Abstract
The impact of oxygen (O2) input at the soil surface and in the rhizosphere of rice (Oryza sativa L.) on the spatial and temporal dynamics of arsenic (As) was investigated in a flooded paddy soil. A soil microcosm and root-mat technique were designed to mimic submerged conditions of paddy fields. Water-filled containers with (planted) or without (unplanted) 27-day-old rice seedlings were fitted for 20 days on top of microcosms containing an As-affected soil (Bangladesh). After the initial establishment of strongly reduced conditions (−230 mV) in both planted and unplanted soils, the redox potential gradually increased until the day 8 to reach + 50 mV at 2 mm from the surface of unplanted soils only. This oxidation was associated with an accumulation of NH4-oxalate extractable As (25.7 mg kg−1) in the 0.5-mm top layer, i.e. at levels above the initial total content of As in the soil (14 mg kg−1) and a subsequent depletion of As in soil solution at 2 mm from soil surface. Root O2-leakage induced the formation of an iron (Fe) plaque in root apoplast, with no evidence of outer rhizosphere oxidation. Arsenic content reached 173 mg kg−1 in the Fe plaque. This accumulation induced a depletion of As in soil solution over several millimetres in the rhizosphere. Arsenic contents in root symplast and shoots (112 and 2.3 mg kg−1, respectively) were significantly lower than in Fe plaque. Despite a large As concentration in soil solution, Fe plaque appeared highly efficient to sequester As and to restrict As acquisition by rice. The oxidation-mediated accumulation of As in the Fe plaque and in the oxidised layer at the top of the soil mobilised 21 and 3% of the initial amount of As in the planted and unplanted soils, respectively. Soil solution As concentration steadily decreased during the last 16 days of the soil stage, likely indicating a decrease in the ability of the soil to re-supply As from the solid-phase to the solution. The driving force of As dynamic in soil was therefore attributed to the As diffusion from reduced to oxidised soil layers. These results suggest a large mobility of As in the soil during the flooded period, controlled by the setting of oxic/anoxic interfaces at the surface of soil in contact with flooding water and in the rhizosphere of rice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As)-rich groundwater is widely used in Southeast Asia for the irrigation of submerged rice (Oryza sativa L.) during the dry season leading to an increase of As content in paddy soils (van Geen et al. 2006). This practice leads to As accumulation in rice grains and may contribute to 17–30% of As intake by population in As-affected area of Bangladesh (Meharg and Rahman 2003).
In flooded paddy soils, the As enrichment of plant is attributed to the high mobility of arsenite [As(III)], the main As species in soil solution under reduced conditions. Arsenic speciation and dynamics are mainly controlled by redox potential, through the reductive dissolution of iron (Fe) and manganese (Mn) oxides (Masscheleyn et al. 1991; Takahashi et al. 2004; van Geen et al. 2004). The term Fe (and Mn) oxide will be used here in the broad sense given by Cornel and Schwertmann (2003) to account for all possible Fe (and Mn) oxides, oxyhydroxides and hydroxides that occur in soils. The redox status of flooded paddy soils is far from homogeneous. Liesack et al. (2000) therefore described two oxidised compartments at micro-scale, the surface of soil and the rhizosphere of rice, involving the setting of two oxic/anoxic interfaces.
Micro-scale profiles of oxygen (O2) at the surface of unplanted flooded paddy soils were characterised by a complete depletion of O2 between 2 to 2.5-mm depth, which was attributed to the active micro-organism respiration and the low diffusivity of O2 in the surface water layer (Revsbech et al. 1999; Lüdemann et al. 2000). This oxidation at the soil surface induced specific gradients of Fe(II) and Fe(III) in the soil solid-phase (Ratering and Schnell 2000), underlying the control of the dynamic of Fe in the soil profile through oxidation and precipitation–dissolution processes.
Similar O2 input was observed in the rhizosphere of rice growing in submerged paddy soils and was due to O2 leakage from roots (Ando et al. 1983). This root-induced oxidation results in the precipitation of Fe and Mn oxides in the root apoplast, the so-called Fe plaque (Bacha and Hossner 1977). There is little direct evidence for root-induced oxidation of the outer rhizosphere. Flessa and Fischer (1992) and Revsbech et al. (1999) showed that redox potential increased only at the very close surface of rice roots. In contrast, rice plants were shown able to oxidise their rhizosphere up to several millimetres away from roots, according to the observed increase in Fe(III)/Fe(II) ratio (Begg et al. 1994).
Iron plaque in roots of wetland plants is mainly composed of Fe oxides, such as ferrihydrite (81–100%) and goethite (0–19%) (Hansel et al. 2001; Liu et al. 2006) which are known for their large arsenate [As(V)] adsorption capacity (Meng et al. 2002). Chen et al. (2005) observed that Fe plaque formation decreased the uptake kinetics of arsenate, which is the dominant As species in Fe plaque according to Liu et al. (2006). Iron plaque is therefore considered as able to sequester As, acting as a buffer to restrict As accumulation in rice plant (Liu et al. 2004a, b; Meharg and Rahman 2003; Liu et al. 2005). Most of these previous works have however been conducted in hydroponically grown rice.
