Abstract
Modern durum wheat (AABB) is more sensitive to zinc (Zn) deficiency than bread wheat (AABBDD). One strategy to increase productivity and expansion of durum wheat industry in Zn-deficient soils is to improve its ability to grow and yield in such soils. This ability is termed Zn efficiency. In a growth room experiment using soil culture, we assessed the potential of Triticum turgidum L. subsp. dicoccon (Shrank) Thell. (domesticated emmer wheat, AABB) as a genetic resource for further improvement of Zn efficiency in modern durum wheat. Twenty four accessions of domesticated emmer wheat, four durum landraces/cultivars, and two bread wheat cultivars/ advanced breeders lines of known Zn efficiency were tested under Zn deficiency and Zn sufficiency. Significant variation was observed among genotypes in Zn deficiency symptoms, dry matter production, shoot Zn concentration, shoot Zn content and Zn utilisation efficiency (physiological efficiency). We identified domesticated emmer wheat accessions with greater Zn efficiency than modern durum wheat and even bread wheat genotypes. These accessions could be used in breeding programs to improve Zn efficiency of durum wheat. The results suggest that Zn efficiency of durum or bread wheat is likely to be determined collectively by its progenitors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Durum wheat, Triticum turgidum subsp. durum (Desf.) Husn. (2n = 4x = 28, AABB), is a high quality wheat that is predominantly used in making pasta and semolina flour. The major durum producing regions are the European Union, Canada, United States, Turkey, Syria, Kazakhstan, India, Australia and Mexico (Anonymous 2001). Durum wheat production in Australia is relatively small but it has increased rapidly from the mid 1990s with production averaging 500,000 tonnes per year (http://www.daff.gov.au). It is primarily grown in New South Wales and South Australia, and expanding to Queensland and Western Australia.
Deficiencies in micronutrients characterize a number of the regions where durum wheat is grown, but particularly on calcareous and alkaline soils that occur in southern Australia, Turkey and west Asia (Alloway 2006; http://www.zinc-crops.org). A survey of the nutrient status of soils in 30 countries, carried out for the Food and Agriculture Organisation of the United Nations between 1974 and 1982, found that 30% of the soils were Zn-deficient (Sillanpää 1982). A follow up study with field experiments in 15 countries found that Zn deficiency had a higher frequency (in about half of the total of 190 field sites) than deficiencies of any of the other six micronutrients included in the experiments (Sillanpää 1990).
Durum wheat is generally more sensitive to micronutrient deficiencies than bread wheat, including zinc (Graham et al. 1992; Cakmak et al. 1997) and manganese (Khabaz-Saberi et al. 2002), which causes significant reductions in yield. One strategy to tackle these nutritional problems is to improve the ability of durum wheat to grow and yield well in soils too deficient for a standard genotype, which is termed as nutrient efficient genotype (Graham 1984). This sustainable approach to overcoming a nutritional limitation offers the potential for increased productivity and further expansion of the durum industry in southern Australia and elsewhere. However, development of nutrient efficient cultivars requires (1) a robust screening method, (2) genetic variation, and (3) understanding of genetic control of this trait. There are already a number of screening methods (e.g. soil, solution and field) available for use in various crop species (Rengel and Graham 1996; Cakmak et al. 1997; Genc et al. 2002). Although durum wheat has been considered the least Zn-efficient of all cereal species (Cakmak et al. 1998; Ekiz et al. 1998), from a limited number of studies, there appears genetic variation in Zn efficiency amongst its wild relatives (Zn efficiency range of 17–53%; Cakmak et al. 1999a), landraces and cultivars (30–50%; Cakmak et al. 2001). However, the lower Zn efficiency in durum wheat (36%) than in bread wheat (65%) in the former study, and Zn efficiency in the range of 20–50% in the latter study clearly indicates that there is potential to improve further the current levels of Zn efficiency in durum wheat. Recently, in our work with synthetic hexaploid wheats (2n = 6x = 42, AABBDD), we identified synthetic hexaploid accessions with significantly greater Zn efficiency than in modern bread wheat genotypes (Genc and McDonald 2004). This result led us to consider alternative sources such as domesticated emmer wheat [T. turgidum L. subsp. dicoccon (Shrank) Thell.] as a candidate for improvement of Zn efficiency in modern durum wheat, which was the subject of the present study.
