Abstract
Pristine peatlands covered by Histosols (bogs and fens) with high water table and a restricted oxygen (O2) availability are known to have low emissions of nitrous oxide (N2O) but may be a significant source for atmospheric methane (CH4) which are both important greenhouse gases. For the first time N2O and CH4 fluxes of a pristine slope mire in the German Harz Mountains have been monitored. Previously reported peatlands are characterised by anaerobic conditions due to high water table levels. Slope mires monitored here receive O2 through slope water inflow. Gas fluxes have been monitored deploying closed chamber method on a central non-forested area and a forested area at the periphery of the slope mire. By means of groundwater piezometers water table levels, ammonium and nitrate contents as well as hydro-chemical variables like oxygen content and redox potential of the mire pore water have been concurrently measured with trace gas fluxes at both monitoring sites of the slope mire. The slope mire took up small amounts of atmospheric methane at a rate of −0.02 ± 0.01 kg C ha−1 year−1 revealing no significant difference between the forested and non-forested site. Higher uptake rates were observed during low water table level. In contrast to pristine peatlands influx of oxygen containing pore water into slope mire does limit reduction processes and resultant CH4 emission. N2O fluxes of the forested and non-forested sites of the slope mire did not differ and amounted to 0.25 ± 0.44 kg N ha−1 year−1. Higher emissions were observed at low water table levels and during thawing periods. In spite of favourable conditions N2O fluxes of the slope mire have been comparable to those of pristine peatlands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural peatlands, covered by Histosols (FAO/ISRIC/ISSS 1998), are important sinks for carbon (C) and nitrogen (N) due to prevailing permanent anaerobic conditions and resultant low rates of decomposition (Moore et al. 2004). They represent only about 3% (3.88–4.38 * 106 km2) of the world’s area but comprise up to 30% of their C and N reserves (Martikainen et al. 1993). Thus, peatlands play an important role in the global C and N cycle and they may also contribute significantly to the production and consumption of the greenhouse gases nitrous oxide (N2O; Martikainen et al. 1993; Regina et al. 1996; Regina et al. 2004) and methane (CH4; Augustin et al. 1996; Khalil 1999) but have not been considered in respective global estimates (Brumme et al. 2005) due to large uncertainties resulting from the diversity of peatlands.
The global warming potentials (GWP) of nitrous oxide (N2O) and methane (CH4) are 310 and 21 times, respectively, than that of CO2 during a 100 year time scale (IPCC 2007). N2O is mainly produced in soils due to autotrophic nitrification and heterotrophic denitrification (Davidson 1991). Virgin peatlands with natural high water table levels show low N2O fluxes or consumption of N2O due to limitation of oxygen and hence absence of nitrification resulting in low NO3 − concentrations (Martikainen et al. 1993; Augustin et al. 1996; Regina et al. 1996; Aerts 1997; Brumme et al. 1999).
The greenhouse gas CH4 is produced during the anaerobic decomposition processes in anoxic parts of peats by methanogenic bacteria whereas it is oxidized in the presence of free oxygen by methanotrophic bacteria (Joabsson et al. 1999). Pristine peatlands are a significant source of CH4 to the atmosphere. Three emission pathways have been reported for CH4: molecular diffusion of dissolved CH4, ebullition (bubbling) of CH4 and transport of CH4 through plant roots and shoots (Tokida et al. 2005).
Previous studies have pointed out that both groundwater table levels and temperature mostly affected the fluxes of trace gases in peatlands (Augustin et al. 1996; Koponen and Martikainen 2004). The few studies of greenhouse gas emissions from virgin peatlands have been conducted in classical bogs and fens without permanent water inflow (e. g. Martikainen et al. 1993; Regina et al. 1996; Heikkinen et al. 2002). Reports of N2O and CH4 fluxes from slope mires, a peatland type widespread in mountain forests are lacking. Globally slope mires represent 1% of the world’s peatlands comprising an area of about 4 million hectare (Joosten, 2007, personal communication). Solely the different movement of soil water in slope mires should change controlling factors of gas formation and resulting greenhouse gas fluxes. We hypothesize that slope mires release more N2O because of increased influx of O2 which inhibits N2O reductase and concurrently restricts N2 formation. The influx of O2 might adversely affect CH4 formation and might favour CH4 oxidation resulting in lower CH4 emission from slope mires than that of pristine bogs and fens.