It can be therefore hypothesized that oxidation due to O2 input at the soil surface or in the rhizosphere of rice would have a drastic impact on As dynamics at micro-scale, as related to the dynamics of Fe and Mn. However, to our knowledge, there is no direct evidence of the establishment of As gradients at the oxic/anoxic interfaces occurring in flooded paddy soils.
To address this question, the soil and rhizosphere chemical properties need to be assessed at appropriate temporal and spatial scales, as commonly performed with microcosm and root-mat approaches (Begg et al. 1994; Hinsinger and Gilkes 1995; Ratering and Schnell 2000; Wenzel et al. 2001b). Therefore, a specific soil microcosm either planted or not with a root-mat technique was designed to mimic flooded conditions of paddy fields. This approach combined thin-slicing of the soil and in situ micro-probes for both spatial and temporal monitoring of soil and soil solution chemical properties. The present study investigated the dynamics of As, Fe and Mn, and the establishment of gradients at the soil surface and in the rhizosphere of rice grown in an As-affected paddy soil collected in Bangladesh. This paper should thereby contribute to elucidate the major processes involved in As dynamics in flooded soils and increase our understanding of As acquisition by rice plant.
Materials and methods
Soil sampling and handling – soil conditions
The topsoil of an eutric gleysol (FAO 1999), containing a total As content of 14 mg kg−1 (all values are expressed relative to dry weight), was sampled in a submerged paddy field in Bangladesh, in the district of Araihazar, 30 km east from Dhaka in January 2005 (Table 1). This level of As contamination was the result of successive flooding with As-contaminated irrigation waters (van Geen et al. 2006). After sampling, the soil was preserved in its initial reduced condition, Eh ≈ −230 mV as measured in the field by Garnier et al. (personal communication), in airtight glass bottles flushed with N2. In laboratory, soil samples were homogenized under N2 atmosphere in a glove box (Plas labs, USA).
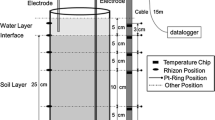
Perspex cylinders (30 mm height, 70 mm internal diameter), sealed with silicone (Rubson®) onto a PVC plate, were equipped with three platinum (Pt) micro-electrodes (20 mm length and 0.5 mm diameter for Pt wire; Cornu et al. 2007) and three soil solution samplers (Rhizon® MOM, 2.5 mm diameter, 70 mm length, Rhizosphere Research Products/SDEC France). Each kind of probe was inserted at 2, 11 and 21 mm from the surface of cylinders (Fig. 1a). Cylinders were then carefully filled with about 260 g moist soil (45% water content) under N2 atmosphere in a glove box. The microcosms were then kept for equilibrating for 3 days in such conditions before the start of the experiment.
Planted soil microcosm (a) and harvested planar mat of roots at the end of the soil stage (b) showing the homogeneous reddish-brown colour of roots. For ( a ) (1) PVC lid, (2) deionised water layer, (3) silicone grease, (4) root mat, (5) nylon mesh (30 μm pore diameter), (6) hole for redox reference electrode insertion, (7) soil, (8) Rhizon® soil solution samplers, (9) Pt redox micro-electrode, (10) CaCl2 10−3 M, (11) hole for water and salt bridge and (12) Perspex cylinders (30 × 70 mm); the two other Pt micro-electrodes and the last Rhizon® were set up in the perpendicular plan
Hydroponic and soil stages – plant growth conditions
After surface sterilisation (H2O2 6%, 15 min), seeds (Boro rice, Orysa sativa L. cv. BRRI dhan 29) were rinsed with deionised water and germinated on filter paper moistened with 600 μM CaCl2 and 2 μM H3BO3. This was conducted in the dark in a growth chamber (day/night: 16:8 h, 25°C/20°C, 70% humidity, photon flux of 480–520 μmol photons m−2 s−1 at 400–800 nm). Rice seedlings were then placed in Perspex cylinders (six plants per container) with a nylon mesh (30 μm pore diameter) glued at the bottom so that roots formed a dense, planar mat (Fig. 1a and b). A PVC lid, with six holes to enable rice seedlings to grow through, was glued into the containers with silicone (Rubson®). Seedlings in plant containers were then grown in 6-dm3 buckets (three containers per bucket) during 27 days with the following nutrient solution (μM), adapted from Cheng et al. (2004) and Calba et al. (2004): KNO3 300, NH4NO3 150, MgSO4 200, CaCl2 100, FeNaEDTA 100, K2SO4 50, KH2PO4 10, H3BO3 10, MnCl2 2, CuCl2 1, ZnSO4 1, CoCl2 0.07, Na2MoO4 0.05. The nutrient solution was changed every third day and pH was daily adjusted to 5.7 with 10−2 M NaOH. In parallel, three Perspex containers were prepared without plant to be used with the unplanted soil microcosms during the second stage of the experiment.
After the hydroponic stage, five containers with plants and three containers without plant were firmly pressed on top of soil-containing cylinders and sealed with silicone under N2 atmosphere in a glove box to prevent re-oxidation of the soil. The second part of the experiment was thereafter called soil stage. All containers were airtight close by putting silicone grease (Molykote 111, Dow Corning Corporation) at the base of each rice stem in the planted microcosms and in the holes of the PVC lids in the unplanted microcosms. In addition, a 15-mm layer of deionised water was poured onto the PVC lid. Each soil microcosm (planted and unplanted) was finally put in a capped box filled with CaCl2 10−3 M and transferred to the growth chamber for another 20 days. Microcosms with and without rice plants were thereafter called planted and unplanted soils, respectively.