Materials and methods
A Zn-deficient siliceous sand (DTPA-extractable Zn = 0.08 mg kg−1 soil), collected near Mount Compass (South Australia), was used in the experiment. Soil preparation, packing into polyethylene-lined cylindrical PVC pots and nutrient additions were as described previously (Genc and McDonald 2004). The basal nutrients (in mg kg-1 dry soil) were as follows: NH4NO3 (350), KH2PO4 (75), K2SO4 (120), MgSO4·7H2O (90), MnSO4·H2O (7), CuSO4·5H2O (5), H3BO3 (0.5), CoSO4·7H2O (1); FeSO4·7H2O (15.8), H2MoO4·H2O (0.5), and NiSO4·6H2O (0.15). There were two Zn treatments: 0.05 and 1 mg kg−1 dry soil (deficient and adequate, respectively).
Twenty four accessions (acc.) of domesticated emmer wheat, three durum cultivars, one durum landrace, one bread wheat cultivar and one bread wheat advanced breeder’s line of known Zn efficiency (Table 1) were multiplied in a potting mix (Barker et al. 1998) prior to the experiment. This was done in order to minimize variation in Zn seed content amongst the genotypes studied since high seed Zn content can significantly affect growth under Zn- deficient conditions (Rengel and Graham 1995a, b; Genc et al. 2000). Seed treatment, germination and transplanting into pots were the same as before (Genc and McDonald 2004). The average kernel weight of the accessions was 38.9 mg and ranged from 32.9 to 47.9 mg. Seed Zn concentration was not affected by kernel weight (r = 0.05, n = 24).
Plants were grown in a growth cabinet at 20/15°C day/night temperature, 14/10 light/dark cycle, and 300 μmol m−2 s light intensity at plant height. At 36 days after transplanting (DAT), the whole tops (all above-ground matter) were harvested. The samples were oven-dried at 65°C for 48 h and weighed. Nutrient analyses of plant samples were performed using Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES; Zarcinas et al. 1987).
Visual symptoms of Zn deficiency were recorded using a scale of 1 (no symptoms) to 8 (severe symptoms; see Genc and McDonald (2004) for detailed description of the scale) between the time when they first became visible and harvest. Zn efficiency (relative shoot growth) was calculated as the ratio of shoot dry matter at deficient Zn supply to that at adequate Zn supply, and expressed as percent. Shoot Zn content was determined by multiplying shoot dry matter with Zn concentration in the shoot. Zn utilisation efficiency, shoot dry matter produced per unit of shoot Zn concentration [g DM/(mg Zn kg−1 DW)] (Genc et al. 2006) was used as an index to assess genotypic variation in efficiency of utilisation of Zn at the whole-plant level.
The experiment was set up as a completely randomized block design with three replicates. Results were analysed by GENSTAT (Windows Version), and Tukey’s Honestly Significant Differences (HSD) at P = 0.05 was used in pairwise comparisons of means (Steel and Torrie 1960). To overcome non-homogeneity of variances, data were transformed where necessary prior to analysis of variance.
Results
Visual deficiency symptoms
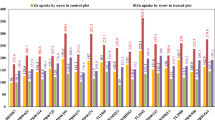
As early as 21 DAT, symptoms of Zn deficiency (e.g. linear chlorotic stripes visible first on young leaves) became apparent in emmer wheat acc. 3734 and 3740. At this point, similar symptoms were evident in durum wheat cultivars Tamaroi and Kalka, while the bread wheat genotypes showed either reduction in growth (RAC875-2) or no symptoms (Stylet). As expected, deficiency symptoms became more severe as growth progressed, and at harvest (36 DAT), there was a range of deficiency symptoms across the genotypes studied (Fig. 1): no visible symptoms in acc. 3735, 3738, 19385 and 22286, severe symptoms in acc. 3734, 3740 and 3804 and moderate symptoms in the remaining accessions. Of the durum cultivars, Kalka, Lagost-3 and Chacan showed slight to moderate symptoms, while Tamaroi developed severe symptoms. As for bread wheat, deficiency symptoms were slight in Zn-efficient Stylet, but severe in Zn-inefficient RAC875-2.