To test these hypotheses we conducted a field experiment in a pristine slope mire at the Harz Mountains with the following objectives:
-
1.
To quantify and to compare fluxes of N2O and CH4 in mire zones at a central non-forested and a peripheral forested area representing different nutrient status, water table levels and oxygen input caused by slope water inflow
-
2.
To monitor N contents and other relevant features of mire pore water in order to identify relationships to the production and consumption of N2O and CH4
-
3.
To find out seasonal dynamics of N2O and CH4 fluxes
Materials and methods
Study site
The study was conducted in the Harz mountains, central Germany (federal state Saxony-Anhalt), close to the highest peak of the Harz mountain (Mt Brocken, 1,142 m a.s.l., 51°48′N, 10°37′E) at an elevation of 843–860 m a.s.l. Springs of the Ilse stream characterize features of the studied area (Böhlmann et al. 2005). Average annual precipitation (1951–1980) amounts to 1,609 mm and mean annual temperature is +2.8°C (Wegener and Kison 2002). The annual bulk N deposition at the mire was about 51 kg ha−1 year−1 in 2003 of which 25 kg ha−1 year−1 was deposited as nitrate (Böhlmann 2004).
The investigated mire comprising Fibric Histosols covers a slope of 0.015 km2 which is part of a catchment of 0.13 km2. The mire was formed about 1,000 years ago (14C analysis, Böhlmann 2004) on granite debris of the Mt Brocken. The thickness of the peat layer is up to 1.4 m. The largest part of the slope mire is a swamp overgrown with spruce plants (0.01 km2) and a Calamagrostio villosae-Piceetum-community. Vegetation of the central part of the mire is dominated by a Eriophorum angustifolium-community and is mostly free of spruce trees (Böhlmann et al. 2005).
Field sampling and analysis
Fluxes of N2O and CH4 were measured biweekly with the closed chamber method during the study period that lasted from 19 June 2002 to 9 July 2003. Measurements have not been made at days with high snow cover during winter from November to March. For flux measurements, 10 chambers consisting of PVC rings (30 cm height, 30 cm diameter) were installed into the peat and remained throughout the entire study period. Four chambers were set up at the peripheral forested site and six chambers at the central non-forested site of the slope mire. Gas samples were taken at 0, 20 and 40 min after closing the chambers with a lid using evacuated glass flasks (100 ml). Before sampling, air pressure of the glass flasks was checked by pressure sensor (Loftfield et al. 1997). Air temperature was measured at a height of 2 m and soil temperatures were measured in 2.5, 5, 10 cm soil depth during gas sampling periods. The gas samples were analysed within few days by an automated gas chromatographic system consisting of a computer-controlled gas chromatograph (Carlo Erba) equipped with a flame ionization detector (FID) for CH4, an electron capture detector (ECD) for N2O measurements, and an autosampler for 64 sample flasks (Loftfield et al. 1997).
Peat samples were taken at the beginning of measurements in June 2002 next to the chambers from a depth of 0–20 cm. Air-dried peat samples were analysed for total C (Ct) and N (Nt) with an elemental analyzer (Hanau, Germany). The pH of the peat samples was determined after shaking with 0.01 M CaCl2. NH4 +and NO3 − of the peat samples have been extracted with a 2 M KCl-solution and analysed with a continuous-flow-analyser (Skalar, Netherlands) using the indophenol-blue method.