Soil redox monitoring
Redox potential (Eh) for each Pt micro-electrode was measured daily (before soil solution sampling was carried out) with a reference electrode (Ag/AgCl) dipped into the CaCl2 solution. The salt bridge between soil solution and CaCl2 solution was ensured by the hole made at the bottom of soil containers (Fig. 1a). Due to the small diameter of the Pt-wire, the exact locations of redox potential measurement were 2, 11 and 21 (±0.5) mm from the soil surface and accounted for a small volume of soil along the 15-mm length of the Pt-wire contacting the soil. Redox potential expressed relative to H2 reference electrode was deduced by adding 198.7 mV to the measured Eh (Pansu and Gautheyrou 2003). Before being used, the Pt wire of each micro-electrode was treated according to Pansu and Gautheyrou (2003): 15 min in H2O2 30%, 15 min in HNO3 (d = 1.37), 15 min in HCl (d = 1.19) and 2 h in deionised water. All Pt micro-electrodes had initially been checked against a redox solution buffer (230 mV, 20°C, Schott Geräte). Two reference electrodes were successively used (Ref. AA02, Bioblock Scientific, up to day 9; Ref. 6.0259.100, Metrohm from day 10). The replacement took place once realising that the first electrode failed at giving correct measurements. The suitability of the second reference electrode was then checked every day with an additional Pt micro-electrode and the redox buffer. Data obtained from the first reference electrode were corrected by adding 202 mV to each measurements. This correction was based on the difference in Eh observed between the days 9 and 10 for the micro-electrodes which did not show any Eh variation before (day 8) and after (day 11) the replacement.
Soil solution collection and analyses
Rhizon® samplers were used to collect soil solution (Fig. 1a). Each Rhizon® had been previously cleaned by sucking 0.5 dm3 of deionised water before setting up in devices. Soil solution was collected after 1, 4, 7, 10, 13, 16 and 20 days of soil stage by connecting Rhizon® samplers to vacuum vials (Vacuette No-additive 10-cm3 vials, Greiner). At each sampling, the first 0.5 cm3 (dead volume) was discarded before collecting 1 cm3 of soil solution. Due to Rhizon® diameter (2.5 mm) and sampling volume, the exact depth of sampling was estimated to be about 0–6.5, 6.5–15.5 and 16.5–25.5 mm for the samplers inserted at 2, 11 and 21 mm from soil surface, respectively. Soil solution pH was measured immediately after sampling using a combined micro-electrode (Ref. 6.0224.100, Metrohm). Soil solution samples were stabilized with 1% ultra pur HCl (33–36%). The concentrations of As, Fe and Mn in soil solution were measured by inductively coupled plasma atomic emission spectrometry (ICP-AES, Ultima C, Jobin Yvon Horiba).
Soil slicing, extractions and analyses
At the end of the 20-day soil stage, soil cylinders were cut in thin slices parallel to the root mat with a purpose-built microtome (Hinsinger and Gilkes 1995): the thickness of the slices was 0.5 mm for the first four slices from soil surface, 2 mm for the two following slices and 5 mm for the last two slices. To prevent oxidation, soil slicing and subsequent extractions were conducted in a glove box under N2 atmosphere. Chemical extractions were adapted from the sequential procedure of Wenzel et al. (2001a) but were carried out as a single-step, parallel extractions under N2 atmosphere in a glove box to prevent oxidation: 0.5 g fresh soil (about 0.3 g dry mass) was mixed with 8 cm3 NH4H2PO4 0.05 M or NH4-oxalate 0.2 M (pH 3.25), shaken at 20°C (16 h for NH4H2PO4 and 4 h in the dark for NH4-oxalate) and then centrifuged for 15 min at 15,000×g. NH4H2PO4 extraction was chosen to target As specifically and non-specifically sorbed, while NH4-oxalate was chosen to recover As bound to amorphous and poorly crystalline Fe and Mn oxides (Wenzel et al. 2001a). Arsenic, Fe and Mn concentrations in soil extracts were measured by ICP-AES.
Plant analyses
The shoots and the root mat of plants in containers (five replicates) were harvested after soil stage and at the end of hydroponic stage to serve as a reference for calculating the uptake of As, Fe and Mn during the soil stage. Root mats appeared reddish-brown at the final harvest (Fig. 1b). To check the formation of Fe plaque and evaluate its As content, half of each root mat was treated with dithionite–citrate–bicarbonate (DCB) according to Liu et al. (2004b). Briefly, rice roots were incubated for 60 min at room temperature (20–25°C) in 40 cm3 solution containing: 0.03 M sodium citrate (Na3C6H5O7·2H2O), 0.125 M sodium bicarbonate (NaHCO3) and 0.6 g sodium dithionite (Na2S2O4). The compartment of DCB-treated roots, i.e. roots without Fe plaque or root symplast, was thereafter called roots for simplicity. Roots were rinsed thrice with deionized water. DCB- and non-treated roots and shoots were dried at 65°C, then finely ground (MM 2000, Retsch). Plant material was then digested in a microwave oven at 180°C for 10 min under 20 bar pressure (Ethos Touch Control, Milestone) after adding 1.5 cm3 HNO3 (65%, reagent grade) to 50 mg dry matter in Teflon vessels.