Shoot dry matter and zinc efficiency
Shoot dry matter was influenced by genotype and Zn fertilization, and significant genetic differences were evident at both deficient and adequate Zn supply. Shoot dry matter ranged from 0.755 g plant−1 in Chacan to 1.690 g plant−1 in 19385 at deficient Zn supply, and 0.993 g plant−1 in Chacan to 2.168 g plant−1 in Lagost-3 at adequate Zn supply. Interestingly, shoot dry matter of some accessions (19385 and 22287) at deficient Zn supply was still higher than other accessions (22286, 22292 and 22293) at adequate Zn supply (Fig. 2). The majority of emmer wheat accessions had higher shoot dry matter than the durum cultivars under deficient and adequate Zn supply. There was a strong and positive relationship between shoot dry matter at deficient and adequate Zn supply (r = 0.792, P < 0.001, n = 30; Fig. 3).
The relationship between shoot dry matter at deficient (Zn0.05) and adequate Zn (Zn1) supply in emmer wheat accessions, landraces, cultivars, and modern genotypes of durum and bread wheat at 36 DAT (r = 0.792, P < 0.001, n = 30). Empty circles indicate genotypes which are Zn efficient and also responsive to Zn fertilizer (acc. 19385 and 22287) and those which are Zn efficient but not responsive to Zn fertilizer (acc. 22286 and 22293), while closed circles represent remainder of genotypes studied
Zn efficiency ranged from 68% in 3734 to 97% in 22286 (Fig. 2). Similar to absolute shoot dry matter responses, some of the emmer wheat accessions had greater Zn efficiency (≥95%) than the durum cultivars (75–78%) and even the Zn-efficient bread wheat Stylet (88%).
Zn concentration and content in the shoots
Large genotypic differences in shoot Zn concentration were measured under low Zn supply (6–12 mg Zn kg−1 DW) and at adequate Zn supply (40–90 mg Zn kg−1 DW) (Table 2). At deficient Zn, acc. 3717 had the lowest concentration (5.7 mg kg−1 DW), and the Zn concentration in the majority of genotypes ranged from 7–9 mg kg−1 DW except for the durum genotypes Kalka and Chacan which had slightly higher values (11–12 mg kg−1 DW). At adequate Zn, acc. 22286 had a higher Zn concentration (90 mg kg−1 DW) than acc. 3731, 21758, 3717, 3740, 3734, modern durum wheat Lagost-3 and modern bread wheat RAC875-2 (<60 mg kg−1 DW), while the remaining genotypes had intermediate concentrations.
Zinc content followed a similar pattern to that of Zn concentration, and ranged from 8 μg plant−1 in acc. 22293 to 14 μg plant−1 in acc. 19385, and 73 μg plant−1 in Chacan to 137 μg plant−1 in acc. 19385 at deficient and adequate Zn, respectively. At deficient Zn supply, acc. 3717, 3804, 19592, 22292, 22293 and Chacan had accumulated lower Zn than the remaining entries, while acc. 3717, 3740, 17643, 22293, 21758, Chacan, Lagost-3 and RAC875-2 were amongst the low Zn accumulating genotypes at adequate Zn supply (Table 2).
Zn utilization efficiency
The ability of genotypes to produce shoot dry matter at a given tissue Zn concentration (utilization) also varied with Zn fertilization, and was five-fold greater at deficient than sufficient Zn supply (Table 2). Under Zn deficiency, acc. 3735, 3804, 22286, 22290, 22293, Kalka, Chacan, Tamaroi, RAC875-2 and Stylet had lower utilization than the remaining genotypes. Under Zn sufficiency, acc. 22286, 22290, 22293, Chacan, Tamaroi and Stylet achieved higher utilization than the other genotypes.
Relationships with Zn efficiency
Of the traits that were assessed, visual symptoms of Zn deficiency and kernel weight were significantly and negatively correlated with Zn efficiency (Table 3). Genotypes showing fewer symptoms tended to have high Zn efficiency, while lines with smaller kernel weights also tended to be more efficient. There was no significant correlation between Zn efficiency and seed Zn concentration.
Discussion
This study demonstrated considerable genotypic variation in Zn efficiency within the domesticated emmer wheat. The rationale for choosing the domesticated emmer wheat over its progenitor, wild emmer wheat [T. turgidum subsp. dicoccoides (Körn.) Thell.)], as a candidate germplasm was the better agronomic attributes of the former. If the former were shown to possess higher Zn efficiency than durum wheat, from a breeding point of view it would be a better candidate for improving Zn efficiency of durum wheat. However, further improvement of Zn efficiency beyond the levels of domesticated emmer wheat would depend on other genetic resources such as wild emmer wheat, which has been shown to be an important source of Zn efficiency for cultivated wheats (Peleg et al. 2008).
