needs to be based on the simultaneous flux rates of all three greenhouse gases, CO2, N2O and CH4.
References
Adams JM, Faire H, Faire-Richard L, Mcglade JM, Woodward FI (1990) Increases in terrestrial carbon storage from the last glacial maximum to the present. Nature 348:711–714
Allard V, Soussana JF, Falcimagne R, Berbigier P, Bonnefond JM, Ceschia E et al (2007) The role of grazing management for the net biome productivity and greenhouse gas budget (CO2, N2O and CH4) of semi-natural grassland. Agric Ecosyst Environ 121:47–58
Anderson TH, Domsch KH (1985) Determination of ecophysiological maintenance carbon requirements of soil microorganisms in a dormant state. Biol Fertil Soils 1:81–89
Bao XK, Cao GM, Gao YX (1995) Accumulation of organic matter in Cryo-sod soil. Approach on Chinese Soil Taxonomy 152–160
Bouwman AF (1996) Direct emission of nitrous oxide from agricultural soils. Nutr Cycl Agroecosyst 46:53–70
Cao GM, Li YN, Bao XK (1998) The feature of moisture retention for high Mountain soil. Soil 130:27–30
Cao GM, Tang YH, Mo WH, Wang YS, Li YN, Zhao XQ (2004) Grazing intensity alters soil respiration in an alpine meadow on the Tibetan plateau. Soil Biol Biochem 36:237–243
Cao GM, Long RJ, Xu XL, Wang QL, Wang CT, Du YG et al (2008) Annual methane emissions from two alpine meadow ecosystems in the Qinghai–Tibet Plateau. Soil Biol Biochem (in press).
Chinese Soil Taxonomy Research Group (1995) Chinese Soil Taxonomy, pp 58–147
Crutzen PJ (1981) Atmospheric chemical processes of the oxides of nitrogen including nitrous oxide. In: Delwiche CC (ed) Denitrification, nitrification and atmospheric N2O. Wiley, Chichester, pp 17–44
Del Prado A, Merino P, Estavillo JM, Pinto M, Gonzalez-Murua G (2006) N2O and NO emissions from different N source and under a range of soil water contents. Nutr Cycl Agroecosyst 74(3):229–243
Du R (2006) Effects of soil moisture and temperature on N2O production rate of meadow grassland soil. Chin J Appl Ecol 17(11):2170–2174
Du R, Wang GC, Lù DR (2001) Effect of grazing on microbiological process of N2O production in grassland soils. Environ Sci 22(4):11–15
Finlayson-Pitts BJ Pitts Jr (2000) Chemistry of the upper and lower atmosphere. Academic, San Diego, CA 969
Flessa H, Ruser R, Schilling R, Loftfield N, Munch JC, Kaiser EA et al (2002) N2O and CH4 fluxes in potato fields: automated measurement, management effects and temporal variation. Geoderma 106:307–325
Flückiger J, Dällenbach A, Blunier T, Stauffer B, Stocker TF, Raynaud D et al (1999) Variations in atmospheric N2O concentration during abrupt climate changes. Science 285:227–230
Goshima N, Mukai T, Suemori M, Takahashi M, Caboche M, Morikawa H (1999) Emission of nitrous oxide (N2O) from transgenic tobacco expressing antisense NiR mRNA. Plant J 19:75–80
Houghton JT, Callander BA, Varney SK (1992) Clim Change :1992. the supplementary report to the IPCC scientific assessment. Cambridge University Press.
Houghton JT, Meiro Filho LG, Callander BA, Harris N, Kattenburg A, Maskell K (1995) Climate Change 1995: The Science of Climate Change Cambridge University Press.