The concentrations of As, Fe and Mn in plant digests were measured by ICP-AES. Six blanks, four standard reference materials of maize shoots (Zea mays L., V463, Bureau InterProfessionnel d’Etudes Analytiques, France) and two standard reference materials of olive leaves (Olea europaea L., n° 62, Community Bureau of Reference, Commission of the European Communities) were included during digestion and analysis to check the accuracy of measurements. Arsenic content in the latter reference material (0.2 mg kg−1) was lower than our working limit (1.7 mg kg−1). However, samples were not polluted as As was not detected in the six blanks. In addition, As volatilisation was unlikely to occur as Ultra et al. (2007) obtained correct concentrations after a digestion procedure conducted at 300°C. Consequently, our measurements of As contents are relevant particularly for root material which exhibited concentrations 60- to 240-fold larger than our working limit.
Statistics
Analysis of variance, using LSD Fisher test, were performed with Statistica (version 6, StatSoft).
Results
Temporal and spatial patterns of redox potential in soil
During the first 5 days, Eh values globally decreased at 2, 11 and 21 mm from the surface of both unplanted and planted soils (Fig. 2). They reached a basal Eh level close to −230 mV, indicative of the setting of reducing conditions in soil. From day 7 onwards, the Eh values measured at 2 and 11 mm from the surface of unplanted soil increased up to +50 and −20 mV, respectively, and were significantly higher than Eh values at 21 mm (P < 0.01, Fig. 2a). This suggests that an O2 input and consequent oxidation occurred at the surface of unplanted soils, possibly due to gas exchanges through failures in the silicone grease sealing the holes of the PVC lid in unplanted soils. This effect was not observed in the rice-planted soils, where strongly reduced conditions were maintained without any significant Eh difference at 2, 11 and 21 mm from the soil surface (Fig. 2b).
Temporal and spatial patterns of redox potential in unplanted (a) and planted (b) soils. Micro-electrodes were set up at 2, 11 and 21 mm from the surface of soil compartment. Horizontal and vertical lines indicate 0 mV and the date where reference electrode was changed (see “Materials and methods”), respectively. Error bars represent standard errors
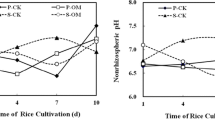
Temporal and spatial patterns of pH in soil solution
Whatever the location in soil, soil solution pH slightly decreased (≈0.2 pH unit) but remained neutral, between 6.8 to 7.2, during soil stage both in unplanted and planted soils (Fig. 3a and b). From day 4 onwards, pH was slightly but significantly lower (P < 0.001) in planted than unplanted soils suggesting the occurrence of root-mediated acidification of the rhizosphere and substantial diffusion of protons in soil (up to at least 21 mm).
Temporal and spatial patterns of Fe, Mn and As concentrations in soil solution
Both in unplanted and planted soils, Fe concentrations in soil solution steeply increased from the first day of soil stage up to day 7 (Fig. 4a and b), which is likely related to the simultaneous Eh decrease that occurred during the first days of soil stage. From day 7 onwards, Fe concentrations were stable at all depths and equal to 30 (±6) and 15–23 mg Fe dm−3 in planted and unplanted soils, respectively. All along the soil stage, Mn concentrations remained stable at all depths and equal to 10 (±2) and 5.4–8 mg Mn dm−3 in planted and unplanted soils, respectively (Fig. 4c and d). From day 7 onwards, Fe and Mn concentrations at 2 and 11 mm from the soil surface were significantly (P < 0.01) higher in planted than unplanted soils (Fig. 4a,b,c and d). From day 10 onwards, Fe and Mn concentrations in unplanted soils ranked as follows: [ ]2 mm ≤ [ ]11 mm < [ ]21 mm (P < 0.01, Fig. 4a and c). This pattern could be attributed to the localised oxidation occurring at the surface of unplanted soils.
Whatever the Rhizon® depth (2, 11 or 21 mm) and soil (planted or unplanted), As concentration in soil solution increased up to 3.3 mg dm−3 at depth 21 mm at day 4, which is likely related to the reductive dissolution of Fe oxides, and then decreased to less than 1 mg dm−3 (Fig. 4e and f). Both in planted and unplanted soils during the whole soil stage, As concentration in soil solution ranked as follows: [As]2 mm < [As]11 mm ≤ [As]21 mm (P < 0.01, Fig. 4e and f). Arsenic concentration were in the range 0.5–1.4 mg dm−3 at 2 mm from the root mat. No significant plant effect could be noted.