Genotypic variation within the domesticated emmer wheat appeared to be mainly genetic and not affected by differences in seed Zn content as evidenced by the lack of correlation between seed Zn content and Zn efficiency among the accessions (r = 0.161, n = 24). A few accessions had Zn efficiency higher than modern durum cultivars and the Zn-efficient bread wheat (Stylet). Earlier studies reported durum wheat to be the least Zn-efficient of all cereal species, and this was partly attributed to lack of the D genome (Aegilops tauschii; Cakmak et al. 1999a). However, further studies in Aegilops tauschii (DD) demonstrated genetic variation in Zn efficiency within this species as well (Cakmak et al. 1999b; Lisa Merry, personal communication). In the present study, the existence of Zn-inefficient bread wheat genotypes (Genc et al. 2006) despite the presence of the D genome, and equivalent or greater Zn efficiency in some tetraploid accessions compared to bread wheat in the present study all indicate that the D genome may not necessarily be the only source of Zn efficiency. It is more likely that Zn efficiency in a given bread wheat genotype is collectively determined by both ancestors, Ae. tauschii and T. turgidum.
Even with the precaution of growing all the lines under standard conditions in the glasshouse to produce uniform seed, there was a range in seed Zn concentrations among the 28 T. durum genotypes. This suggests there is genetic variation in grain Zn concentration among these durum accessions which may partly be related to differences in grain yield (McDonald et al. 2008). However, the yield of the lines was not measured so the magnitude of this effect is not known. Seed Zn concentration can be important to the early growth of seedlings growing under low soil Zn (Genc et al. 2000) and may influence the assessment of Zn efficiency. However, seed Zn concentration and Zn efficiency were unrelated in these durum lines, suggesting that high Zn efficiency may not necessarily result in increased grain Zn concentrations. This lack of relationship has been demonstrated previously at the phenotypic level (Cakmak et al. 2004) and suggests that Zn efficiency and high seed Zn concentration are mainly under independent genetic control. Consequently breeding for these two traits should be considered as separate breeding objectives.
Emmer wheat accessions with high Zn efficiency can be exploited in a breeding program to improve the current levels of Zn efficiency in durum cultivars. To this end, acc. 3717, 19385 and 22287 are currently being backcrossed to advanced breeder’s lines in order to improve Zn efficiency of adapted material. At present, the assessment of Zn efficiency in a breeding sense is based on visual scores and/or shoot dry matter production. However, this type of assessment can be time consuming and expensive when a large number of individuals are considered, therefore, identification of physiological traits associated with Zn efficiency and closely linked molecular markers in the near future would help speed up the breeding for this complex trait.
The higher Zn efficiency of emmer wheat accessions can also be transferred to hexaploid bread wheat through bridge crosses “synthetic hexaploid wheat” derived from the cross between Zn-efficient emmer wheat and Zn-efficient Ae. tauschii. However, this targeted breeding approach requires screening of a large number of accessions of both species for identification of sources of Zn efficiency. In such screening studies, it is important to remember that donors should be selected based on their performance under both Zn deficient and Zn sufficient conditions. It is obvious that the genotypes which are high yielding under Zn deficiency and also responsive to Zn fertilizer (acc. 19385 and 22287) are highly desirable for cropping on Zn-deficient soils (Cakmak et al. 2001) (Fig. 3), while those with high Zn efficiency simply due to low yield potential under Zn sufficiency are not (acc. 22286 and 22293).
Genotypes with high yield under Zn deficiency, and also responsive to Zn fertilizers can be identified simultaneously by the two level testing where the second level aims to identify Zn-efficient and responsive genotypes (Fig. 3). However, the two-level testing may be costly and difficult to manage in breeding programs where large numbers of individuals are handled. In such circumstances, an initial low Zn screening based on visual symptoms of Zn deficiency is recommended since there was a good correlation between Zn efficiency and visual scores of severity of Zn deficiency (Table 3) and elsewhere (Cakmak et al. 1998; Genc et al. 2006).