Groundwater piezometers were placed in the peat layer adjacent to the chambers. The groundwater piezometers were used to measure water table levels (WT), redox potentials (Eh, WTW, Germany), and oxygen levels (O2, WTW, Germany) at the time of gas measurements. Measurements were performed at a depth of 30 dcm. Furthermore, water samples were collected and analysed for ammonium, nitrate, pH (WTW, Germany) and electric conductivity (EC) (WTW, Germany).
Statistical analyses
All statistical analyses were performed using a SAS software package [SAS Institute (1999–2001), Inc., Cary, USA, Release 8.2] and Statistica for Windows v. 5.1 (StatSoft, Inc. 1996, Tulsa). Pearson correlation coefficients were calculated to elucidate relationships between gas fluxes, soil temperature and variables of mire pore water. One-way analysis of variance (ANOVA) has been used to test significance of differences between gas fluxes at the forested and non-forested part of the mire. Data were tested for normality and equality of variance before statistical analyses.
Results
Soil temperature, water table, peat soil, mire pore water
Thickness of peat differed significantly (p < 0.05, n = 4, Table 1) between the central and peripheral site of the mire being 105 and 56 cm respectively. Top layer (0–20 cm) of peat at both locations showed similar pH, bulk density, total N and C:N ratio while nitrate content was higher in the forested area of the mire (p < 0.05, n = 4, Table 1).
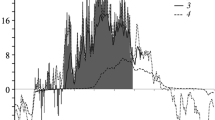
Not being different between soil depths soil temperatures ranged from −0.1 to 19.6°C for the dates monitored (Fig. 1a). Water table levels differed significantly between the forested and non-forested areas (p < 0.05, n = 16, Fig. 2a). At the peripheral forested area, water table levels varied between −1 and −10 cm below the mire surface and between −4 and −27 cm at the central non-forested part of the mire. The highest water table levels were found during snow melting in April 2003, the lowest in July 2002.
Pattern of pore water characteristics followed the temporal variation of the water level. Values of O2 being low during low water table levels in summer increased with water table in winter and peaked in April. On average the redox potential was 150 mV and 14 mV and O2 content averaged 2.6 mg L−1 and 1.4 mg L−1 at the forested and non-forested area, respectively. Resulting from a considerable spatial variation of O2 and Eh in pore water (Table 2) these values did not significantly differ. Values of pH, ammonium, total N and EC remained relatively constant during the study (not shown) while nitrate showed some variations with lowest values in May 2003 (Fig. 2b). In contrast to pH and values of ammonium and nitrate concentrations the conductivity of pore water differed significantly (p < 0.05, n = 16) between the forested and non-forested areas of the mire averaging 53 and 34 µS cm−1 respectively.
N2O
The N2O emissions ranged between −0.9 and +37.1 µg m−2 h−1 with peak emissions at the 19th June 2002 and 6th May 2003 (Fig. 1b).
No relationships were found between N2O release of the mire and soil temperature, water table level, Eh and ammonium concentration. Negative correlations were observed between N2O release and O2 contents at the central part of the mire and between N2O release and nitrate for the one measuring point only at the peripheral part of the mire (Table 3). Mean annual N2O emissions did not differ significantly between the sites and amounted to 0.4 ± 0.5 kg N ha−1 year−1 at the forested and 0.2 ± 0.4 at the non-forested site.
CH4
The mire was found to be a CH4 sink at almost all sampling dates (Fig. 1c). The annual CH4 uptake of the mire adds up to −0.02 ± 0.01 kg ha−1 CH4-C. The highest uptake of −88.7 µg m−2 h−1 occurred during low level of water table at the 19th June 2002. At all other observation dates CH4 fluxes of the mire varied around zero with CH4 rates of −1.8 to 0.005 µg m−2 h−1. The exceptional singular high peak uptake does cause the significant correlation between CH4 uptake and soil temperature (Table 3). None of the other variables revealed a significant correlation to CH4 fluxes. CH4 fluxes amounted to −0.01 ± 0.01 kg ha−1 year−1 at the forested and −0.02 ± 0.01 kg ha−1 year−1 at the non-forested areas and did not differ significantly.