Spatial pattern of Fe, Mn and As contents in the soil solid-phase
Iron and Mn contents in NH4H2PO4 extracts were small, especially Fe which was rather steady around 3 mg kg−1, with no difference between planted and unplanted soils (data not shown). Iron and Mn contents in NH4-oxalate extracts also showed little significant difference between planted and unplanted soils (Fig. 5a and b). For Mn-oxalate, there was no variation all along the soil profile in the planted soil and a steep, two-fold increase in the first soil slice (<0.5 mm). For Fe-oxalate, there was a general trend towards an increase with increasing depth in both planted and unplanted soils, which significantly differed only in the first soil slice (<0.5 mm): there, the Fe-oxalate content in unplanted soils (2,947 ± 128 mg kg−1) was higher than the Fe-oxalate content in the rhizosphere (2,269 ± 178 mg kg−1).
Spatial patterns of Fe (a), Mn (b) and As (c and d) contents in NH4-oxalate (Fe, Mn and As) and NH4H2PO4 (As only) soil extracts as a function of the distance from the surface of unplanted and planted soils. Dashed line in graphic (d) indicates the initial total content of As in soil. Error bars represent standard errors
Arsenic contents in NH4H2PO4 (As-H2PO4) and NH4-oxalate (As-oxalate) extracts exhibited much higher values (7.8 ± 0.4 mg As-H2PO4 kg−1 and 25.7 ± 2.8 mg As-oxalate kg−1) in the first 0.5 mm of unplanted soils (Fig. 5c and d) than deeper in the soil where concentrations remained almost constant. Remarkably, the contents of As-oxalate at the surface of the unplanted soil cylinders were even greater than the total content of As measured in soil samples before the beginning of the experiment (14 mg kg−1). In contrast, no such high content occurred in the first millimetre of planted soils but rather constant As contents (2.8 ± 0.5 mg As-H2PO4 kg−1 and 6.9 ± 1.1 mg As-oxalate kg−1) were observed all along the profile (Fig. 5c and d).
Contents and distributions of Fe, Mn and As in Fe plaque, roots and shoots
Iron and Mn contents in the DCB extracts (expressed relative to root dry biomass) significantly increased between the end of hydroponics and the end of soil stage (Fig. 6a and b). They finally amounted to 7.5 (±0.6) g Fe kg−1 and 0.29 (±0.09) g Mn kg−1. Iron and Mn were distributed in plant as follows: FeFe plaque >> FeRoots> FeShoots and MnFe plaque = MnShoots > MnRoots (P < 0.01, Fig. 6a and b).
Contents and distributions of Fe (a), Mn (b) and As (c) in Fe plaque (DCB extracts), root symplast (Roots) and shoots of rice after hydroponic (DCB extracts only, white column) and soil (dark columns) stages. Different letters indicate a significant difference (P < 0.01, n = 5). The stars indicate a significant difference of As, Fe or Mn in DCB extracts between hydroponic and soil stages (P < 0.01, n = 5). Arsenic was not detected in DCB extracts after hydroponic stage (n.d. stands for not detectable, <1.7 mg As kg−1). Error bars represent standard errors
Arsenic was not detected (<1.7 mg kg−1) in shoots, DCB- and non-treated roots at the end of hydroponics. Arsenic was not detected in shoots for three out of five of the replicates after the soil stage. For the average calculation of As content in shoots, 1.7 mg As kg−1 was arbitrarily assigned to these replicates. Arsenic content reached 2.3 (±0.8), 112 (±15) and 173 (±29) mg kg−1 in shoots, roots and Fe plaque respectively and was therefore distributed similarly to Fe: AsFe plaque > AsRoots ≫ AsShoots (P < 0.01, Fig. 6c).
Discussion
Arsenic dynamics at the surface of a flooded paddy soil
Although the bulk of unplanted soils remained under strongly reduced conditions during the main part of soil stage, Eh values at 2 and 11 mm from the surface of soil increased, indicating that some oxidation occurred (Fig. 2a). The O2 input was mediated by the entrance of deionised water containing dissolved O2 at the surface of soil cylinders. This phenomenon which was observed in unplanted soils was similar to that usually reported under field conditions at the surface of flooded paddy soils. Formerly, Revsbech et al. (1999) and Lüdemann et al. (2000) noted the penetration of dissolved O2 originating in the flooding water into the first 2–2.5 mm of soil profiles, leading to the setting of an oxic/anoxic interface.
Ratering and Schnell (2000) reported an increase in Fe(III) extracted by 0.5 M HCl due to the oxidation of Fe(II) to Fe(III) within this oxic/anoxic interface and to the subsequent precipitation of Fe oxides. Similarly, the increase in As-H2PO4 and As-oxalate in the first millimetres of unplanted soils (Fig. 5c and d) suggests that some As adsorption and/or co-precipitation with freshly precipitated Fe and Mn oxides took place. The concomitant changes of Fe- and more so Mn-oxalate at the surface (<0.5 mm) of unplanted soils provided another indication of Fe and Mn oxide precipitation in this oxic layer (Fig. 5a and b). This was definitively the case for Mn, while for Fe, the higher Fe-oxalate content near the surface was rather small in unplanted soils (but large relative to the planted soils). However, it amounted about 3,000 mg Fe kg−1 in the bulk of the soil, which means that some additional precipitation of Fe oxide may not be easy to distinguish from this background value. The redox potential measured at 2 mm (≤ + 50 mV) was not high enough to enable the oxidation of As, Fe or Mn, suggesting that the redox potential reached higher values (up to +100 or +200 mV at least; Masscheleyn et al. 1991) in the surface layer (<2 mm) of unplanted soils. This oxidised layer acted as a sink for As, Fe and Mn and might explain the depletion of As, Fe and Mn which occurred in soil solution monitored as revealed by the samplers located at 2 mm from the surface of unplanted soils (Fig. 4a, c and e). This depletion profile would be the driving force to the diffusion of As, Fe and Mn from the well established anoxic bulk soil to the oxidised layer. The accumulation of As-oxalate in the first 0.5 mm of unplanted soils up to values which were well over the initial total content of soil As can only be explained by this diffusive mechanism (Fig. 5d). It was also noted that, after an initial increase of As in soil solution due to the reductive dissolution of Fe and Mn oxides, As in soil solution steadily decreased up to the end of the soil stage (Fig. 4e). This could be partly explained by the diffusion of As towards the surface of unplanted soils which involved the transport of about 75 (±27) μg As (as inferred from As-oxalate increase), i.e. roughly 3% of the initial amount of As in the whole soil cylinders.