Seedling test is most useful in breeding programs where large numbers of individuals are screened in a short period of time and space. Obviously, the rate of success depends on how well the seedling test is able to predict yield responses. Previous studies in bread wheat (Kalayci et al. 1999) and barley (Genc et al. 2002) found significant correlations between seedling responses and yield, although not all genotypes behaved in the same manner. A few genotypes had higher Zn efficiency at the seedling stage than maturity or vice versa, which not only reduces reliability of the seedling test but results in some misclassification of Zn efficiency (Genc et al. 2002). However, this must be considered acceptable as long as some efficient genotypes are identified and enter the crossing program or the next generation (Graham 1984). Although speculative, as there were no yield data, we would expect a reasonable agreement between the seedling responses and yield had the study been extended to maturity.
Zn efficiency in a given genotype is determined collectively by a number of Zn efficiency mechanisms such as Zn uptake by the roots, translocation to the shoots and physiological efficiency (utilization) (Rengel 1999; Gao et al. 2005; Genc et al. 2006). In the present study we did not sample the roots, thus could not determine Zn uptake and translocation to the shoots. Therefore, it is not possible to estimate the relative importance of these individual components in overall Zn efficiency. However, it is likely that Zn uptake is also a major determinant of Zn efficiency in emmer wheat as shown in several other cereal species (Graham and Rengel 1993; Cakmak et al. 1998; Gao et al. 2005; Genc et al. 2006). We estimated physiological efficiency which indicates the ability to maintain metabolic functions with limited Zn. Physiological efficiency differed among the accessions, and explained only 13% of the variation in Zn efficiency suggesting a small contribution to Zn efficiency as reported in bread wheat (Genc et al. 2006).
In conclusion, domesticated emmer wheat is an important genetic resource of Zn efficiency for improvement of the current levels of Zn efficiency in durum wheat. Only 24 accessions were tested in the present study, and testing of more accessions in the future may reveal higher Zn efficiency values than those identified here. In addition, seedling responses measured in the present study need to be confirmed at maturity in future studies.
References
Alloway BJ (2006) Zinc in soils and crop nutrition. http://www.zinccrops.org/Crops/Alloway-all.php
Anonymous (2001) Durum wheat: outlook, Bi-wkly Bull 14:20
Barker SJ, Stummer B, Gao L, Dsipain I, O’Conner PJ, Smith SE (1998) A mutant in Lycopersicon esculentum Mill. with highly reduced VA mycorrhizal colonisation: isolation and preliminary characterization. Plant J 15:791–797
Cakmak I, Ekiz H, Yilmaz A, Torun B, Koleli N, Gultekin I, Alkan A, Eker S (1997) Differential response of rye, triticale, bread and durum wheats to zinc deficiency in calcareous soils. Plant Soil 188:1–10
Cakmak I, Torun B, Erenoglu B, Ozturk L, Marschner H, Kalayci M, Ekiz H, Yilmaz A (1998) Morphological and physiological differences in the response of cereals to zinc deficiency. Euphytica 100:349–357
Cakmak I, Tolay I, Ozdemir A, Ozkan H, Ozturk L, Kling CI (1999a) Differences in zinc efficiency among and within diploid, tetraploid and hexaploid wheats. Ann Bot 84:163–171
Cakmak I, Tolay I, Ozkan H, Ozdemir A, Braun HJ (1999b) Variation in zinc efficiency among and within Aegilops species. J Plant Nutr Soil Sci 162:257–262
Cakmak O, Ozturk L, Karanlik S, Ozkan H, Kaya Z, Cakmak I (2001) Tolerance of 65 durum wheat genotypes to zinc deficiency in a calcareous soil. J Plant Nutr 24:1831–1847
Cakmak I, Torun A, Millet E, Feldman M, Fahima T, Korol A, Nevo E, Braun HJ, Ozkan H (2004) Triticum dicoccoides: An Important genetic resource for increasing zinc and iron concentration in modern cultivated wheat. Soil Sci Plant Nutr 50:1047–1054
Ekiz H, Bagci SA, Kiral AS, Eker S, Gültekin I, Alkan A, Cakmak I (1998) Effects of zinc fertilization and irrigation on grain yield and zinc concentration of various cereals grown in zinc-deficient calcareous soils. J Plant Nutr 21:2245–2256