Discussion
Temporal pattern of N2O emission
Two N2O emission peaks observed in this study could be classified as event-related emissions according to Brumme et al. (1999). One N2O peak of 30 µg m−2 h−1 was measured at the central part of the mire on 19th June 2002 during low water table level. The second high N2O release on 06th May 2003 followed a freezing–thawing periods. Increased N2O emissions during the freezing–thawing cycles have been reported by several other studies (Teepe et al. 2000; Koponen and Martikainen 2004; Koponen et al. 2006). Soil thawing has been suggested to favour denitrification processes due to high water saturation and limited O2 diffusion (Teepe et al. 2000). Furthermore freezing of soils destroys microbial cells providing substrates for denitrifying microbes (Papen and Butterbach-Bahl 1999). Inhibition of N2O reductase at low temperatures was discussed also as a reason for high N2O release during freezing–thawing cycles (Melin and Nommik 1983). Several authors reported that increasing nitrate concentrations in liquid water during frost enhance N2O production (Stähli and Stadler 1997).
With the exception of the event-related emissions N2O fluxes remained low at both sites despite differences in water table levels. N2O release of peatlands has been reported to be mainly affected by water table and nutrition status (Martikainen et al. 1993; Augustin et al. 1996; Regina et al. 1996; Brumme et al. 1999). Negative relationships between emitted N2O amounts and water table levels have been established in various studies (Maljanen et al. 2003; Drösler 2005) due to increases of O2 availability promoting nitrification in drier peat that either directly increased N2O formation or produce NO3 − as precursor of denitrification (Seitzinger 1994; Regina et al. 1996). Very high N2O emissions between 25 and 72 kg N2O-N ha−1 within 46 weeks were observed in single chambers at a drained alder forest in Bornhöved, Germany (Brumme et al. 1999). N2O emissions up to 27 kg N2O-N ha−1 year−1 have been monitored at newly drained peatlands with a decreasing water table level down to −60 cm (Augustin et al. 1998). Similar drainage caused high N2O releases up to 79 µg m−2 h−1 for a short time (Dorwick et al. 1999). Several authors observed annual N2O emission rates of >1 kg N ha−1 at mean water table levels of −23 to −50 cm (Regina et al. 1996; Augustin et al. 1998; Drösler 2005). Commonly found higher water table levels in our study might be the reason for the generally observed low N2O emission.
Annual N2O fluxes
Annual mean N2O fluxes amounting to 0.25 kg N2O-N ha−1 did not statistically differ between the monitoring sites at the mire. These fluxes are in line with those reported for various studies of pristine bogs and fens with high water table levels (Martikainen et al. 1993; Augustin et al. 1996; Regina et al. 1996; Drösler 2005). Hence our hypothesis that raising O2 input through flowing water increases N2O release from mire water below the water table level by decreasing N2 formation could not be confirmed. Likewise assumptions that observed high O2 concentrations in the pore water are assumed to restrict N2 formation when nitrate is not limiting denitrification processes due to high atmospheric input (Davidson 1991; Granli and Bøckman 1994, Scholefield et al. 1997) have to be questioned. Denitrification processes resulting in N2 formation in water and sediments require O2 contents of <0.1 to a maximum of 0.8 mg L−1 (Granli and Bøckman 1994; Mühlherr and Hiscock 1998; Venterink et al. 2003). Accordingly nitrate should not have been reduced to N2 in large amounts in our slope mire during the study period since the oxygen contents mostly exceeded these values. In addition low pH of the mire pore water should have restricted N2 formation. Acidity inhibits N2O reductase and thus increases the N2O:N2 ratio (Granli and Bøckman 1994; Aerts 1997). At the prevailing hydro-chemical conditions of our slope mire formation of N2O should be favoured compared to N2. Various authors reported that groundwater was oversaturated with N2O which emitted to the atmosphere when the water reached the surface (Mühlherr and Hiscock 1998; Hefting et al. 2006). N2O captured in groundwaters below our slope mire is likely to be emitted when those groundwaters are rising to the surface in the spring which obviously requires further investigation.