According to the redox conditions occurring in natural flooded paddy fields, the results reported here from unplanted soils suggest a transport and an accumulation of As in the top-soil in association with freshly precipitated Fe and Mn oxides.
Arsenic dynamics in the rhizosphere of rice
In planted soils, no oxidation was observed in the first millimetres of soil in contact with the root mat, i.e. in the outer rhizosphere (Figs. 2b and 5). However, O2 leakage from rice roots induced the formation of Fe and Mn oxides in the root apoplast and at the surface of roots (Fig. 1b), the so-called Fe plaque, as evidenced by the increase in DCB-extractable Fe and Mn in rice roots (Fig. 6a and b). This plaque appeared mainly composed of Fe oxides as the Fe/Mn ratio of the DCB extract was about 25. The absence of oxidation in the outer rhizosphere is in agreement with previous findings: Flessa and Fischer (1992) and Revsbech et al. (1999) noted through Eh and O2 measurements that oxidation was mostly restricted to root surface and did not extend further than 0.4 mm from roots in the outer rhizosphere. These results suggest a very high consumption of O2 released by rice roots in the rhizosphere, leading to a very thin oxidized layer, the Fe plaque, mostly located within the root apoplast and at the surface of roots. Begg et al. (1994), Saleque and Kirk (1995) and Kirk and Bajita (1995) nevertheless found an increase in Fe(III) concentration up to two millimetres away from the soil–root mat interface, and a decrease in Fe(II) which extended further away.
Plant roots also induced a slight pH decrease (≈0.2 pH unit) in the rhizosphere compared with unplanted soils (Fig. 3). Since the localised oxidation at the surface of unplanted soils did not induce any significant pH gradient, the observed rhizosphere acidification in the planted soils was likely a consequence of plant nutrition. Under reduced conditions, rice plant takes up nitrogen mainly as ammonium, involving a root-induced release of protons to balance the uptake of excess cations over anions (Begg et al. 1994, Hinsinger et al. 2003). Kirk and Bajita (1995) and Revsbech et al. (1999) also noted that rhizosphere acidification was limited to 0.2 pH unit in neutral soils, likely due to the pH buffering capacity of these soils, while Begg et al. (1994) and Saleque and Kirk (1995) reported acidifications up to 2 pH units in more acidic soils. In the present work, As, Fe and Mn dynamics in the rhizosphere were most probably little affected by this minor pH change at the root–soil interface and thus mostly controlled by the oxidation-mediated development of the so-called Fe plaque.
Arsenic accumulated in Fe plaque with an Fe-plaque/soil As concentration factor of 12. Liu et al. (2006) measured concentration factors up to 60 for mature rice plants. Consequently, Fe plaque acted as a sink for As through As adsorption and/or co-precipitation onto Fe and Mn oxides in a similar manner as the oxidised layer at the surface of unplanted soils. This process may have generated the observed depletion of As in solution at the soil–root mat interface up to at least two millimetres (Fig. 4f) and, hence, induced the diffusion of As from the anoxic bulk soil to the Fe plaque. Arsenic accumulation in plant (roots plus shoots) involved the diffusive transport of 520 (±140) μg As, i.e. 21% of the initial amount of As in the whole soil cylinders. Similarly to what occurred in unplanted soils, the large flux of As to the root mat could be responsible for the steady decrease in As concentration which was observed in soil solution for the last 16 days of the soil stage. This result suggests a root-induced decrease in the ability of the soil to re-supply As from the solid phase to the solution, mainly due to Fe plaque (60% of the whole As recovered in rice plant). Fitz et al. (2003) already reported a root-induced decrease of As re-supply from the soil solid-phase in the rhizosphere of the As hyperaccumulator Pteris vittata L. However, compared with the maximal root density of 2 mg cm−3 measured in the 3-cm top-soil of a flooded paddy soil (Frenzel et al. 1999), the root density was 7.5 times higher in the present work. Although the overall effect of Fe plaque on As dynamics in the rhizosphere is clearly described in the root-mat approach used in the present work, this sink effect is likely over-estimated as compared to what could occur under field conditions.