Gao X, Zou C, Zhang F, van der Zee SEATM, Hoffland E (2005) Tolerance to zinc deficiency in rice correlates with zinc uptake and translocation. Plant Soil 278:253–261
Genc Y, McDonald GK (2004) The potential of synthetic hexaploid wheats to improve zinc efficiency in modern bread wheat. Plant Soil 262:23–32
Genc Y, McDonald GK, Graham RD (2000) Effect of seed zinc content on early growth of barley (Hordeum vulgare L.) under low and adequate soil zinc supply. Australian Journal of Agricultural Research 51:37–45
Genc Y, McDonald GK, Graham RD (2002) A soil-based method to screen for zinc efficiency in seedlings and its ability to predict yield responses to zinc. Australian Journal of Agricultural Research 53:409–421
Genc Y, McDonald GK, Graham RD (2006) Contribution of different mechanisms to zinc efficiency in bread wheat during early vegetative stage. Plant Soil 281:353–367
Graham RD (1984) Breeding for nutritional characteristics in cereals. Adv Plant Nutr 1:57–102
Graham RD, Rengel Z (1993) Genotypic variation in zinc uptake and utilization by plants. In: Robson AD (ed) Zn in soils and plants. Kluwer, Dordrecht, The Netherlands, pp 107–114
Graham RD, Ascher JS, Hynes CS (1992) Selecting for Zn-efficient cereal genotypes for soils of low zinc status. Plant Soil 146:241–250
Kalayci M, Torun B, Eker S, Aydin M, Ozturk L, Cakmak I (1999) Grain yield, zinc efficiency and zinc concentration of wheat cultivars grown in a zinc-deficient calcareous soil in field and greenhouse. Field Crops Res 63:87–98
Khabaz-Saberi H, Graham RD, Pallotta MA, Rathjen AJ, Williams KJ (2002) Genetic markers for manganese efficiency in durum wheat. Plant Breed 121:224–227
McDonald GK, Genc Y, Graham RD (2008) A simple method to evaluate genetic variation in grain zinc concentration by correcting for differences in grain yield. Plant Soil 306:49–55
Peleg Z, Saranga Y, Yazici A, Fahima T, Ozturk L, Cakmak I (2008) Grain zinc, iron and protein concentrations and zinc efficiency in wild emmer wheat under contrasting irrigation regimes. Plant Soil 306:57–67
Rengel Z (1999) Physiological mechanisms underlying differential nutrient efficiency of crop genotypes. In: Rengel Z (ed) Mineral nutrition of crops-fundamental mechanisms and implications. Food Products, New York, pp 227–265
Rengel Z, Graham RD (1995a) Importance of seed Zn content for growth on Zn-deficient soil. I. Vegetative growth. Plant Soil 173:259–266
Rengel Z, Graham RD (1995b) Importance of seed Zn content for growth on Zn-deficient soil. II. Grain yield. Plant and Soil 173:267–274
Rengel Z, Graham RD (1996) Uptake of Zn from chelate-buffered nutrient solutions by wheat genotypes differing in Zn efficiency. J Exp Bot 47:217–226
Sillanpää M (1982) Micronutrients and the nutrient status of soils: a global study. FAO Soils Bulletin No. 48, FAO, Rome
Sillanpää M (1990) Micronutrient Assessment at Country Level: An International Study. FAO Soils Bulletin No. 63, FAO, Rome
Steel RGD, Torrie JH (1960) Principles and procedures of statistics. McGraw-Hill, New York
Zarcinas BA, Cartwright B, Spouncer LR (1987) Nitric acid digestion and multi-element analysis of plant material by inductively coupled plasma spectrometry. Commun Soil Sci Plant Anal 18:131–146
Acknowledgements
We wish to thank Mrs. Teresa Fowles and Mr. Lyndon Palmer (Waite Analytical Services, The University of Adelaide) for their help with ICP-OES analysis and Dr. Hugh Wallwork (South Australian Research and Development Institute) for kindly providing the seed of emmer wheat accessions used in this study. We greatly appreciate constructive comments from the editor and anonymous reviewers. This work was supported by Grains Research and Development Corporation, Molecular Plant Breeding Cooperative Research Centre, and The University of Adelaide, Australia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ismail Cakmak.
Rights and permissions
About this article
Cite this article
Genc, Y., McDonald, G.K. Domesticated emmer wheat [T. turgidum L. subsp. dicoccon (Schrank) Thell.] as a source for improvement of zinc efficiency in durum wheat. Plant Soil 310, 67–75 (2008). https://doi.org/10.1007/s11104-008-9630-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9630-4