Temporal pattern of CH4 emissions
CH4 fluxes of the mire didn’t show any seasonal pattern. Hydro-chemical variables of the mire pore water did not explain fluxes except probably the high CH4 uptake rates of 88 µg m−2 h−1 at the central part of the mire on the day of 19th June 2002. The high uptake occurred during low water table level of −27 cm and was assumed to be the result of CH4 oxidation by aerobic methanotrophic bacteria when aerobic conditions in peat could be enhanced. Water table has been identified to be the major controlling factor of CH4 emission rates in peatlands. With the water table up to 62% of the variability of CH4 fluxes have been explained (MacDonald et al. 1998; Drösler 2005). Different water table levels have been reported at which peatlands may change from CH4 release to CH4 uptake. Some authors reported a critical water table level of >−10 cm (Christensen et al. 2003; Drösler 2005). In contrast Augustin et al. (1996) observed still small CH4 releases of 0.6–3.5 kg C ha−1 year−1 in drained mires with water table levels down to −60 cm. Beside water table levels plant species composition and cover of vascular plants are known to affect CH4 release from peatlands. Plant species with aerenchymous tissue are able to transport CH4 through the root surface without being oxidized by methanotrophic bacteria (Joabsson and Christensen 2001). Even though these CH4 fluxes would be included in our measurements the mire showed no significant CH4 release. Thus plant mediated CH4 fluxes seems not to be important at the study site although our slope mire show a high cover of vascular plants. Similarly positive impact of available nutrients and labile carbon on the CH4 release (Joabsson and Christensen 2001; Yavitt et al. 2005) could not be confirmed in our study.
Annual CH4 fluxes
Annual CH4 flux revealed an uptake of −0.02kg CH4-C ha−1 not differing between the forested and non forested parts of the slope mire. The small annual consumption of CH4 is in contrast to reported CH4 releases from peatlands with similar high water table levels (Augustin et al. 1996; Drösler 2005). Restricted oxygen diffusion causing strictly anaerobic conditions of Eh values lower than −250 mV and high thickness of peat enhancing anaerobic peat layer capable of CH4 production were discussed to be reasons for high methane fluxes in peatlands with high water table levels (Mitsch and Gosselink 2000). The uptake of CH4 at our slope mire might be the result of a restricted CH4 production due to O2 input through groundwater movement causing redox potential far above the necessary value for methane production. A weak positive correlation between water inflow and oxygen content in pore water (R = 0.52, p < 0.05, n = 16) does indicate the importance of flowing groundwater for methane oxidation in slope mires. An additional explanation for low CH4 emission rates might be the thin peat layer of our slope mire with low total production of methane not exceeding methane oxidation in the aerobic part of the slope mire.
Conclusions
In contrast to peatlands without permanent water inflow the studied slope mire showed oxygen influx through inflowing pore water restricting reduction processes. Compared to pristine peatlands the influx of oxygen is assumed to restrict CH4 production of the slope mire explaining its weak CH4 consumption. N2O release from the slope mire didn’t differ from those of reported peatlands with high water table levels and low oxygen availability. Nevertheless slope mires have a high potential for the N2O formation due to available NO3 −. Our assumption that areas of slope mires with springs may be hot spots for N2O releases requires further research.