IPCC (2007) Technical Summary. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate Change 2007: The physical science basis. Contribution of Working Group 1 to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom
Kato T, Tang YH, Gu S, Cui XY, Hirota M, Du MY et al (2004) Carbon dioxide exchange between the atmosphere and an alpine meadow ecosystem on the Qinghai–Tibetan Plateau, China. Agric For Meteorol 124:121–134
Klein JA, Harte J, Zhao XQ (2007) Experimental warming, not grazing, decreases rangeland quality on the Tibetan plateau. Ecol Appl 17(2):541–557
Kuzyakov Y, Cheng W (2001) Photosynthesis controls of rhizosphere respiration and organic matter decomposition. Soil Biol Biochem 33(14):1915–1925
Li YN, Zhao XQ, Cao GM, Zhao L, Wang QX (2004) Analyses on climates and vegetation productivity background at Haibei alpine meadow ecosystem research station. Plat Meteorol 23:558–567
Mosier AR (1994) Nitrous oxide emissions from agriculture soils. Fert Res 37:191–200
Oenema O, Gebauer G, Rodriguez M (1998) Controlling nitrous oxide emissions from grassland livestock production systems. Nutr Cycl Agroecosyst 52:141–149
Pei ZY, Ouyang H, Zhou CP, Xu XL (2004) N2O exchange within a soil and atmosphere profile in alpine grasslands on the Qinghai-Xining Plateau. Acta Bot Sin 46:20–28
Soussana JF, Allard V, Pilegaard K, Ambus P, Amman C, Campbell C et al (2007) Full accounting of the greenhouse gas (CO2, N2O, CH4) budget of nine European grassland sites. Agric Ecosyst Environ 121:121–134
Stehfest E, Bouwman L (2006) N2O and NO emission from agricultural fields and soils under natural vegetation: summarizing available measurement data and modeling of global annual emissions. Nutr Cycl Agroecosyst 74:207–228
Velthof GL, Oenema O (1997) Nitrous oxide emission from dairy farming systems in the Netherlands. J Agric Sci 46:257–267
Verville JH, Hobbie SE, ChapinIii FS (1998) Response of tundra CH4 and CO2 flux to manipulation of temperature and vegetation. Biogeochemistry 41:215–235
Wang YS, Wang YH (2003) Quick measurement of CH4, CO2 and N2O from a short-plant ecosystem. Adv Atmos Sci 22:842–844
Wang YS, Ji BM, Huang Y (2001) Effects of grazing and cultivating on emission of nitrous oxide, carbon dioxide and uptake of methane from grasslands. Environ Sci 22:7–13
Wang YS, Liu GR, Wang YH, Sun Y, Xue M (2003) Simultaneous measurement of CO2, CH4 and N2O emission from terrestrial ecosystem with one improved gas chromatography. Tech Equip Environ Pollut Contr 4:84–90
Wang CK, Lu XG, Zhou HR, Cai ZC, Luo Y (2004) Studies on methane oxidation by bog soils in Zoige Plateau. China Environ Sci 24:646–649
Williams DL, Ineson P, Coward PA (1999) Temporal variations in nitrous oxide fluxes from urine-affected grassland. Soil Biol Biochem 31:779–788
Xu-Ri, Wang YS, Zheng XH, Ji BM, Wang MX (2003) A comparison between measured and modeled N2O emissions from Inner Mongolian semi-arid grassland. Plant Soil 255:513–528
Yan W, Zhang XZ, Shi PL, Yang ZL, He YT, Xu LL (2006) Net ecosystem carbon dioxide exchange and water use efficiency of alpine meadow in the Tibetan Plateau. J Nat Res 21(5):756–767
Yu KW, Chen GX, Yang SH (1995) The role of several upland crops in N2O emission from farmland and its response to environmental factors. Chin J App Ecol 6(4):387–391
Zhang XJ, Jiang PW (2006) Advance in the research of N2O emission from plants. J Shenyang Coll Edu 8(1):119–121
Zhang S, Qi YC, Manfred D, Dieter S, Dong YS (2000) N2O emissions from agricultural soils in the North China Plain: the effect of chemical nitrogen fertilizer and organic manure. Environ Sci 12(4):463–468
Zhao L, Gu S, Xu SX, Zhao XQ, Li YN (2007) Carbon flux and controlling process of alpine meadow on Qinghai–Tibetan Plateau. Acta Bot Boreal Occident Sin 27(5):1054–1060
Zheng D, Zhu L (2000) Formation and Evolution, Environmental Changes and Sustainable Development on the Tibetan Plateau. Beijing Academy Press, Beijing
Zhou XM, Wu ZL (2006) Vegetation and Plants Searchers List on The Haibei Research Station of Alpine Meadow Ecosystem, the Chinese Academy of Science. Qinghai People's Press
Zou JW, Huang Y, Sun WJ, Zheng XH, Wang YS (2005) Contribution of plants to N2O emissions in soil-winter wheat ecosystem: pot and field experiments. Plant Soil 269:205–211
Acknowledgments
We thank Qiwu Hu, Qin Wu, Dong Li, Quande Yang, Jianzhen Liu and Funian Zhang for their sampling and measurement of nitrous oxide concentration. We also thank the two anonymous reviewers for their constructive comments. This work was supported by National Natural Science Key Foundation of China (30730069), GEF program CHA-GS-Y-4, the Knowledge Innovation Program of Chinese Academy of Sciences (KZCX1-SW-01) and National Natural Science Foundation of China (40471133).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ute Skiba.
Rights and permissions
About this article
Cite this article
Du, Y., Cui, Y., Xu, X. et al. Nitrous oxide emissions from two alpine meadows in the Qinghai–Tibetan Plateau. Plant Soil 311, 245–254 (2008). https://doi.org/10.1007/s11104-008-9727-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9727-9