Arsenic acquisition by rice plant as affected by Fe plaque
Despite the accumulation of As in the Fe plaque, As content of root symplast (i.e. DCB-treated roots) reached 112 mg kg−1. According to the lower content of Fe recovered in the DCB extracts than previously reported in the literature (Taylor and Crowder 1983; Liu et al. 2004a, b, 2005), part of the Fe plaque may not have been dissolved. Consequently, some of the As bound to Fe plaque was possibly not released by DCB treatment and therefore considered as part of the root-symplast fraction. Nevertheless, the very low As content of rice shoots (2–3 mg kg−1) clearly demonstrated that Fe plaque acted as an efficient barrier against As translocation towards rice shoots, given the fairly large As concentrations found in soil solution (up to 1.4 mg dm−3 at 2 mm from the root–soil interface) in the present work. Arsenic and Fe dynamics could also be linked within the plant as these two elements showed similar distributions (Fig. 6a and c), in agreement with the linear correlations between As and Fe reported by Liu et al. (2004a) in Fe plaque and shoots of different rice cultivars grown in hydroponics.
Conclusion
Through a microcosm approach which combined thin-slicing of the investigated soil and in situ micro-probes for both spatial and temporal monitoring of soil biogeochemistry, this study explored the impact of O2 input at the surface of soil and in the rhizosphere of rice on As dynamics in a flooded paddy soil. The oxidation was spatially restricted to the first millimetre of the soil surface in unplanted soils and to the Fe plaque formed in the apoplast and at the surface of rice roots. In addition, the setting of these two oxic/anoxic interfaces appeared as the key process controlling the mobility and the fate of As in flooded soils. Due to their affinity for As, oxides precipitating at the soil surface or in the Fe plaque acted as a sink, thereby scavenging As from deeper in the anoxic soil. This resulted in an As gradient in the soil solution extending over several millimetres from the oxic layer and in the rhizosphere. This depletion of As in soil solution was the driving force for the diffusion and the accumulation of As in the oxic layer and the Fe plaque. Despite the large As concentration in soil solution, As concentration in rice shoots was small, suggesting that the Fe plaque was efficiently sequestering As and restricting As acquisition. The observation of a limited extent of rhizosphere oxidation remains a matter of concern as it could play a prominent role in the capacity of rice plant to withstand As contamination. The magnitude of redox changes in the rhizosphere mainly depends on the O2-leakage capacity of rice roots (Liu et al. 2004a) and the buffering capacity of soils (Flessa and Fischer 1992). Further research is therefore needed with a broad range of soils and rice genotypes.
References
Ando T, Yoshida S, Nishiyama I (1983) Nature of oxidizing power of rice roots. Plant Soil 72:57–71
Bacha RE, Hossner LR (1977) Characteristics of coatings formed on rice roots as affected by iron and manganese additions. Soil Sci Soc Am J 41:931–935
Begg MCB, Kirk GJD, Mackenzie AF, Neue HU (1994) Root-induced iron oxidation and pH changes in the lowland rice rhizosphere. New Phytol 128:469–477
Calba H, Firdaus, Cazevieille P, Thée C, Poss R, Jaillard B (2004) The dynamics of protons, aluminium, and calcium of maize cultivated in tropical acid soils: experimental study and modelling. Plant Soil 260:33–46
Chen Z, Zhu YG, Liu WJ, Meharg AA (2005) Direct evidence showing the effect of root surface iron plaque on arsenite and arsenate uptake into rice (Oryza sativa) roots. New Phytol 165:91–97
Cheng Y, Howieson JG, O’Hara GW, Watkin ELJ, Souche G, Jaillard B, Hinsinger P (2004) proton release by roots of Medicago murex and Medicago sativa growing in acidic conditions, and implications for rhizosphere pH changes and nodulation at low pH. Soil Biol Biochem 36:1357–1365
Cornell RM, Schwertmann U (2003) The iron oxides, 2ndnd edn. Wiley, Weinheim, New York
Cornu JY, Staunton S, Hinsinger P (2007) Copper concentration in plants and in the rhizosphere as influenced by the iron status of tomato (Lycopersicon esculentum L.). Plant Soil 292:63–77
FAO (1999) World reference base for soil resources. FAO, Roma, p 96
Fitz WJ, Wenzel WW, Zhang H, Nurmi J, Stipek K, Fischerova Z, Schweiger P, Kollensperger G, Ma LQ, Stingeder G (2003) Rhizosphere characteristics of the Arsenic hyperaccumulator Pteris vittata L. and monitoring of phytoremoval efficiency. Environ Sci Technol 37:5008–5014
Flessa H, Fischer WR (1992) Plant-induced changes in the redox potentials of rice rhizospheres. Plant Soil 143:55–60
Frenzel P, Bosse U, Janssen PH (1999) Rice roots and methanogenesis in a paddy soil: ferric iron as an alternative electron acceptor in the rooted soil. Soil Biol Biochem 31:421–430