References
Aerts R (1997) Atmospheric nitrogen deposition affects potential denitrification and N2O emission from peat soils in the Netherlands. Soil Biol Biochem 29:1153–1156
Augustin J, Merbach W, Schmidt W, Reining E (1996) Effect of changing temperature and water table on trace gas emission from minerotrophic mires. Angew Bot 70:45–51
Augustin J, Merbach W, Steffens L, Snelinski B (1998) Nitrous oxide fluxes of disturbed minerotrophic peatlands. Agribiol Res 51:47–57
Brumme R, Borken W, Finke S (1999) Hierarchical control on nitrous oxide emission in forest ecosystems. Glob Biogeochem Cycles 13:1137–1148
Brumme R, Verchot LV, Martikainen PJ, Potter CS (2005) Contribution of trace gases nitrous oxide (N2O) and methane (CH4) to the atmospheric warming balance of forest biomes. In: Griffiths H, Jarvis PG (eds) The carbon balance of forest biomes. Taylor & Francis, Oxon, New York, pp 293–318
Böhlmann N (2004) Wasser- und Stickstoffhaushalt eines soligenen Hangmoores im Hochharz am Beispiel des Ilsemoores. UFZ-Bericht 21, pp 252
Böhlmann N, Meissner R, Bernsdorf S, Böhme F, Russow R, Wegener U (2005) Studies of atmospheric nitrogen deposition in a mire of the German National Park Hochharz Mountains using two different methods. Water Air Soil Pollut 168:17–32
Christensen TR, Ekberg A, Ström L, Mastepanov M (2003) Factors controlling large scale variations in methane emissions from wetlands. Geophys Res Lett 30:1414–1419
Davidson EA (1991) Fluxes of nitrous oxide and nitric oxide from terrestrial ecosystems. In: Rogers JE, Whitman WB (eds) Microbial production and consumption of greenhouse gases: Methane, nitrogene oxides and halomethanes. American Society of microbiology, Washington, pp 219–235
Dorwick DD, Hughes S, Freeman C, Lock MA, Reynolds B, Hudson JA (1999) Nitrous oxide emissions from a gully mire in mid-Wales, UK, under simulated summer drought. Biogeochemistry 44:151–162
Drösler M (2005) Trace gas exchange and climatic relevance of bog ecosystems, Southern Germany. DS thesis, Technical University Munich, Munich, pp 179
FAO/ISRIC/ISSS (1998) World reference base for soil resources. World soil resources report, Vol. 84. FAO, Rome
Granli T, Bøckman OC (1994) Nitrous oxide from agriculture. Norw J Agric Sci 12:1–128
Hefting MM, Bobbink R, Janssens MP (2006) Spatial variation in denitrification and N2O emission in relation to nitrate removal efficiency in a N-stressed riparian buffer zone. Ecosystems 9:550–563
Heikkinen JEP, Elsakov V, Martikainen PJ (2002) Carbon dioxide and methane dynamics and annual carbon balance in tundra wetland in NE Europe, Russia. Global Biogeochem Cycles 16, DOI 10.1029/2002GB001930
IPCC (2007) Changes in atmospheric constituents and in radiative forcing. In: Solomon S, Quin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, New York, pp 212–213
Joabsson A, Christensen TR (2001) Methane emissions from wetlands and their relationship with vascular plants: an arctic example. Glob Chang Biol 7:919–932
Joabsson A, Christensen TR, Wallén B (1999) Vascular plant control on methane emissions from northern peatforming wetlands. Trends Ecol Evol 14:385–388
Khalil MAK (1999) Non-CO2 greenhouse gases in the atmosphere. Annu Rev Energy Environ 24:645–661
Koponen HT, Martikainen PJ (2004) Soil water content and freezing temperature affect freeze–thaw related N2O production in organic soil. Nutr Cycl Agroecosyst 69:213–219
Koponen HT, Duran CE, Maljanen M, Hytönen J, Martikainen PJ (2006) Temperature responses of NO and N2O emissions from boreal organic soil. Soil Biol Biochem 38:1779–1787
Loftfield N, Flessa H, Augustin J, Beese F (1997) Automated gas chromatographic system for rapid analysis of the atmospheric trace gases methane, carbon dioxide, and nitrous oxide. J Environ Qual 26:560–564
Macdonald JA, Fowler D, Hargreaves KJ, Skiba U, Leith ID, Murray MB (1998) Methane emission rates from a northern wetland; response to temperature, water table and transport. Atmos Environ 32:3219–3227
Maljanen M, Liikanen A, Silvola J, Martikainen PJ (2003) Nitrous oxide emissions from boreal organic soil under different land-use. Soil Biol Biochem 35:1–12
Martikainen PJ, Nykänen H, Crill P, Silvola J (1993) Effect of a lowered water table on nitrous oxide fluxes from northern peatlands. Nature 366:51–53
Melin J, Nommik H (1983) Denitrification measurements in intact soil cores. Acta Agric Scand 33:145–151
Mitsch JM, Gosselink JG (2000) Wetlands. Wiley, New York, p 920
Moore T, Blodau C, Turunen J, Roulet N, Richard PJH (2004) Patterns of nitrogen and sulfur accumulation and retention in ombrotrophic bogs, eastern Canada. Glob Chang Biol 11:356–367
Mühlherr IH, Hiscock KM (1998) Nitrous oxide production and consumption in British limestone aquifers. J Hydrol 211:126–139
Papen H, Butterbach-Bahl K (1999) 3-year continuous record of N-trace gas fluxes from untreated and limed soil of a N-saturated spruce and beech forest in Germany: I-N2O emissions. J Geophys Res 104:18487–18503
Regina K, Nykänen H, Silvola J, Martikainen PJ (1996) Fluxes of nitrous oxide from boreal peatlands as affected by peatland type, water table level and nitrification capacity. Biogeochemistry 35:401–418
Regina K, Syväsalo E, Hannukkala A, Esala M (2004) Fluxes of N2O from farmed peat soils in Finland. Eur J Soil Sci 55:591–599
Scholefield D, Hawkins JMB, Jackson SM (1997) Use of a flowing helium atmosphere incubation technique to measure the effects of denitrification controls applied to intact cores of a clay soil. Soil Biol Biochem 29:1337–1344
Seitzinger SP (1994) Linkages between organic matter mineralization and denitrification in eight riparian wetlands. Biogeochemistry 25:19–39
Stähli M, Stadler D (1997) Measurement of water solute dynamics in freezing soil columns with time domain reflectometry. J Hydrol 195:352–369
Teepe R, Brumme R, Beese F (2000) Nitrous oxide emissions from frozen soils under agricultural, fallow and forest land. Soil Biol Biogeochem 32:1807–1810
Tokida T, Miyazaki T, Mizoguchi M, Seki K (2005) In situ accumulation of methane bubbles in a natural wetland soil. Eur J Soil Sci 56:389–395
Venterink HO, Hummelink E, Van Den Hoorn MW (2003) Denitrification potential of a river floodplain during flooding with nitrate-rich water: grasslands versus reedbeds. Biogeochemistry 65:233–244
Wegener U, Kison HU (2002) Die Vegetation des Brockens im Nationalpark Hochharz (Exkursion G). Tüxenia 22:243–267
Yavitt JB, Williams CJ, Wieder RK (2005) Soil chemistry versus environmental controls on production of CH4 and CO2 in northern peatlands. Eur J Soil Sci 56:169–178
Acknowledgments
We would like to thank the German National Park Harz Mountains for its support of the studies, and the Federal State of Saxony-Anhalt and the Helmholtz Centre for Environmental Research-UFZ for post-graduate and financial support. Furthermore we thank Dr. Samia El-Guindy, National Water Research Center, APP (Kairo) and Prof. Neue, Helmholtz Centre for Environmental Research-UFZ for improving the English of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Bernard Nicolardot.
Rights and permissions
About this article
Cite this article
Tauchnitz, N., Brumme, R., Bernsdorf, S. et al. Nitrous oxide and methane fluxes of a pristine slope mire in the German National Park Harz Mountains. Plant Soil 303, 131–138 (2008). https://doi.org/10.1007/s11104-007-9493-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-007-9493-0