Hansel CM, Fendorf S, Sutton S, Newville M (2001) Characterization of Fe plaque and associated metals on the roots of mine-wasted impacted aquatic plants. Environ Sci Technol 35:3863–3868
Hinsinger P, Gilkes RJ (1995) Root-induced dissolution of phosphate rock in the rhizosphere of lupins grown in alkaline soil. Aust J Soil Res 33:477–489
Hinsinger P, Plassard C, Tang C, Jaillard B (2003) Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant Soil 248:43–59
Kirk GJD, Bajita JB (1995) Root-induced iron oxidation, pH changes and zinc solubilization in the rhizosphere of lowland rice. New Phytol 131:129–137
Liesack W, Schnell S, Revsbech NP (2000) Microbiology of flooded rice paddies. FEMS Microbiol Rev 24:625–645
Liu WJ, Zhu YG, Smith FA, Smith SE (2004a) Do iron plaque and genotypes affect arsenate uptake and translocation by rice seedlings (Oryza sativa L.) grown in solution culture? J Exp Bot 55:1707–1713
Liu WJ, Zhu YG, Smith FA, Smith SE (2004b) Do phosphorus nutrition and iron plaque alter arsenate (As) uptake by rice seedlings in hydroponic culture? New Phytol 162:481–488
Liu WJ, Zhu YG, Smith FA (2005) Effects of iron and manganese plaques on arsenic uptake by rice seedlings (Oryza sativa L.) grown in solution culture supplied with arsenate and arsenite. Plant Soil 277:127–138
Liu WJ, Zhu YG, Hu Y, Williams PN, Gault AG, Meharg AA, Charnock JM, Smith FA (2006) Arsenic sequestration in iron plaque, its accumulation and speciation in mature rice plants (Oryza sativa L.). Environ Sci Technol 40:5730–5736
Lüdemann H, Arth I, Liesack W (2000) Spatial changes in the bacterial community structure along a vertical oxygen gradient in flooded paddy soil cores. Appl Environ Microb 66:754–762
Masscheleyn PH, Delaune RD, Patrick WH Jr (1991) Effect of redox potential and pH on arsenic speciation and solubility in a contaminated soil. Environ Sci Technol 25:1414–1419
Meharg AA, Rahman M (2003) Arsenic contamination of Bangladesh paddy field soils: implications for rice contribution to arsenic consumption. Environ Sci Technol 37:229–234
Meng X, Korfiatis GP, Bang S, Bang KW (2002) Combined effects of anions on arsenic removal by iron hydroxides. Toxicol Lett 133:103–111
Pansu M, Gautheyrou J (2003) L’analyse du sol minéralogique, organique et minérale. Springer, France, p 1012
Ratering S, Schnell S (2000) Localization of iron-reducing activity in paddy soil by profile studies. Biogeochemistry 48:341–365
Revsbech NP, Pedersen O, Reichardt W, Briones A (1999) Microsensor analysis of oxygen and pH in the rice rhizosphere under field and laboratory conditions. Biol Fertil Soils 29:379–385
Saleque MA, Kirk GJD (1995) Root-induced solubilization of phosphate in the rhizosphere of lowland rice. New Phytol 129:325–336
Takahashi Y, Minamikawa R, Hattori KH, Kurishima K, Kihou N, Yuita K (2004) Arsenic behavior in paddy fields during the cycle of flooded and non-flooded periods. Environ Sci Technol 38:1038–1044
Taylor GJ, Crowder AA (1983) Use of DCB technique for extraction of hydrous iron oxides from roots of wetland plants. Am J Bot 70:1254–1257
Ultra Jr VU, Tanaka S, Sakurai K, Iwasaki K (2007) Effects of arbuscular mycorrhiza and phosphorus application on arsenic toxicity in sunflower (Helianthus annuus L.) and on the transformation of arsenic in the rhizosphere. Plant Soil 290:29–41
van Geen A, Rose J, Thoral S, Garnier JM, Zheng Y, Bottero JY (2004) Decoupling of As and Fe release to Bangladesh groundwater under reducing conditions. Part II: evidence from sediment incubation. Geochim Cosmochim Acta 68:3475–3486
van Geen A, Zheng Y, Cheng Z, He Y, Dhar RK, Garnier JM, Rose J, Seddique A, Hoque MA, Ahmed KM (2006) Impact of irrigating rice paddies with groundwater containing arsenic in Bangladesh. Sci Total Environ 367:769–777
Wenzel WW, Kirchbaumer N, Prohaska T, Stingeder G, Lombi E, Adriano CD (2001a) Arsenic fractionation in soils using an improved sequential extraction procedure. Anal Chim Acta 436:309–323
Wenzel WW, Wieshammer G, Fitz WJ, Puschenreiter M (2001b) Novel rhizobox design to assess rhizosphere characteristics at high spatial resolution. Plant Soil 237:37–45
Acknowledgments
This research was funded by the ACI “Ecodyn” (French Ministry of Research). We deeply thank Michaël Clairotte and Jean-Louis Aznar for the analyses, Nicole Balsera and Didier Arnal for the technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Yongguan Zhu.
Rights and permissions
About this article
Cite this article
Bravin, M.N., Travassac, F., Le Floch, M. et al. Oxygen input controls the spatial and temporal dynamics of arsenic at the surface of a flooded paddy soil and in the rhizosphere of lowland rice (Oryza sativa L.): a microcosm study. Plant Soil 312, 207–218 (2008). https://doi.org/10.1007/s11104-007-9532-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-007-9532-x